Abstract
Ketamine is an NMDA receptor antagonist with a variety of uses, ranging from recreational drug to pediatric anesthetic and chronic pain reliever. Despite its value in the clinical setting, little is known about the immediate and long-lasting effects of repeated ketamine treatment. We assessed the effects of chronic administration of a subanesthetic dose of ketamine on contextual fear conditioning, detection of pitch deviants and auditory gating. After four, but not two, weeks of daily ketamine injections, mice exhibited decreased freezing in the fear conditioning paradigm. Gating of the P80 component of auditory evoked potentials was also significantly altered by treatment condition, as ketamine caused a significant decrease in S1 amplitude. Additionally, P20 latency was significantly increased as a result of ketamine treatment. Though no interactions were found involving test week, stimulus and treatment condition, these results suggest that repeated ketamine administration impairs fear memory and has lasting effects on encoding of sensory stimuli.
Keywords: ketamine, event related potential, auditory evoked potential, gating, fear conditioning, schizophrenia, drug abuse
Introduction
The N-methyl-D-aspartate (NMDA) receptor antagonist ketamine is a pediatric and veterinary anesthetic whose recreational use in the United States has escalated dramatically since the 1990s (DAWN 2003). Though ketamine-naïve, non-drug abusers enjoy the effects of the drug at a low dose (Morgan, Mofeez et al. 2004), ketamine has repeatedly been shown to elicit and exacerbate psychotic symptoms (Krystal, Karper et al. 1994; Malhotra, Pinals et al. 1997; Newcomer, Farber et al. 1999; Morgan, Mofeez et al. 2004). These symptoms, which include auditory hallucinations, blunted affect and withdrawal, are all common to patients with schizophrenia. Therefore, studies verifying the psychotic effects of ketamine not only lend support to the NMDA hypofunction model of schizophrenia but also confirm its ability to replicate behavioral analogues of the disease in non-human studies (Lindsley, Shipe et al. 2006; Javitt 2007).
The induction of schizophrenia-related behaviors upon treatment with acute ketamine has been demonstrated in a wide range of rodent studies. In such animals acute ketamine has been shown to disrupt fear conditioning, mismatch negativity (MMN) and auditory gating, which all serve as endophenotypes for schizophrenia (Brown, Gordon et al. 2000; Javitt 2000; Michie 2001; Winterer, Egan et al. 2001; Ong, Brody et al. 2005; Maxwell, Ehrlichman et al. 2006; Pietersen, Bosker et al. 2007; Ehrlichman, Maxwell et al. 2008). Episodic memory impairments are the most severe cognitive dysfunctions associated with schizophrenia (Heinrichs and Zakzanis 1998). For this reason, the fear conditioning paradigm, in which an initially neutral (conditioned) stimulus is paired with an aversive (unconditioned) stimulus, is often used to assess memory in animal models of the disease.
In the MMN paradigm, a set of repeated stimuli is followed by a deviant, such as a tone of differing frequency. People with schizophrenia have a decreased ability to detect auditory deviants as measured by changes to the N100 and mismatch negativity (MMN) of their auditory evoked potentials (AEPs) (Javitt 2000; Michie 2001; Davalos, Kisley et al. 2003). The N100 is the negative-most deflection in the human AEP, a modality of event-related potentials (ERPs) occurring approximately 100 milliseconds (ms) post-stimulus whose mouse analog is termed the N40 (Yingling and Nethercut 1983; Covington and Polich 1996; Shelley, Silipo et al. 1999). The MMN generally occurs immediately following the N100 and is manifest as an amplified negative deflection to deviant stimuli (May, Tiitinen et al. 1999; Haenschel, Vernon et al. 2005). Gating refers to a reduction in the second of two successive stimuli. Patients with schizophrenia also exhibit impaired auditory gating of the N100, P50 and P200 AEP components (Shenton, Faux et al. 1989; Brockhaus-Dumke, Schultze-Lutter et al. 2008). The latter two components are positive midlatency ERP constituents occurring 50 and 200 ms post-stimulus whose mouse analogs are the P20 and P80, respectively. The method for assessing auditory gating is very similar in both humans and in mice, making this paradigm a highly translatable measure of schizophrenia-like deficits.
Despite its usage as an anesthetic agent, recreational drug and more recently as a treatment for chronic pain and depression, relatively few studies have addressed the effects of repeated ketamine administration (Berman, Cappiello et al. 2000; Zarate, Singh et al. 2006; Okon 2007; Reuben and Buvanendran 2007; Cvrcek 2008; Sinner and Graf 2008). However, several clinical studies have found that chronic ketamine administration for the treatment of pain has a variety of central nervous system side effects (Kannan, Saxena et al. 2002; Cvrcek 2008). In the present study, we use the three different paradigms discussed above in order to assess the immediate and lasting effects of chronic ketamine treatment in mice. A wealth of studies has demonstrated that repeated exposure to subanesthetic doses of ketamine leads to apoptosis, as well as lasting changes in brain physiology, suggesting the presence of irreversible changes in the brain (Scallet, Schmued et al. 2004; Young, Jevtovic-Todorovic et al. 2005; Slikker, Zou et al. 2007; Majewski-Tiedeken, Rabin et al. 2008; Zou, Patterson et al. 2009). We therefore hypothesized that chronic ketamine would impair contextual fear conditioning, novelty detection and sensory gating at all time points after cessation of treatment by such irreversible changes, rather than acute NMDA receptor (NMDAR) blockade.
