Abstract
Objective
To evaluate the efficacy of subconjunctival nanoparticle carboplatin in the treatment of transgenic murine retinoblastoma.
Methods
Dendrimeric nanoparticles loaded with carboplatin were prepared. Forty LHβ-Tag mice were randomly assigned into 4 groups, and treated at 10 weeks of age. Each mouse received a single subconjunctival injection in one eye, and the opposite eye was left untreated as a control. Group 1 (high-dose nanoparticle) received 37.5 mg/ml nanoparticle carboplatin; Group 2 (low-dose nanoparticle) received 10 mg/ml nanoparticle carboplatin; Group 3 (conventional carboplatin) received 10 mg/ml of carboplatin in aqueous solution, and group 4 (phosphate-buffered saline; PBS) received PBS. Mice were euthanized on day 22 after treatment. Eyes were serially sectioned, and retinal tumor burden was quantified by histopathologic analysis.
Results
Mean tumor burden in the treated eyes was significantly smaller compared to the untreated eyes in the same mice in both nanoparticle carboplatin groups (Group 1, P=0.02; Group 2, P=0.02), to the treated eyes in conventional carboplatin group (Group 1 vs. 3, P<0.01; Group 2 vs. 3, P=0.01), and PBS group (Group 1 vs. 4, P<0.01; Group 2 vs. 4, P=0.01). The untreated eyes in high-dose nanoparticle carboplatin showed significantly smaller tumor mass compared to conventional carboplatin (P=0.03) and PBS group (P=0.04). No toxicity was observed in any of the groups.
Conclusions and Clinical Relevance
A single injection of subconjunctival nanoparticle carboplatin was effective in the treatment of transgenic murine retinoblastoma with no associated toxicity. The higher dose of subconjunctival nanoparticle carboplatin decreased the tumor burden in the contralateral eye.
Retinoblastoma is the most common eye cancer in children, accounting for 11% of all infant cancers and 3% of cancers developing in children younger than 15 years old. There are 300 new cases of retinoblastoma per year in the U.S 1. For unilateral, sporadic cases, enucleation is the treatment of choice, whereas other treatment modalities are considered for the familial, bilateral cases 2–3. Radiation therapy and/or systemic chemotherapy with carboplatin, etoposide, and vincristine are preferred treatment options, which are associated with increased incidence of systemic adverse effects and secondary malignancies 4–8.
In order to minimize the systemic complications, local delivery of the chemotherapeutic agent to the eye has been proposed 9,10. Subconjunctival injection of carboplatin initially showed promising results11, followed by reports of local toxicity of the periocular carboplatin such as orbital fat necrosis, ocular motility changes12, and ischemic necrosis and atrophy of the optic nerve13. It was assumed that these toxic effects were caused by the rapid dispersion of the aqueous solution of carboplatin to the surrounding orbital tissue with subsequent undesired adverse effects. Subconjunctival injection of carboplatin in fibrin sealant showed the advantage of sustained release with longer duration and minimal local toxicities14.
Nanoparticles are widely introduced into the medical field as a therapeutic or diagnostic agent, and have lower therapeutic toxicity and extend product half-life cycle15. Dendrimers are novel class of hyperbranched polymers with nanosize and a number of surface functional groups. Poly (amidoamine) (PAMAM) dendrimers have been widely used as delivery systems for various therapeutics in cancer and arthritis16,17. In this study, we evaluated the therapeutic effects of a single subconjunctival injection of carboplatin loaded PAMAM dendrimeric nanoparticles in murine transgenic retinoblastoma.
MATERIALS AND METHODS
Animals
All animal procedures were approved by Institutional Animal Care and Use Committee of Emory University and conformed to the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. The LHβ-Tag mouse model used in this study carries a transgene composed of the coding region of the simian virus 40 large T-antigen (Tag) driven by the human luteinizing hormone β–subunit promoter gene (LHβ), developing multifocal, bilateral retinal tumors analogous to human retinoblastoma18. Mice in this study were treated at 10 weeks of age.
