Abstract
In Caenorhabditis elegans, the dauer pheromone, which consists of a number of derivatives of the 3,6-dideoxysugar ascarylose, is the primary cue for entry into the stress-resistant, ‘non-aging’ dauer larval stage. Here, using activity-guided fractionation and NMR-based structure elucidation, a structurally novel, indole-3-carboxyl-modified ascaroside is identified that promotes dauer formation at low nanomolar concentrations, but inhibits dauer formation at higher concentrations.
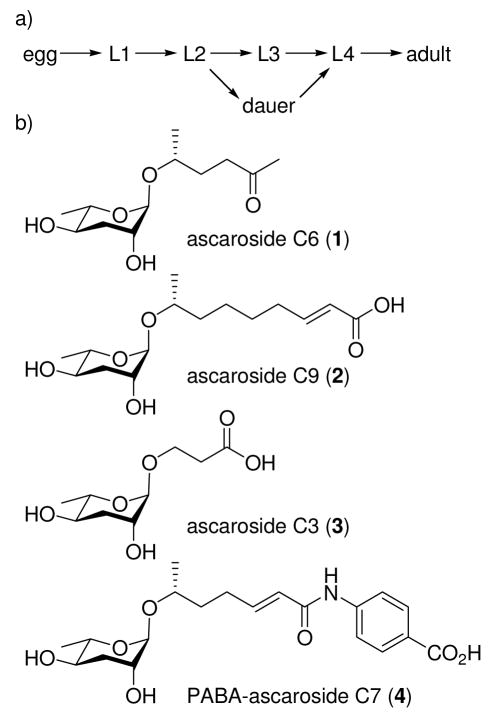
Caenorhabditis elegans, a small nematode (round worm) that has become an important model organism in development, neurobiology, and aging research, senses the world primarily through small molecules. In response to unfavorable growth conditions, such as high population density, C. elegans enters the dauer larval stage, which is specialized for survival under harsh conditions1 (Figure 1a). Dauer formation is controlled by the insulin/IGF-12 and TGFβ pathways3, which also control metabolism, stress resistance, and aging throughout the lifecycle. In order to monitor its population density and trigger entry into the dauer stage, C. elegans uses the dauer pheromone that it secretes into its environment and senses using chemosensory organs in its head.4 The dauer pheromone consists of a number of structurally related derivatives of the 3,6-dideoxysugar ascarylose with fatty acid-like side chains5 (Figure 1b). We have been naming these ascarosides based on the carbon chain length of the fatty acid-like portion. Minor modifications to the structures of the ascarosides dramatically affect their ability to induce dauer formation.5a Synergism between some of the ascarosides suggests that they may have different receptors.5b Furthermore, different ascarosides have differently shaped titration curves and show different temperature dependencies.5b Here, we report the structure of another dauer pheromone component, indolecarboxyl-ascaroside C5 (5) (Figure 2), its total synthesis, and characterization of its dauer-inducing activity.
Figure 1.
(a) Under favorable environmental conditions (low population density, high food availability, low temperature), C. elegans undergoes reproductive growth, progressing through four larval stages (L1–L4) to the adult. Under unfavorable conditions (high population density, low food availability, high temperature), C. elegans will instead progress from the L2 larval stage to a stress-resistant alternative L3 stage, the dauer. (b) C. elegans senses its population density using dauer-inducing ascarosides (1–4) (para-aminobenzoic acid, PABA).
Figure 2.
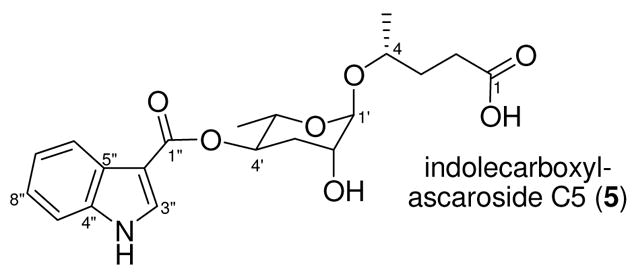
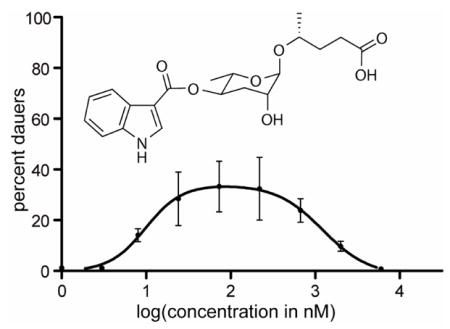
The chemical structure of indolecarboxyl-ascaroside C5 (5).