Materials & Methods
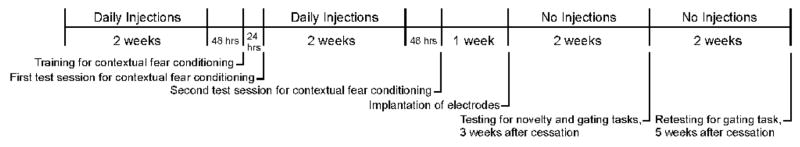
The overall study design is depicted in Figure 1.
Figure 1.
Schedule of injections and experimental testing.
Animals
Twenty-two male C57BL/6J (B6) mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA) at 5 to 6 weeks of age. Mice were acclimated to the housing facility for approximately one week prior to the commencement of injections. Four test sessions were conducted on mice between the following age ranges: 8 to 9 weeks; 10 to 11 weeks; 13 to 14 weeks; and 15 to 16 weeks. Mice were housed four or five per cage until electrode implantation, after which each mouse was housed singly. One ketamine-treated mouse died between contextual fear conditioning experiments, five saline- and one additional ketamine-treated mouse died before EEG recording began, and 1 ketamine-treated mouse died between EEG sessions. Water and standard rodent chow were available ad libitum. All protocols were conducted in accordance with University Laboratory Animal Resources (ULAR) and Institutional Laboratory Animal Care and Use Committee (IACUC) guidelines.
Injections
Following acclimation, mice received a daily intraperitoneal (i.p.) injection of either 0.09% saline (n=8) or 5.0 mg/kg ketamine (n=8) for fourteen days. Mice received no injections for approximately 48 hours prior to behavioral testing in the contextual fear conditioning paradigm. 48 hours were allowed before test sessions in order to minimize the likelihood that drug was bound to NMDARs during testing. After completion of the first fear conditioning session, mice again received a daily i.p. injection of saline (n=8) or ketamine (n=7) for an additional fourteen days. Mice received the same treatment of either saline or ketamine during all twenty-eight days of injections. Drug dosage was chosen based on previous studies indicating that repeated exposure of mice to 5 mg/kg ketamine both induces apoptosis and causes significant auditory gating deficits (Maxwell, Ehrlichman et al. 2006; Majewski-Tiedeken, Rabin et al. 2008).
Contextual Fear Conditioning Paradigm
In order to minimize the acute effects of ketamine, mice underwent training for contextual fear conditioning 48 hours after the last injections on day 14. In training, a 2-s, 1.5-mA scrambled footshock was delivered two and 2.5 minutes after being placed in the chamber. Baseline freezing was measured during the first two minutes (pre-shock), while the immediate, unconditioned freezing response was recorded for one minute post-shock. Freezing, defined as the absence of visible movement except for respiration, was recorded once every five seconds (Graves, Heller et al. 2003). Twenty-four hours after training, mice were placed back into the same chamber (conditioned context) in the absence of footshock. Freezing was recorded for five minutes, for a total of 60 observations. Mice were then returned to their home cages and received fourteen more days of daily injections. Forty-eight hours after the last injection, freezing was measured in the conditioned context for another five minutes. It is important to note that prior to the second testing session, animals did not undergo another training session, thus increasing the difficulty of the task and isolating memory retrieval.
Surgery
Approximately one week after the last injection, animals underwent stereotaxic implantation of electrodes (Plastic Products, Roanoke, VA, USA) for the recording of auditory evoked potentials. Animals were anesthetized with isoflurane prior to and during surgery. Unipolar recording electrodes were placed unilaterally in the right CA3 region of the hippocampus (1.4 mm posterior, 2.65 mm lateral and 2.75 mm deep relative to bregma) and referenced to the ipsilateral frontal sinus. The electrode pedestal was secured to the skull with ethyl cyanoacrylate (Loctite, Henkel KGaA, Duesseldorf, Germany) and dental cement (Ortho Jet, Lang Dental, Wheeling IL, USA). All surgical procedures were consistent with previously published methodologies (Connolly, Maxwell et al. 2003; Siegel, Connolly et al. 2003; Connolly, Maxwell et al. 2004; Maxwell, Kanes et al. 2004; Maxwell, Liang et al. 2004; Siegel, Maxwell et al. 2005; Metzger, Maxwell et al. 2007).
Novelty AEPs
Testing in the novelty paradigm occurred three weeks after the last injections of either saline (n=5) or ketamine (n=6). EEG recordings were conducted in the home cage environment, which was placed in a Faraday cage in order to eliminate signal contamination. Auditory stimuli were generated by Micro1401 hardware and Spike2, version 6.0 software (Cambridge Electronic Design, Cambridge, UK) and were delivered through speakers attached to the cage top. The stimulus protocol consisted of 24 standard tones (7 kHz) followed by one novel tone that was at a randomly-selected frequency between 5 and 9 kHz in increments of 100 Hz (excluding 7 kHz). Tones were presented at 85 dB compared to a 70-dB white-noise background. These tones were sinusoidal, 50 ms in duration and separated by a 500-ms interstimulus interval. The series of 25 tones was repeated forty times so that half of the deviant tones were of higher and half of lower frequency than the standard tone. Each deviant tone was used only once. Individual waveforms were sampled at 1667 Hz, filtered between 1 and 500 Hz and rejected for movement artifact based on the criterion of two times the root mean squared amplitude per mouse. For each mouse an average standard and novel tone waveform was created, each of which was baseline corrected to 0 μV. The standard tone waveform consisted of the average response to the 24th standard tone in the series to keep the number of trials constant between novel and standard conditions. These conditions were chosen according to previous results from our lab indicating that MMN and the N1 are sensitive to ketamine treatment under such parameters (Ehrlichman, Maxwell et al. 2008).