Preparation and characterization of carboplatin loaded dendrimeric nanoparticles
For preparing carboplatin loaded nanoparticles, half-generation poly (amidoamine) dendrimer (G3.5 PAMAM, 32 carboxyl end groups) was used. First, a weighed quantity of carboplatin (27.94 mg) was dissolved in 20 mL of deionized water and stirred well till a clear solution was obtained. Then 0.375 mL of G3.5 PAMAM solution (30.43 mg) was dissolved in 4 mL of deionized water and exposed to a stream of nitrogen for 30 minutes to evaporate the trace amount of methanol. The dendrimer solution was slowly added to the drug solution and stirred for 24 hours at room temperature. The stirred solution was dialyzed against deionized water for 4 hours to remove the unentrapped drug. The final dialyzate was lyophilized and characterized for particle size and drug loading.
Treatments
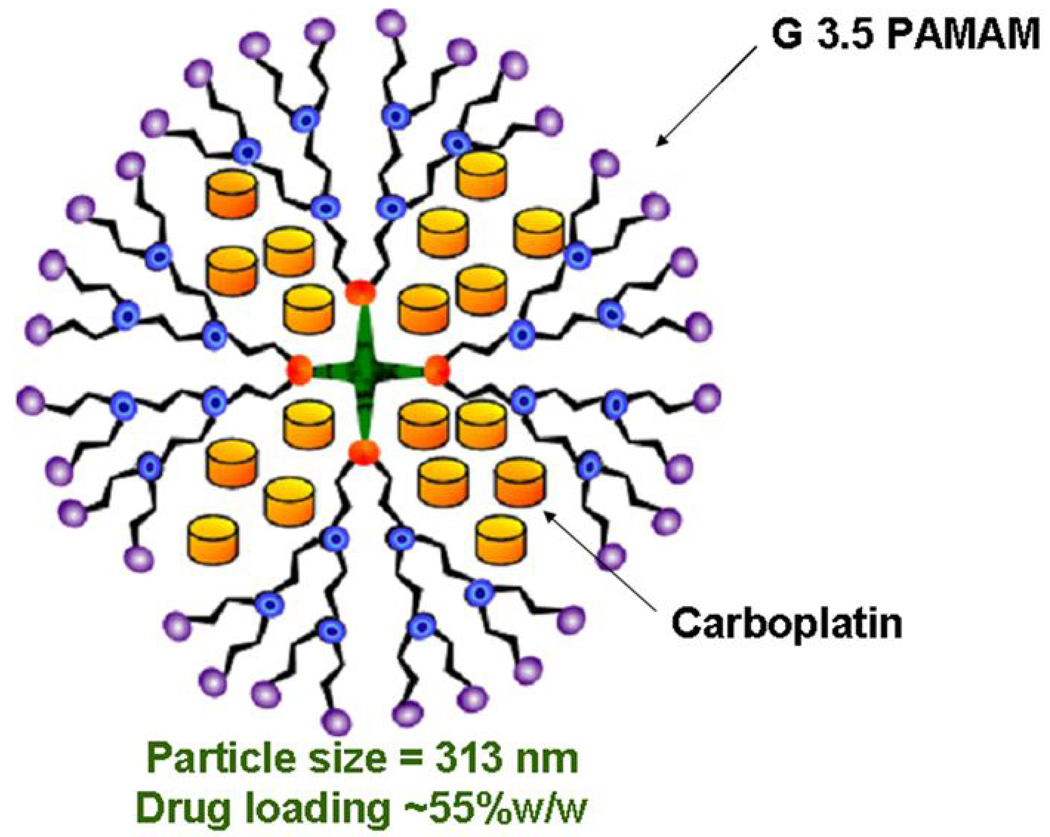
Mice were randomly assigned into 4 groups of 10 animals each. Mice were first anesthetized with a mixture of ketamine and xylazine, and then received a single 30 µl injection of each solution in the superior temporal subconjunctival space of the right eye, and the left eye was untreated as an internal control. Group 1 (high-dose nanoparticle group) received nanoparticle carboplatin with the concentration of 37.5 mg/ml, the same concentration as the low-dose carboplatin used by van Quill and et al14. We used dendrimer type of nanoparticles shown in Figure 1. Group 2 (low-dose nanoparticle group) received 10 mg/ml nanoparticle carboplatin, the same concentration as the conventional carboplatin solution. Conventional carboplatin solution (10 mg/ml) was prepared by adding carboplatin (C2528, Sigma-Aldrich, St. Louis, MO) to PBS. Group 3 (conventional group) received 10 mg/ml carboplatin in aqueous solution, and group 4 (PBS group) received 30 µl of PBS. Mice were killed on day 22, and both eyes were enucleated.