As described previously, we generated a crude dauer pheromone by cultivating C. elegans in liquid culture for approximately two weeks at 25 °C and by collecting, lyophilizing, and extracting the growth medium.5b To summarize our fractionation scheme, we fractionated the crude extract using C18 extraction, followed by silica gel chromatography, and high pressure liquid chromatography on a C18 column (see Supporting Information). Activity was followed using the dauer formation assay in which fractions are incorporated into an agar plate, a small amount of food is added, and adult worms are allowed to lay 50–100 eggs before removal. The eggs are then allowed to develop, and the dauer/adult ratio is determined. In addition to the primary dauer-inducing components of the extract, ascarosides C6 and C9, a number of minor components contributed to the overall activity. One of these components was associated with a major UV peak at 281 nm, suggesting that it might be structurally distinct from the known ascarosides. This component was isolated at approximately 7.5 μg per liter of growth medium. Although activity-guided fractionation suggested that this component was less of a contributor to the overall activity than either ascaroside C6 or C9, the fact that the component was present at a very low concentration in the culture medium (~19 nM based on isolated yield) suggested that its specific activity might be very high.
HRESIMS analysis of the dauer pheromone component (m/z 414.1513) indicated a molecular formula of C20H25NO7 (calcd. m/z for [M+Na]+, 414.1529). NMR analysis included proton, dqf-COSY, gHSQC, gHMBC, and NOESY experiments (Table 1 and Figure S1). Due to the small amount of compound isolated, NMR experiments were performed using a 5 mm symmetrical microtube (Shigemi) in a 600 MHz NMR instrument equipped with a cryoprobe. The dqf-COSY spectrum indicated three distinct spin systems– a 3-substituted indole unit, an ascarylose sugar, and a 4-carbon aliphatic chain (Figure S2a). The long-range HMBC correlation between the ascarylose C-4′ proton and the C-1″ carbonyl carbon, as well as the proton and carbon shifts of the indole, suggested that the indole unit was connected via an ester linkage to the ascarylose sugar at its C-4′ position. Long-range HMBC correlations also established that the aliphatic chain was in fact a pentanoic acid subunit that was linked via an alcohol at the ω-1 position to the anomeric carbon of the ascarylose sugar. Coupling constants and NOESY correlations were used to assign tentatively the relative stereochemistry of the sugar, as well as the β configuration at the anomeric carbon and the relative configuration of the pentanoic acid ω-1 alcohol (Figure S2b). The structure and absolute configuration of indolecarboxyl-ascaroside C5 (5) (Figure 2) were then confirmed through total synthesis.
Table 1.
NMR shifts derived from 1H, dqf-COSY, gHSQC, and gHMBC spectra of natural indolecarboxyl-ascaroside C5 salt in methanol-d4.