Gating AEPs
Testing in the gating paradigm took place both three and five weeks after the last injections. As in the novelty paradigm, recordings were conducted in the home cage environment, which was placed in a Faraday cage. Auditory stimuli were generated by Micro1401 hardware and Spike2, version 6.0 software delivered through speakers on the cage top. The stimulus protocol in the gating paradigm consisted of fifty paired white-noise bursts (10-ms duration, 500-ms intra-pair interval) with a 9-s inter-pair interval presented at 85 dB compared to a 70-dB white-noise background. Each 10-ms white-noise burst was composed of fifty randomly selected 0.2-ms square waves. Again, individual waveforms were sampled at 1667 Hz, filtered between 1 and 500 Hz and rejected for movement artifact based on the criterion of two times the root mean squared amplitude per mouse. Average waveforms for individual mice were also baseline corrected to 0 μV. Grand average waves were then produced from 0 to 200 ms following stimulus presentation.
Contextual Fear Conditioning Analyses
The freezing behavior of each subject was measured by calculating the fraction of time that the animal exhibited freezing (ratio of time freezing divided by total observation time). The freezing ratio for one ketamine-treated mouse fell outside two standard deviations of the mean and therefore its data was excluded from analyses. Freezing ratios were analyzed using separate general linear model one-way ANOVAS after two and four weeks of ketamine treatment.
Novelty Analyses
Average AEPs were divided into 25-ms bins from 0 to 200 ms for a total of eight sequential epochs as previously described (Umbricht, Vyssotki et al. 2005; Ehrlichman, Maxwell et al. 2008). We calculated the difference waveform by subtracting the average AEP in response to standard tones from that of the frequency deviants (Difference = Deviant - Standard). The area under the difference curve was then computed for each epoch. According to this methodology, a value of zero for the difference wave indicates that there was not a deviance-elicited change during a given epoch. A t test was therefore used to compare the difference waves for each treatment condition against a value of zero. We also performed general linear model repeated measures ANOVAs (rmANOVAs) for each epoch in order to determine whether ketamine disrupted any potential deviance-elicited changes in the AEP. Our hypothesis, based on previously published data using acute ketamine, was that chronic ketamine would attenuate the deviance-elicited AEP response immediately following the N40 component, between 50 and 75 ms (Ehrlichman, Maxwell et al. 2008).
Gating Analyses
The amplitudes of the P20 and P80 components of the AEP waveform were chosen by determining the maximum positive, post-stimulus deflection between 10 and 30 ms and 60 and 200 ms, respectively. The amplitude of the N40 component was chosen by determining the maximum negative deflection between 20 and 70 ms post-stimulus. The latency of each of these components was defined as the time post-stimulus at which its maximum deflection occurred. These parameters were chosen based on a previous study published by our group on the immediate and last effects of chronic ketamine treatment on mouse AEPs (Maxwell, Ehrlichman et al. 2006). Such temporal limits enabled us to identify drug-induced latency shifts. The AEP of one saline-treated mouse was rejected after the second gating recording session due to artifact. Individual rmANOVAs were performed on baseline corrected data for the amplitude of each AEP component in order to identify possible interactions between treatment condition, stimulus and test week. rmANOVAs were also performed on the latencies of each component in response to stimulus 1 (S1) in order to identify potential treatment differences at or between test weeks. Significant interactions were subsequently submitted to Fisher’s LSD post hoc analysis. All data analyses were conducted using Statistica 6.1 (Statsoft, Inc., Tulsa, OK).
Results
Contextual Fear Conditioning
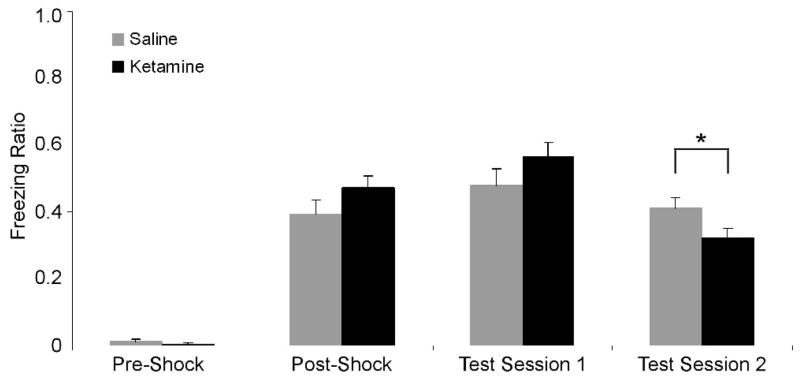
As expected, one-way ANOVAs on pre- and post-shock data gathered during training did not reveal any significant differences between the two treatment groups. There was also no difference between the two groups during the first test session (24 hours after training), suggesting that two weeks of daily ketamine treatment did not impair acquisition or initial retrieval of CFC. However, during the second testing session, ketamine-treated mice displayed significantly reduced freezing behavior as compared with saline-treated controls (Figure 2) [F(1, 17)=12.01, p=0.003]. These data indicate that retrieval of CFC was impaired after a longer interval (17 days as compared to one) between training and testing.