Figure 1.
Schematic diagram of nanosize carboplatin loaded G3.5 PAMAM dendrimer used in this study.
Determination of Ocular Tumor Burden
After enucleation, the eyes were fixed in 10% neutral-buffered formalin, processed routinely, and serially sectioned and stained with hematoxylin-eosin. The slides were obtained from at least 10 different levels throughout each eye. For each eye, one section from each level was examined by light microscopy and all tumor foci were identified and digitally imaged at X100 magnification. The cross-sectional area of each tumor focus was measured in square pixels with ImageJ program19, and the total area of all tumor foci was divided by the number of levels examined to calculate the mean tumor burden.
For the statistical analysis of ocular tumor burden, unpaired, two-tailed t-test was used was used to compare mean tumor burden per level among the four groups. Statistically significant difference was defined as P<0.05.
RESULTS
Carboplatin loaded dendrimeric nanoparticles had a mean particle size of 258 ± 5.7 nm with a very narrow polydispersity index of 0.072 ± 0.05. Carboplatin content was found to be 0.475 mg/mg of nanoparticles (47.54% w/w drug loading) as estimated by a previously described HPLC method20.
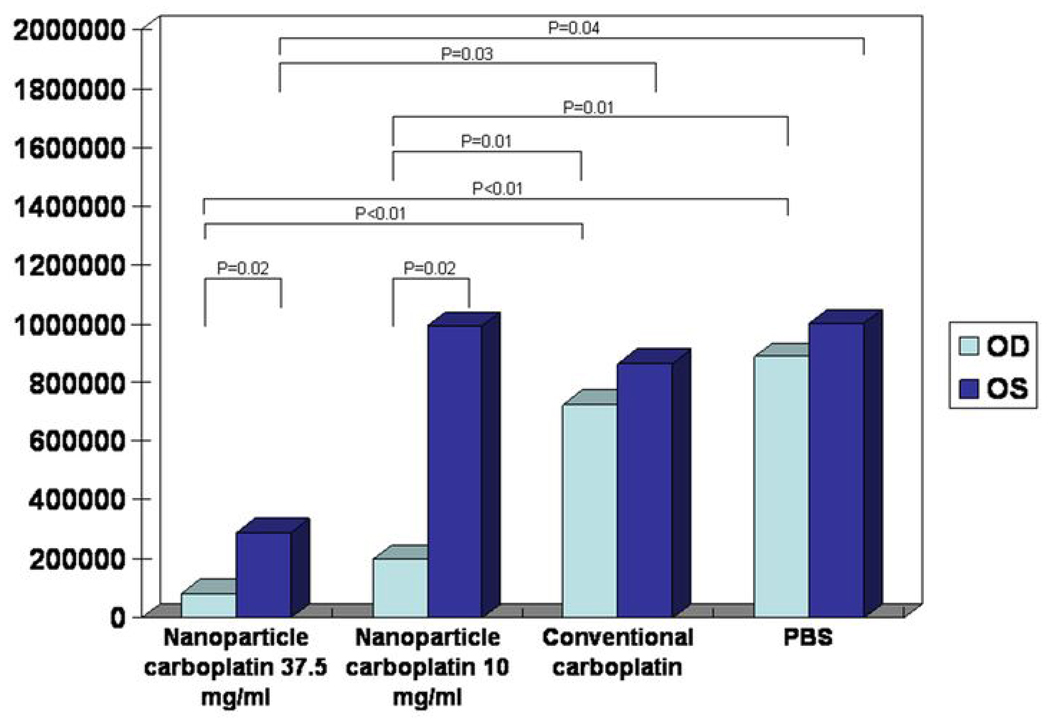
There were no clinically significant complications after subconjunctival injection in all the mice, and there was no toxicity to the retina, optic nerve, or other ocular tissues. The results of the tumor burden analysis are summarized in Figure 2 and Figure 3. The mean tumor burden in treated eyes in group 1 (high-dose