| no. | δH mult. (J (Hz)) | δC | HMBC |
|---|---|---|---|
| 1 | 182.34 | ||
| 2a | 2.27, m (J2a,2b=14.2) | 35.48 | C-1,3,4 |
| 2b | 2.41, m | C-1,3,4 | |
| 3a | 1.85, m | 35.21 | C-1,2,4,5 |
| 3b | 1.91, m | C-1,2,4,5 | |
| 4 | 3.88, m | 72.41 | C-2,1′ |
| 5 | 1.19, d (J4,5=6.0) | 18.81 | C-3,4 |
| 1′ | 4.75, br s | 97.16 | C-4,3′,5′ |
| 2′ | 3.80, dt (J1′,2′=2.7) | 69.47 | C-4′ |
| 3′ax | 2.05, ddd (J2′,3′ax=3.4, J3′ax,3′eq=12.7) | 33.17 | C-4′ |
| 3′eq | 2.19, ddd (J2′,3′eq=2.7) | C-1′,4′ | |
| 4′ | 5.11, ddd (J3′ax,4′=11.0, J3′eq,4′=4.7) | 70.36 | C-5′,6′,1″ |
| 5′ | 4.09, dq (J4′,5′=9.7) | 68.51 | C-4′ |
| 6′ | 1.24, d (J5′,6′=6.3) | 18.06 | C-4′,5′ |
| 1″ | 166.36 | ||
| 2″ | 108.11 | ||
| 3″ | 7.99, s | 133.26 | C-2″,4″,5″ |
| 4″ | 138.26 | ||
| 5″ | 127.22 | ||
| 6″ | 8.04, m | 121.64 | C-4″,8″ |
| 7″ | 7.19, m | 122.32 | C-5″,6″ |
| 8″ | 7.20, m | 123.42 | C-4″,9″ |
| 9″ | 7.43, m | 112.75 | C-5″,7″ |
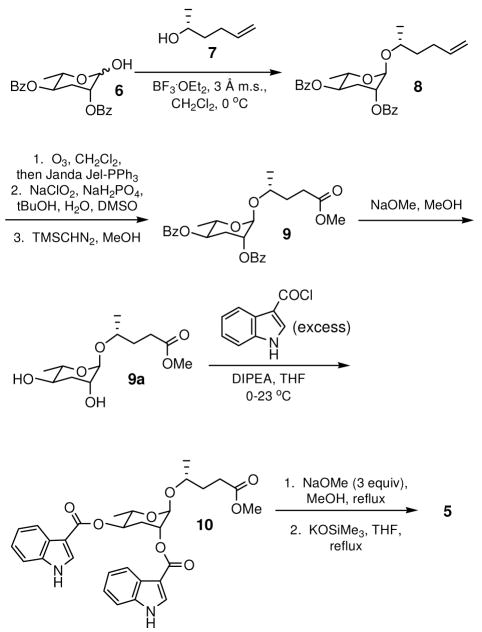
The synthesis of indolecarboxyl-ascaroside C5 proceeded from dibenzoyl ascarylose6 (6) and commercially available (R)-5-hexen-2-ol (7) (Scheme 1 and Supporting Information). In brief, glycosylation of 6 with 7 proceeded under the conditions reported by Jeong et. al. in good yield (73%) to afford 8.6 Attempted cleavage of the olefin of 8 with KMnO4 resulted in an inseparable mixture of desired product carboxylic acid and unidentified impurities. A more selective protocol employed the ozonolysis of 8 followed by treatment with polymer-supported PPh3 (Janda Jel-PPh3) to afford the aldehyde intermediate, which was subsequently oxidized to a carboxylic acid and then converted to methyl ester 9 via treatment with TMSCHN2 (60%, 3 steps). Methanolytic removal of the benzoyl protecting groups of 9 afforded diol 9a, which was then subjected to acylation conditions with indole-3-carboxyl chloride. Surprisingly, attempts at the monoacylation of 9a resulted primarily in the formation of a product acylated at the 2 position of the ascarylose ring. On the other hand, the doubly acylated product 10 could be produced in good yield upon treatment with a large excess of indole-3-carboxyl chloride and diisopropylethylamine. Methanolysis of 10 produced the methyl ester precursor to indolecarboxyl-ascaroside C5 as the only detectable monoacylated product in 26% yield. Finally, the methyl ester was converted to the final product with potassium trimethylsilanolate in refluxing THF. Since the natural indolecarboxyl-ascaroside C5 was isolated as a salt, the synthetic molecule was converted to a salt before obtaining NMR spectra, including proton, dqf-COSY, gHSQC, gHMBC, and NOESY, for comparison purposes (Figure S3). The NMR data of the natural and synthetic indolecarboxyl-ascaroside C5 were virtually identical (see Table 1 and Table S1 and Figure S1 and S3), and analysis of the natural and synthetic molecules by CD spectroscopy indicated that they shared the same absolute configuration (Supporting Information).