Figure 2.
Freezing ratios of mice chronically treated with either saline (gray) or 5.0 mg/kg ketamine (black) in contextual fear conditioning. Pre- and post-shock data represent baseline and immediate, unconditioned responses before and after footshock, respectively. Freezing behavior in the conditioned context during the first test session (following 2 weeks of ketamine) and second test session (17 days after training) are also shown. Error bars represent standard error of the mean (±SEM). Ketamine resulted in a significant impairment of contextual fear conditioning during the second test session (asterisk) but not the first.
Novelty
A t test on the epochs generated from baseline corrected novel and standard tone difference waveforms did not produce any significant results. The rmANOVAs on each epoch did not identify an interaction between ketamine condition and stimulus type (novel vs. standard) within the MMN epochs (50–75) [F(1, 13)=1.74, p=0.210] and (75–100 ms) [F(1, 13)=0.40, p=0.540].
Amplitude, Gating and Latency
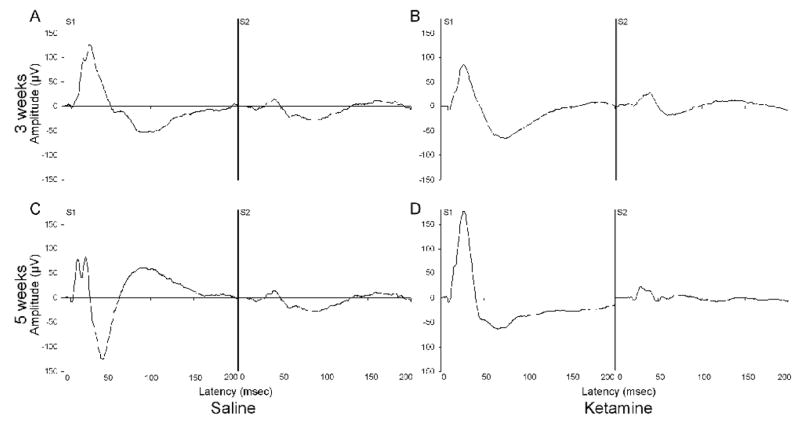
The overall pattern of auditory event related potentials for the P20, N40 and P80 is shown in Figure 3.
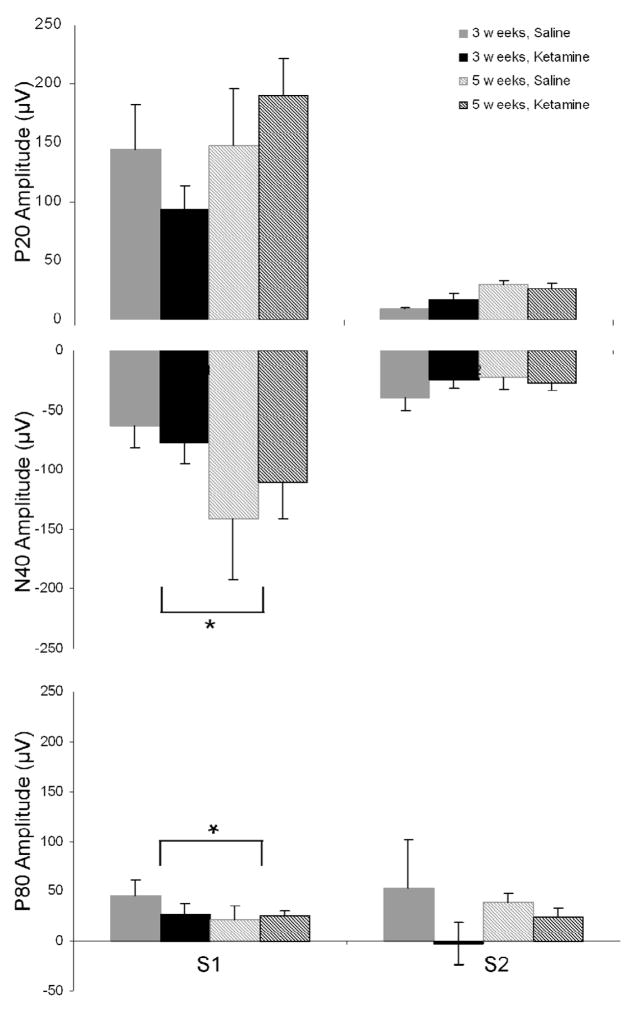
Figure 3.
Grand average AEP readings for each treatment, test session and stimulus condition in the gating task are shown. Grand averages of (A) 0.09% saline and (B) 5.0 mg/kg ketamine treated mice, 3 weeks after the cessation of the 4-week, daily administration schedule. (C) Saline- and (D) ketamine-treated grand averages, 5 weeks after the cessation of treatment. S1 represents the response to the first of two white-noise bursts, while S2 represents the response to the second.