nanoparticle carboplatin; 37.5 mg/ml) and group 2 (low-dose nanoparticle carboplatin; 10 mg/ml) was significantly smaller than that of untreated eyes from the same mice (group 1, 82,064 ± 85,503 vs. 288,634 ± 89,052 square pixels, P=0.02; group 2, 198,392 ± 117,373 vs. 992,633 ± 126,795 square pixels, P=0.02). One of the eyes in group 1 demonstrated zero tumor burden. Eyes treated with carboplatin in aqueous solution (group 3) or PBS (group 4) did not show significant difference compared to the untreated contralateral eyes (group 3, 724,956 ± 186,433 vs. 864,264 ± 305,110 square pixels, P=0.24; group 4, 887,485 ± 165,861 vs. 1,001,467 ± 117,121 square pixels, P=0.53). The mean tumor burden in eyes treated with both high and low-dose nanoparticle carboplatin was significantly smaller than eyes treated with carboplatin in aqueous solution (Group 1 vs. Group 3, P<0.01; Group 2 vs. Group 3, P=0.01), and PBS group (Group 1 vs. Group 4, P<0.01; Group 2 vs. Group 4, P=0.01). There was no significant difference in mean tumor burden between eyes treated with high-dose nanoparticle carboplatin and low-dose nanoparticle carboplatin (Group1 vs. Group 2, P=0.07). A single subconjunctival injection of conventional carboplatin did not show significant decrease in tumor burden compared to the PBS group (Group 3 vs. Group 4, P=0.53).
Figure 2.
Ocular tumor burden in LHβ-Tag transgenic mice treated with subconjunctival nanoparticle carboplatin. The x axis shows the different treatment group, and the y axis shows the mean tumor burden per level in square pixels.
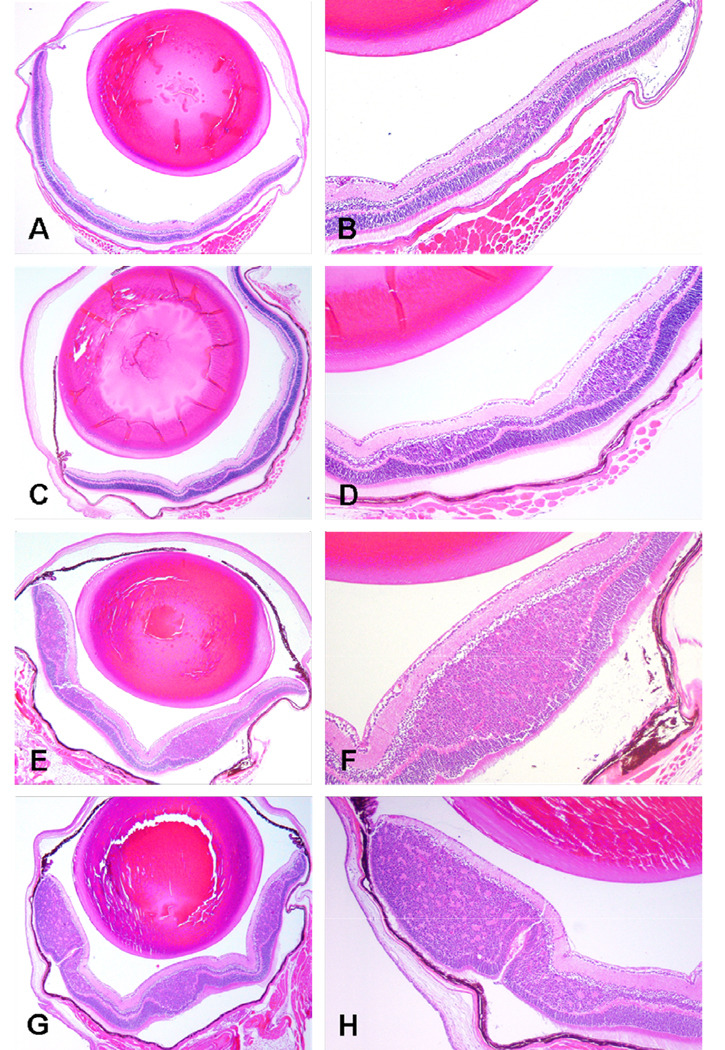
Figure 3.