Scheme 1.
Synthesis of indolecarboxyl-ascaroside C5 (5).
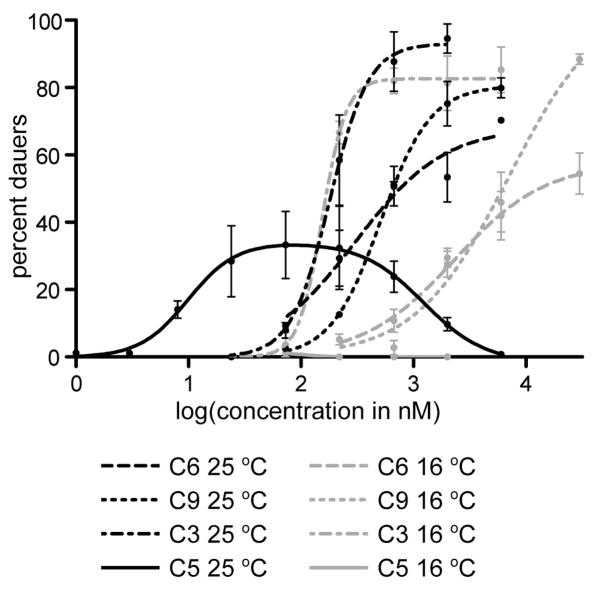
The synthetic indolecarboxyl-ascaroside C5 was titrated in the dauer formation assay alongside ascarosides C3, C6, and C9. Given that other ascarosides had previously shown different temperature dependencies,5b all ascarosides were titrated at both a lower (16 °C) and a higher (25 °C) temperature (Figure 3). As was seen before, the EC50 of ascaroside C3 is relatively temperature independent, whereas the EC50’s of ascarosides C6 and C9 are smaller at higher temperatures (indicating that C6 and C9 are much more active at higher temperatures). Indolecarboxyl-ascaroside C5 has no observable activity at 16 °C, but is the most potent of all the tested ascarosides at 25 °C (with an EC50 of approximately 9.9 nM). Interestingly, at higher concentrations, indolecarboxyl-ascaroside C5 seems to block its own activity, resulting in a bell-shaped titration curve. In order to test whether a high concentration of indolecarboxyl-ascaroside C5 blocks the activity of the other ascarosides, we tested the dauer-inducing activity of each ascaroside in combination with a high concentration (6 μM) of indolecarboxyl-ascaroside C5 (Figure S4). These results, however, suggest that a high concentration of indolecarboxyl-ascaroside C5 does not block the activity of other ascarosides. In order to test whether a low concentration of indolecarboxyl-ascaroside C5 works additively or synergistically with the other ascarosides, we tested the dauer-inducing activity of the different ascarosides either alone or in combination with other ascarosides (Figure S5). These results suggest that indolecarboxyl-ascaroside C5 displays some synergism with ascaroside C3, but not with other ascarosides.
Figure 3.
Titration of ascaroside C6 (1), ascaroside C9 (2), ascaroside C3 (3), and indolecarboxyl-ascaroside C5 (5) in the dauer formation assay at either 16 °C or 25 °C.
Here, we report the structure elucidation, total synthesis, and biological evaluation of indolecarboxyl-ascaroside C5. The indole-3-carboxyl portion of the molecule is biosynthetically and structurally unusual. Whereas the indole-3-acetyl group, which is derived from tryptophan, is found in many natural products (e.g., auxin)7, the indole-3-carboxyl group, which is derived through an unknown mechanism, has been observed only in a handful of natural products8 (that are otherwise structurally unrelated to indolecarboxyl-ascaroside C5). The bell-shaped titration curve of indolecarboxyl-ascaroside C5 suggests that high concentrations of the molecule differentially modulate its receptor to block dauer formation and suggest that it may be possible to develop more potent molecules that block dauer formation. The fact that low concentrations of indolecarboxyl-ascaroside C5 show synergism with ascaroside C3, coupled with the fact that high concentrations of indolecarboxyl-ascaroside C5 cannot block the activities of other ascarosides, indicates that indolecarboxyl-ascaroside C5 may target a different receptor from the other ascarosides. Identification of new ascarosides such as indolecarboxyl-ascaroside C5 suggests that the chemical language that C. elegans uses to coordinate population-wide activities like dauer formation is very complex. Exploration of the biosynthesis of these molecules will likely reveal equally complex control mechanisms for their production.