P20
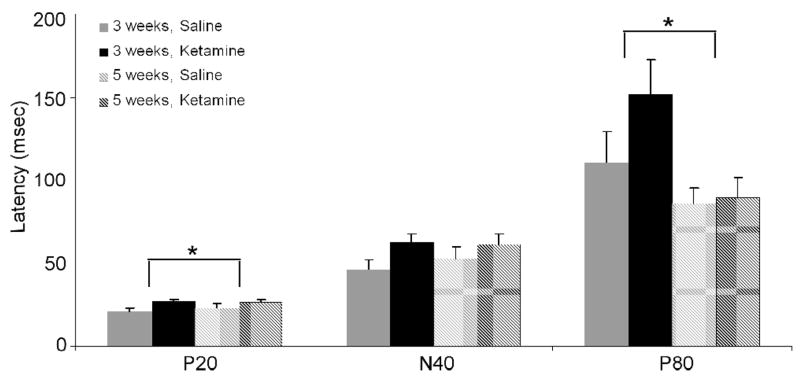
Though a rmANOVA on baseline corrected data for P20 amplitude did not yield any significant interactions, as expected there was a significant main effect of stimulus order (S1 vs. S2), confirming that S2 was gated relative to S1 [F(1, 12)=54.37, p<0.001]. Our analyses also revealed a trend toward increased P20 amplitude 5, as opposed to 3, weeks after the cessation of treatment [F(1, 12)=4.37, p=0.059]. An evaluation of P20 latency also demonstrated that chronic ketamine administration resulted in a significant increase in S1 latency [F(1, 12)=5.80, p=0.033] (Figure 4).
Figure 4.
S1 latencies of the P20, N40 and P80 components of the auditory ERP following chronic saline (gray) or 5.0 mg/kg ketamine (black) treatment. Asterisk indicates either a significant ketamine-induced increase in P20 latency across both test weeks, or a decrease in P80 latency between weeks 3 and 5. Overall, ketamine increased the S1 latency of all components at both time points. Data are presented as mean ± SEM.
N40
A rmANOVA on baseline corrected data for N40 amplitude revealed only a significant interaction between test week and stimulus [F(1, 12)=6.66, p=0.024], as S1 increased between weeks 3 and 5 [MS=2760.5, p<0.001, df=12.00] (Figure 5). In addition, analyses demonstrated a main effect of stimulus, as S2 was again significantly gated relative to S1 [F(1, 12)=25.45, p<0.001], and a trend toward increased N40 amplitude at week 5 [F(1, 12)=4.24, p=0.062].
Figure 5.
Gating data for the P20, N40 and P80 AEP components three and five weeks after cessation of chronic saline (gray) or 5.0 mg/kg ketamine (black) treatment. Asterisk indicates either a significant interaction between N40 gating and test week, or P80 gating and treatment condition. Post hoc analyses revealed that both of these results were mediated by effects on S1 amplitude, which increased between weeks 3 and 5, and was decreased by ketamine. Data are presented as mean ± SEM.
P80
A rmANOVA on baseline corrected data for P80 amplitude revealed a significant interaction between treatment and stimulus, indicating differing effects of saline and ketamine on auditory sensory gating [F(1, 12)=5.02, p=0.045]. Post hoc analyses revealed that ketamine-treated mice exhibited a significant decrease in S1 amplitude as compared to saline-treated subjects [MS=956.69, p=0.016, df=23.34] (Figure 5). The P80 component also displayed a trend toward decreased amplitude when treated with ketamine, though this result did not quite reach significance [F(1, 12)=4.21, p=0.063]. A latency evaluation showed that there was also a significant decrease between weeks 3 and 5 [F(1, 12)=12.38, p=0.004].
Discussion
The current study examines both immediate and long-lasting effects of chronic ketamine administration on contextual fear conditioning, detection of pitch deviants and auditory gating. Murine studies have repeatedly shown that acute ketamine treatment results in impaired contextual fear conditioning (Pietersen, Bosker et al. 2006; Pietersen, Bosker et al. 2007; Calzavara, Medrano et al. 2008). In those studies, the authors suggest that this deficit is caused by the blockage of NMDA receptors (NMDARs), which are requisite for such Pavlovian conditioning. Additionally, ketamine causes widespread apoptotic neurodegeneration after both single and chronic administrations (Scallet, Schmued et al. 2004; Wang, Sadovova et al. 2005; Young, Jevtovic-Todorovic et al. 2005; Majewski-Tiedeken, Rabin et al. 2008). Such neurodegeneration has been identified in the thalamus, amygdala, parietal cortex and hippocampus, among other regions. Because all four of these regions are involved in the fear conditioning pathway, it is possible that ketamine-induced cell death, and not only the blockage of NMDARs, in any of these regions can result in fear conditioning impairments (Bissiere, Plachta et al. 2007; Matus-Amat, Higgins et al. 2007; Zanoveli, Ferreira-Netto et al. 2007; Keene and Bucci 2008). Because experiments in this study were conducted 48 hours after injections and the half-life of ketamine in mice is approximately 13 minutes, it is unlikely that the drug was still bound to NMDARs during data collection (Maxwell, Ehrlichman et al. 2006). Though the pharmacokinetics of ketamine’s metabolites, norketamine and dehydroketamine, have not been studied in mice, data from other small animals suggest that they are largely cleared within 24 hours (Pedraz, Lanao et al. 1985; Pypendop and Ilkiw 2005). Therefore, results in the CFC paradigm support our hypothesis that the detrimental effects of ketamine were caused not by acute NMDAR blockade, but more lasting, potentially apoptotic, mechanisms.