Representative sections showing intraretinal tumor in eyes received high-dose nanoparticle carboplatin (A, B), low-dose nanoparticle carboplatin (C, D), conventional carboplatin (E, F), and PBS (G, H). Hematoxylin-eosin; original magnifications, X40 (A, C, E, G) and X100 (B, D, F, H).
The untreated eyes in the high-dose nanoparticle group showed statistically significant smaller tumor mass compared to the untreated eyes in the conventional carboplatin group and PBS group (Group 1 vs. Group 3, P=0.03; Group 1 vs. Group 4, P=0.04).
COMMENTS
In this study, we evaluated the therapeutic effect of a single subconjunctival injection of nanoparticle carboplatin in murine transgenic retinoblastoma. Our results showed that the subconjunctival nanoparticle carboplatin was more effective compared to the carboplatin in aqueous solution.
To enhance the delivery of the drug to the eye, we used the nanoparticle form of carboplatin. Advantages of nanoparticle-based drug delivery are that it improves the solubility of poorly water-soluble drugs, prolongs the half-life of drug systemic circulation by reducing immunogenicity, releases drugs at a sustained rate, lowers the frequency of administration, delivers drugs in a target manner to minimize systemic side effect, and enables to deliver two or more drugs simultaneously for combination therapy to generate a synergistic effect and suppress drug resistance15,21. We used PAMAM dendrimer nanoparticles with a diameter of 258 nm. The dose of carboplatin in this study was based on the report by Van Quill and coworkers, who injected carboplatin in fibrin sealant into the subconjunctival space of the LHβ-Tag mice using 2 different doses (low-dose, 37.5 mg/ml; high-dose, 75 mg/ml) 14. The low-dose group showed tumor regression in 91% of the eyes, whereas the high dose group showed a similar treatment effect with severe toxicity14. Therefore, we chose the concentration of 37.5 mg/ml for our high-dose nanoparticle carboplatin group. Low-dose nanoparticle carboplatin group used the same concentration as the carboplatin in aqueous media at its highest stable concentration of 10 mg/ml.
The results showed that the eyes treated with both high and low-dose nanoparticle carboplatin showed significant reduction in the mean tumor burden compared to the untreated, contralateral eyes. Even with the same concentration (10 mg/ml), nanoparticle carboplatin showed smaller tumor mass compared to the carboplatin in aqueous solution. This observation can be explained on the basis of prolonged retention of nanoparticles and sustained drug delivery when compared to plain drug.
In a previous study we investigated the influence of particle size on disposition of nanoparticles following periocular injection in a rodent model22. For 20 nm nanoparticles, at 6 hours after periocular injection, we observed particle accumulation in spleen, liver, and cervical, axillary, and mesenteric lymph nodes of the animals, but not in the intraocular tissues including retina and vitreous. Further, there was no detectable transport of 20 nm nanoparticles across excised bovine sclera-choroid-RPE in 24 hours. Thus, small nanoparticles of 20 nm size are not transported well across sclera to the retina and they are rapidly cleared by lymph and/or blood circulation in the periocular space. We observed that only 15% of the administered dose of 20 nm particles is retained in the periocular space after 24 hours in rodents23. However, when 200 nm particles were assessed in the same study, nearly the entire dose of particles was retained at the site of administration for at least 2 months. Therefore, the dendrimer particles administered in this study (258 nm) are not likely to cross the sclera-choroid-RPE in their original form. However, the drug released from these systems can be transported across ocular as well as systemic barriers.
Since the nanoparticles used in this study are aggregates of dendrimer molecules present at <5 nm, we cannot rule out the possibility that the administered dendrimer aggregates dissociate slowly into drug-dendrimer particles <20 nm, thereby entering the circulatory system as well as intraocular tissues. It is anticipated that PAMAM nanoparticle aggregates dissociate eventually, releasing individual nanoparticles. These small particles are expected to be cleared by blood and lymphatic systems. Also, there is a possibility that the smaller particles may traverse sclera to access the intraocular tissues. Ultimately, once in their molecular from, G3.5 PAMAM dendrimers are expected to be removed from the body via glomerular filtration24.