Supplementary Material
Acknowledgments
We kindly thank Greg Heffron and Gerhard Wagner (Harvard Medical School) for NMR time and assistance. We thank Ralph Mazitschek (Massachusetts General Hospital) for helpful conversations regarding synthetic strategy and Edward Kim (Harvard College) for assisting with synthetic steps. We thank Jesse Chen and Jack Szostak (Harvard Medical School) for use of a CD spectropolarimeter and Gary Ruvkun (Harvard Medical School) for use of a microscope. Some worm strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. This work was supported by a grant from NIH CA24487 (J.C.). R.A.B. was the recipient of an NIH Kirschstein National Service Research Award (GM077943) and an NIH K99 Pathway to Independence Award (GM087533).
Footnotes
Supporting Information Available: Supporting data and NMR spectra of natural and synthetic indolecarboxyl-ascaroside C5 (5), as well as synthetic intermediates to 5.
References
- 1.Cassada RC, Russell RL. Dev Biol. 1975;46:326. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 2.(a) Li W, Kennedy SG, Ruvkun G. Genes Dev. 2003;17:844. doi: 10.1101/gad.1066503. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, Ferguson KC, Heller J, Platt DM, Pasquinelli AA, Liu LX, Doberstein SK, Ruvkun G. Genes Dev. 2001;15:672. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. Science. 1997;277:942. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]; (d) Lin K, Dorman JB, Rodan A, Kenyon C. Science. 1997;278:1319. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]; (e) Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. Nature. 1997;389:994. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 3.(a) Georgi LL, Albert PS, Riddle DL. Cell. 1990;61:635. doi: 10.1016/0092-8674(90)90475-t. [DOI] [PubMed] [Google Scholar]; (b) Estevez M, Attisano L, Wrana JL, Albert PS, Massague J, Riddle DL. Nature. 1993;365:644. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]; (c) Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Science. 1996;274:1389. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]; (d) Schackwitz WS, Inoue T, Thomas JH. Neuron. 1996;17:719. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 4.(a) Golden JW, Riddle DL. Science. 1982;218:578. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]; (b) Golden JW, Riddle DLJ. Chem Ecol. 1984;10:1265. doi: 10.1007/BF00988553. [DOI] [PubMed] [Google Scholar]; (c) Bargmann CI, Horvitz HR. Science. 1991;251:1243. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- 5.(a) Butcher RA, Fujita M, Schroeder FC, Clardy J. Nat Chem Biol. 2007;3:420. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]; (b) Butcher RA, Ragains JR, Kim E, Clardy J. Proc Natl Acad Sci U S A. 2008;105:14288. doi: 10.1073/pnas.0806676105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Pungaliya C, Srinivasan J, Fox BW, Malik RU, Ludewig AH, Sternberg PW, Schroeder FC. Proc Natl Acad Sci U S A. 2009;106:7708. doi: 10.1073/pnas.0811918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong E, Lee W, Kim YH, Kim K, Paik YK. Nature. 2005;433:541. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 7.Woodward AW, Bartel B. Ann Bot (London) 2005;95:707. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Bednarek P, Schneider B, Svatos A, Oldham NJ, Hahlbrock K. Plant Physiol. 2005;138:1058. doi: 10.1104/pp.104.057794. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hu JF, Wunderlich D, Sattler I, Hartl A, Papastavrou I, Grond S, Grabley S, Feng XZ, Thiericke R. J Antibiot (Tokyo) 2000;53:944. doi: 10.7164/antibiotics.53.944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.