Despite ketamine’s link to impaired fear conditioning, one caveat of the current study is our inability to identify which phase of the learning process was affected by drug. Studies show that the thalamus, hippocampus and sensory cortex are all involved in conditioned response (CR) acquisition, hippocampus and amygdala are important for memory consolidation, and the amygdala is the primary site of conditioned stimulus (CS)-unconditioned stimulus (US) convergence (LeDoux 1995; Davis and Shi 2000; Maren 2001). The hippocampus and amygdala also play a role in the retrieval, or expression, of contextual fear memories, which is one of the physiological functions ultimately being assessed in our two experimental test sessions. Because ketamine-treated mice exhibited normal fear conditioning after two weeks of injections, it appears that they were able to acquire fear memories and retrieve them at that time point. However, because there was only one training session, the task at the second time point was more difficult and isolated to memory retrieval. The selective impairment during the second test session also suggests that ketamine may alter system consolidation, which is the gradual reorganization of brain connections that support memory. This process is generally slower and longer-lasting than synaptic consolidation, which is complete within hours of training and involves changes in more localized circuits. One of the key characteristics of system consolidation is that memories shift from hippocampus- to prefrontal cortex-dependence. The effect of ketamine on CFC two weeks after training may therefore be due to a decreased ability to strengthen the cortical connections involved in this process (Frankland and Bontempi 2005).
Though CFC testing was only performed at the two discussed time points, data collected in the gating and novelty paradigms also suggest that repeated ketamine administration has lasting effects on brain functioning. Alternatively, our hypothesis regarding MMN was not supported. The lack of a MMN effect in the current study suggest that the disruption of MMN following acute ketamine is due to receptor blockade, rather than the lasting changes secondary to previous exposure. Despite the lack of effect of chronic ketamine on novelty recognition, the drug did produce the predicted results in terms of several different AEP components. For instance, similar to a previously published study from our laboratory, ketamine caused a significant increase in P20 latency, and generally increased the latencies of the N40 and P80 components, as well (Maxwell, Ehrlichman et al. 2006). ERP component latency is generally thought to be a measure of processing speed, which is the rate at which a person responds to a given stimulus. As discussed in Amann et al. 2008, an increase in processing speed would subsequently decrease the likelihood that information held with one’s working memory could be accessed before it is lost or diminished (Fry and Hale 2000; Amann, Phillips et al. 2008). The reported effects of chronic ketamine on ERP component latency are therefore consistent with its effects on CFC, and lend further support to the proposed link between the temporal characteristics of electrophysiological recordings and behavioral outcomes.
Similar to its effects on latency, we also found that chronic ketamine had negative consequences in terms of P80 amplitude and gating, as gating was decreased over both test weeks. The mouse P80 displays morphology, relative latency and pharmacological response properties similar to those of the human P200 (Siegel, Connolly et al. 2003; Maxwell, Liang et al. 2004). Clinical electrophysiology studies consistently show that the P200 exhibits decreased amplitude and inhibition in patients with schizophrenia (Roth, Pfefferbaum et al. 1981; Ford, Roth et al. 1999). Such changes are usually manifest as a reduction in the ability to gate repeated stimuli, as mediated by a decrease in S1 amplitude. Therefore, the effects of chronic ketamine treatment on P80 amplitude and gating support both the NMDAR blockade and chronic ketamine models of abnormal P80/P200 amplitudes after auditory stimuli, as seen in schizophrenia. The P200 has multiple generators and due to their analogous nature, it is believed that the mouse P80 does, as well (Wunderlich and Cone-Wesson 2001; Sheehan, McArthur et al. 2005). However, because the recording technique utilized in this study measures whole-brain activity, it is impossible to determine which regions were affected by ketamine and responsible for the gating deficit observed. Because immunohistochemical analyses were not performed, it is also not possible to confirm that the long-lasting effect of ketamine on P80 amplitude, and P20 latency, were due to mechanisms other than NMDAR blockade.
The current study builds upon previous reports assessing the effects of chronic ketamine treatment on the mouse AEP. Until now, such studies have only evaluated these effects immediately and one week after a 14-day daily administration regimen. Therefore, this study demonstrates that continued ketamine administration has even longer lasting effects on AEP components than previously recognized. Such lasting effects also add support to the belief that repeated recreational ketamine use could have long-term effects on brain function and information processing.