We observed that the untreated left eyes in the high-dose nanoparticle carboplatin showed significantly smaller mean tumor burden compared to the untreated eyes in the conventional carboplatin group and in the PBS group. Tsui and coworkers investigated the effect of subconjunctival topotecan in fibrin sealant for the treatment of transgenic murine retinoblastoma. In mice treated with topotecan, tumor burden in treated eyes and in untreated contralateral eyes did not differ significantly. However, comparison of mean tumor burden in both eyes from topotecan-treated and from control mice demonstrated a statistically significant reduction in tumor burden (P=0.04). The authors concluded that topotecan does not cross the sclera as efficiently as carboplatin in vivo because it is cleared by local vasculature at a significantly higher rate than carboplatin and the major route of drug delivery in this system is hematogenous rather than transscleral25. Our results implicate a similar effect by the size of nanoparticle (larger than 200 nm) and overloading of the nanoparticle carboplatin with higher dose. The higher concentration of nanoparticle carboplatin not only crossed directly through the sclera, but also was retained for a longer period of time, thus cleared by the local vasculature to reach the contralateral untreated eye. This is further supported by an earlier study of ours wherein we observed that following administration of a high dose of celecoxib (3 mg/rat) in the periocular space, drug was detected in the plasma and the retina of the contralateral undosed eyes26.
In summary, a single injection of subconjunctival nanoparticle carboplatin was effective in the treatment of transgenic murine retinoblastoma. A higher dose of subconjunctival nanoparticle carboplatin could reach and decrease the tumor in the untreated, contralateral eye.
ACKNOWLEDGEMENT
This study was supported by grants NIH R24 EY017045-01, P30 EY06360, and departmental grant by Research to Prevent Blindness.
Footnotes
Presented in part at the 2008 annual meeting of the Association for Research in Vision and Ophthalmology at Fort Lauderdale, Florida
None of the authors has proprietary interest in any of the product mentioned in the paper.
REFERENCES
- 1.Young JL, Smith MA, Roffers SD, Liff JM, Bunin GR. Retinoblastoma. In: Ries LAG, Smith MA, Gurney JG, editors. Cancer incidence and survival among children and adolescents: United States SEER Program, 1975–1995. Bethesda, MD: National Cancer Institute, SEER Program. NIH Pub. No.99–4649; 1999. [Accessed January 12, 2009]. Available at: http://seer.cancer.gov/publications/childhood. [Google Scholar]
- 2.Shields JA, Shields CL. Current management of retinoblastoma. Mayo Clin Proc. 1994;69(1):50–56. doi: 10.1016/s0025-6196(12)61612-7. [DOI] [PubMed] [Google Scholar]
- 3.Balmer A, Zografos L, Munier F. Diagnosis and current management of retinoblastoma. Oncogene. 2006;25(38):5341–5349. doi: 10.1038/sj.onc.1209622. [DOI] [PubMed] [Google Scholar]
- 4.Eng C, Li FP, Abramson DH, et al. Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst. 1993;85(14):1121–1128. doi: 10.1093/jnci/85.14.1121. [DOI] [PubMed] [Google Scholar]
- 5.Wong FL, Boice JD, Jr, Abramson DH, et al. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA. 1997;278(15):1262–1267. doi: 10.1001/jama.278.15.1262. [DOI] [PubMed] [Google Scholar]
- 6.Pradhan DG, Sandridge AL, Mullaney P, et al. Radiation therapy for retinoblastoma: a retrospective review of 120 patients. Int J Radiat Oncol Biol Phys. 1997;39(1):3–13. doi: 10.1016/s0360-3016(97)00156-9. [DOI] [PubMed] [Google Scholar]
- 7.Abramson DH, Frank CM. Second nonocular tumors in survivors of bilateral retinoblastoma: a possible age effect on radiation-related risk. Ophthalmology. 1998;105(4):573–579. doi: 10.1016/S0161-6420(98)94006-4. discussion 579–580. [DOI] [PubMed] [Google Scholar]
- 8.Benz MS, Scott IU, Murray TG, Kramer D, Toledano S. Complications of systemic chemotherapy as treatment of retinoblastoma. Arch Ophthalmol. 2000;118(4):577–578. [PubMed] [Google Scholar]
- 9.Murray TG, Cicciarelli N, O’Brien JM, et al. Subconjunctival carboplatin therapy and cryotherapy in the treatment of transgenic murine retinoblastoma. Arch Ophthalmol. 1997;115(10):1286–1290. doi: 10.1001/archopht.1997.01100160456013. [DOI] [PubMed] [Google Scholar]
- 10.Hayden BH, Murray TG, Scott IU, et al. Subconjunctival carboplatin in retinoblastoma: impact of tumor burden and dose schedule. Arch Ophthalmol. 2000;118(11):1549–1554. doi: 10.1001/archopht.118.11.1549. [DOI] [PubMed] [Google Scholar]
- 11.Abramson DH, Frank CM, Dunkel IJ. A phase I/II study of subconjunctival carboplatin for intraocular retinoblastoma. Ophthalmology. 1999;106(10):1947–1950. doi: 10.1016/S0161-6420(99)90406-2. [DOI] [PubMed] [Google Scholar]
- 12.Mulvihill A, Budning A, Jay V, et al. Ocular motility changes after subtenon carboplatin chemotherapy for retinoblastoma. Arch Ophthalmol. 2003;121(8):1120–1124. doi: 10.1001/archopht.121.8.1120. [DOI] [PubMed] [Google Scholar]
- 13.Schmack I, Hubbard GB, Kang SJ, Aaberg TM, Jr, Grossniklaus HE. Ischemic necrosis and atrophy of the optic nerve after periocular carboplatin injection for intraocular retinoblastoma. Am J Ophthalmol. 2006;142(2):310–315. doi: 10.1016/j.ajo.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Van Quill KR, Dioguardi PK, Tong CT, et al. Subconjunctival carboplatin in fibrin sealant in the treatment of transgenic murine retinoblastoma. Ophthalmology. 2005;112(6):1151–1158. doi: 10.1016/j.ophtha.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 16.Tomalia DA, Reyna LA, Svenson S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem Soc Trans. 2007;35(1):61–67. doi: 10.1042/BST0350061. [DOI] [PubMed] [Google Scholar]
- 17.Chandrasekar D, Sistla R, Ahmad FJ, Khar RK, Diwan PV. The development of folate-PAMAM dendrimer conjugates for targeted delivery of anti-arthritic drugs and their pharmacokinetics and biodistribution in arthritic rats. Biomaterials. 2007;28(3):504–512. doi: 10.1016/j.biomaterials.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 18.Windle JJ, Albert DM, O’Brien JM, et al. Retinoblastoma in transgenic mice. Nature. 1990;343(6259):665–669. doi: 10.1038/343665a0. [DOI] [PubMed] [Google Scholar]
- 19.Image J [computer program]. Version 1.41, Bethesda, MD, National Institute of Heath.
- 20.Valière C, Arnaud P, Caroff E, Duaphin JF, Clément G, Brion F. Stability and compatibility study of a carboplatin solution in syringes for continuous ambulatory infusion. Int J Pharm. 1996;138(1):125–128. [Google Scholar]
- 21.Emerich DF, Thanos CG. Targeted nanoparticle-based drug delivery and diagnosis. J Drug Target. 2007;15(3):163–183. doi: 10.1080/10611860701231810. [DOI] [PubMed] [Google Scholar]
- 22.Amrite AC, Edelhauser HF, Singh SR, Kompella UB. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol Vis. 2008;14:150–160. [PMC free article] [PubMed] [Google Scholar]
- 23.Amrite AC, Kompella UB. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J Pharm Pharmacol. 2005;57(12):1555–1563. doi: 10.1211/jpp.57.12.0005. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi H, Brechbiel MW. Nano-sized MRI contrast agents with dendrimer cores. Adv Drug Deliv Rev. 2005;57(15):2271–2286. doi: 10.1016/j.addr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Tsui JY, Dalgard C, Van Quill KR, et al. Subconjunctival topotecan in fibrin sealant in the treatment of transgenic murine retinoblastoma. Invest Ophthalmol Vis Sci. 2008;49(2):490–496. doi: 10.1167/iovs.07-0653. [DOI] [PubMed] [Google Scholar]
- 26.Ayalasomayajual SP, Kompella UB. Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharm Res. 2004;21(10):1797–1804. doi: 10.1023/b:pham.0000045231.51924.e8. [DOI] [PubMed] [Google Scholar]