Acknowledgments
Funding provided by NIDA 1 R01 DA023210-01 (SJ Siegel), NCI P50-CA-084718 (C. Lerman, PI) and NIMH P50 MH064045 (RE Gur, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amann LC, Phillips JM, et al. Male and female mice differ for baseline and nicotine-induced event related potentials. Behav Neurosci. 2008;122(5):982–90. doi: 10.1037/a0012995. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bissiere S, Plachta N, et al. The Rostral Anterior Cingulate Cortex Modulates the Efficiency of Amygdala-Dependent Fear Learning. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Schultze-Lutter F, et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008;64(5):376–84. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Brown K, Gordon E, et al. Misattribution of sensory input reflected in dysfunctional target:non-target ERPs in schizophrenia. Psychol Med. 2000;30(6):1443–9. doi: 10.1017/s0033291799002858. [DOI] [PubMed] [Google Scholar]
- Calzavara MB, Medrano WA, et al. Neuroleptic Drugs Revert the Contextual Fear Conditioning Deficit Presented by Spontaneously Hypertensive Rats: A Potential Animal Model of Emotional Context Processing in Schizophrenia? Schizophr Bull. 2008 doi: 10.1093/schbul/sbn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly PM, Maxwell C, et al. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochem Res. 2004;29(6):1179–88. doi: 10.1023/b:nere.0000023605.68408.fb. [DOI] [PubMed] [Google Scholar]
- Connolly PM, Maxwell CR, et al. Inhibition of auditory evoked potentials and prepulse inhibition of startle in DBA/2J and DBA/2Hsd inbred mouse substrains. Brain Res. 2003;992(1):85–95. doi: 10.1016/j.brainres.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Covington JW, Polich J. P300, stimulus intensity, and modality. Electroencephalogr Clin Neurophysiol. 1996;100(6):579–84. doi: 10.1016/s0168-5597(96)96013-x. [DOI] [PubMed] [Google Scholar]
- Cvrcek P. Side effects of ketamine in the long-term treatment of neuropathic pain. Pain Med. 2008;9(2):253–7. doi: 10.1111/j.1526-4637.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- Davalos DB, Kisley MA, et al. Mismatch negativity in detection of interval duration deviation in schizophrenia. Neuroreport. 2003;14(9):1283–6. doi: 10.1097/00001756-200307010-00019. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The amygdala. Curr Biol. 2000;10(4):R131. doi: 10.1016/s0960-9822(00)00345-6. [DOI] [PubMed] [Google Scholar]
- Ehrlichman RS, Maxwell CR, et al. Deviance-elicited Changes in Event-related Potentials are Attenuated by Ketamine in Mice. J Cogn Neurosci. 2008 doi: 10.1162/jocn.2008.20097. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roth WT, et al. Failures of automatic and strategic processing in schizophrenia: comparisons of event-related brain potential and startle blink modification. Schizophr Res. 1999;37(2):149–63. doi: 10.1016/s0920-9964(98)00148-0. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6(2):119–30. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biol Psychol. 2000;54(1–3):1–34. doi: 10.1016/s0301-0511(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, et al. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10(3):168–76. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenschel C, Vernon DJ, et al. Event-related brain potential correlates of human auditory sensory memory-trace formation. J Neurosci. 2005;25(45):10494–501. doi: 10.1523/JNEUROSCI.1227-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–45. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol Neurootol. 2000;5(3–4):207–15. doi: 10.1159/000013882. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and Schizophrenia: Phencyclidine, N-Methyl-d-Aspartate Receptors, and Dopamine-Glutamate Interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Kannan TR, Saxena A, et al. Oral ketamine as an adjuvant to oral morphine for neuropathic pain in cancer patients. J Pain Symptom Manage. 2002;23(1):60–5. doi: 10.1016/s0885-3924(01)00373-6. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behav Neurosci. 2008;122(1):89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: clues from the brain. Annu Rev Psychol. 1995;46:209–35. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Lindsley CW, Shipe WD, et al. Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr Top Med Chem. 2006;6(8):771–85. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- Majewski-Tiedeken CR, Rabin CR, et al. Ketamine exposure in adult mice leads to increased cell death in C3H, DBA2 and FVB inbred mouse strains. Drug Alcohol Depend. 2008;92(1–3):217–27. doi: 10.1016/j.drugalcdep.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17(3):141–50. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, et al. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121(4):721–31. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Ehrlichman RS, et al. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther. 2006;316(1):315–24. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Kanes SJ, et al. Phosphodiesterase inhibitors: a novel mechanism for receptor-independent antipsychotic medications. Neuroscience. 2004;129(1):101–7. doi: 10.1016/j.neuroscience.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Liang Y, et al. Effects of chronic olanzapine and haloperidol differ on the mouse N1 auditory evoked potential. Neuropsychopharmacology. 2004;29(4):739–46. doi: 10.1038/sj.npp.1300376. [DOI] [PubMed] [Google Scholar]
- May P, Tiitinen H, et al. Frequency change detection in human auditory cortex. J Comput Neurosci. 1999;6(2):99–120. doi: 10.1023/a:1008896417606. [DOI] [PubMed] [Google Scholar]
- Metzger KL, Maxwell CR, et al. Effects of nicotine vary across two auditory evoked potentials in the mouse. Biol Psychiatry. 2007;61(1):23–30. doi: 10.1016/j.biopsych.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Michie PT. What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol. 2001;42(2):177–94. doi: 10.1016/s0167-8760(01)00166-0. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Mofeez A, et al. Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharmacology. 2004;29(1):208–18. doi: 10.1038/sj.npp.1300342. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Mofeez A, et al. Ketamine impairs response inhibition and is positively reinforcing in healthy volunteers: a dose-response study. Psychopharmacology (Berl) 2004;172(3):298–308. doi: 10.1007/s00213-003-1656-y. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20(2):106–18. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Okon T. Ketamine: an introduction for the pain and palliative medicine physician. Pain Physician. 2007;10(3):493–500. [PubMed] [Google Scholar]
- Ong JC, Brody SA, et al. An investigation of the efficacy of mood stabilizers in rodent models of prepulse inhibition. J Pharmacol Exp Ther. 2005;315(3):1163–71. doi: 10.1124/jpet.105.090845. [DOI] [PubMed] [Google Scholar]
- Pedraz JL, Lanao JM, et al. Kinetics of ketamine and its metabolites in rabbits with normal and impaired renal function. Eur J Drug Metab Pharmacokinet. 1985;10(1):33–9. doi: 10.1007/BF03189695. [DOI] [PubMed] [Google Scholar]
- Pietersen CY, Bosker FJ, et al. An animal model of emotional blunting in schizophrenia. PLoS ONE. 2007;2(12):e1360. doi: 10.1371/journal.pone.0001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersen CY, Bosker FJ, et al. Ketamine administration disturbs behavioural and distributed neural correlates of fear conditioning in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1209–18. doi: 10.1016/j.pnpbp.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Pypendop BH, Ilkiw JE. Pharmacokinetics of ketamine and its metabolite, norketamine, after intravenous administration of a bolus of ketamine to isoflurane-anesthetized dogs. Am J Vet Res. 2005;66(12):2034–8. doi: 10.2460/ajvr.2005.66.2034. [DOI] [PubMed] [Google Scholar]
- Reuben SS, Buvanendran A. Preventing the development of chronic pain after orthopaedic surgery with preventive multimodal analgesic techniques. J Bone Joint Surg Am. 2007;89(6):1343–58. doi: 10.2106/JBJS.F.00906. [DOI] [PubMed] [Google Scholar]
- Roth WT, Pfefferbaum A, et al. Auditory event-related potentials in schizophrenia and depression. Psychiatry Res. 1981;4(2):199–212. doi: 10.1016/0165-1781(81)90023-8. [DOI] [PubMed] [Google Scholar]
- Scallet AC, Schmued LC, et al. Developmental neurotoxicity of ketamine: morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci. 2004;81(2):364–70. doi: 10.1093/toxsci/kfh224. [DOI] [PubMed] [Google Scholar]
- Sheehan KA, McArthur GM, et al. Is discrimination training necessary to cause changes in the P2 auditory event-related brain potential to speech sounds? Brain Res Cogn Brain Res. 2005;25(2):547–53. doi: 10.1016/j.cogbrainres.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Shelley AM, Silipo G, et al. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophr Res. 1999;37(1):65–79. doi: 10.1016/s0920-9964(98)00138-8. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Faux SF, et al. Clinical correlations of auditory P200 topography and left temporo-central deficits in schizophrenia: a preliminary study. J Psychiatr Res. 1989;23(1):13–34. doi: 10.1016/0022-3956(89)90014-9. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Connolly P, et al. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology. 2003;28(4):675–82. doi: 10.1038/sj.npp.1300087. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Maxwell CR, et al. Monoamine reuptake inhibition and nicotine receptor antagonism reduce amplitude and gating of auditory evoked potentials. Neuroscience. 2005;133(3):729–38. doi: 10.1016/j.neuroscience.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol. 2008;(182):313–33. doi: 10.1007/978-3-540-74806-9_15. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Zou X, et al. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98(1):145–58. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Vyssotki D, et al. Deviance-related electrophysiological activity in mice: is there mismatch negativity in mice? Clin Neurophysiol. 2005;116(2):353–63. doi: 10.1016/j.clinph.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Wang C, Sadovova N, et al. The role of the N-methyl-D-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience. 2005;132(4):967–77. doi: 10.1016/j.neuroscience.2005.01.053. [DOI] [PubMed] [Google Scholar]
- Winterer G, Egan MF, et al. Event-related potentials and genetic risk for schizophrenia. Biol Psychiatry. 2001;50(6):407–17. doi: 10.1016/s0006-3223(01)01072-1. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK. Effects of stimulus frequency and complexity on the mismatch negativity and other components of the cortical auditory-evoked potential. J Acoust Soc Am. 2001;109(4):1526–37. doi: 10.1121/1.1349184. [DOI] [PubMed] [Google Scholar]
- Yingling CD, Nethercut GE. Evoked responses to frequency shifted tones: tonotopic and contextual determinants. Int J Neurosci. 1983;22(1–2):107–18. doi: 10.3109/00207459308987389. [DOI] [PubMed] [Google Scholar]
- Young C, Jevtovic-Todorovic V, et al. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146(2):189–97. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoveli JM, Ferreira-Netto C, et al. Conditioned place aversion organized in the dorsal periaqueductal gray recruits the laterodorsal nucleus of the thalamus and the basolateral amygdala. Exp Neurol. 2007;208(1):127–36. doi: 10.1016/j.expneurol.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zou X, Patterson TA, et al. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci. 2009;108(1):149–58. doi: 10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]