Abstract
Antibodies to the extracellular region of the ErbB receptors have played key roles in the development of a mechanistic understanding of this family of receptor tyrosine kinases. An extensively studied class of such antibodies inhibits activation of ErbB receptors, and these antibodies have been the focus of intense development as anti-cancer agents. In this review we consider the properties of ErbB receptors antibodies in light of the current structure-based model for ErbB receptor homo- and hetero-dimerization and activation. Crystal structures of the Fab fragments from five different inhibitory antibodies in complex with the extracellular regions of EGFR and ErbB2 have been determined. These structures highlight several different modes of binding and mechanisms of receptor inhibition. Information about antibody interactions with the structurally well-characterized soluble extracellular regions of ErbB receptors can be combined with the rich knowledge of the effects of these antibodies in cultured cells, and in vivo, to provide insights into the conformation and activation of ErbB receptors at the cell surface.
Keywords: EGFR/ErbB1, ErbB2/HER2, Antibody, Trastuzumab/Herceptin, Cetuximab/Erbitux, ErbB receptor inhibition
Introduction
Antibodies have played a crucial role in understanding ErbB receptors since the early mechanistic studies of this family of receptor tyrosine kinases (RTKs). Antibodies were essential for the generation of purified EGFR that was used to demonstrate that ligand-induced dimerization is a critical first step in receptor activation [1]. Others antibodies have provided clues as to the nature of the two ligand affinity “classes” of receptors that exist at the cell surface [2, 3]. For ErbB2 (also known HER2/neu), antibodies have also played a key role in establishing the identity and role of this receptor [4, 5]. ErbB2 antibodies were found to reverse the phenotype of transformed cells by binding to and down-modulating this oncogenic protein [6]. This confirmed the link between the neu oncogene and malignancy, and provided proof of concept that antibodies could have antitumor activity [7].
In the early 1980s, a number of groups generated monoclonal antibodies to the extracellular region of EGFR using as immunogen A431 epidermoid carcinoma cells, which express high levels of cell surface epidermal growth factor receptor (EGFR). The resulting antibodies display various properties. Some had no effect on growth factor activation [8], while others induced receptor aggregation and mimicked the effects of ligand stimulation [9, 10]. A third class of antibodies blocked the ability of growth factor to activate the receptor [11, 12]. Antibodies of this third class have received substantial attention as potential inhibitors of EGFR activation in human tumors [11, 12]. It has been clear for many years that there is a correlation between aberrant activation of members of the ErbB receptor family and the development and progression of cancers [13, 14]. Both the extracellular and intracellular regions of EGFR and ErbB2 are targets of therapeutic agents in active clinical use and/or development [15, 16]. In 2003, when the first special issue on the ErbB/EGF system was published in Experimental Cell Research, the ErbB2 targeted antibody drug trastuzumab/Herceptin had been approved for use in ErbB2 positive breast cancers, and several anti-EGFR directed antibody drugs were in clinical trials. There are now three EGFR antibodies approved for use in various clinical settings (Table 1), and numerous other antibodies against this family of receptors are the focus of active clinical trials. Many excellent reviews focus on the development and clinical application of monoclonal antibodies against the extracellular regions of ErbB receptors [7, 15–18]. In this review we consider the interactions of antibodies with the extracellular region of ErbB receptors in light of structure-based models of ErbB receptor homo- and hetero-dimerization.
Table 1.
Properties of selected conformationally sensitive antibodies to the extracellular regions of ErbB receptors
| mAb | Epitope information | Drug name1 | Comments | Refs.2 |
|---|---|---|---|---|
| EGFR | ||||
| 1. Monoclonal antibodies derived from mice immunized with A431 cell EGFR (intact cells or membrane preparations) | ||||
| 225 | Domain III [65] | Cetuximab/Erbitux | Global approval for use against colorectal and head and neck cancers, wider clinical trials in progress | [11, 12] |
| 425 | Domain III [66] | Matuzumab | Phase II trials | [37, 38] |
| R1 | Domain I/II [96] | Does not block ligand stimulated activation | [8] | |
| 2E9 | Domain I [96] | Blocks low affinity cell surface EGF binding sites | [3] | |
| 108 | Domain III [96] | Blocks high affinity cell surface EGF binding sites | [2] | |
| 13A9 | Domain III [84] | Inhibits TGFα activation (and binding) of EGFR, but does not alter EGF binding or signaling | [97] | |
| 29.1 | Carbohydrate | Used in purification of active EGFR | [98–100] | |
| 2. Monoclonal antibody derived from mice immunized with purified placental EGFR | ||||
| R3 | Linear epitope on domain III (400–410) [101]. | Nimotuzumab (TheraCIM) | Not conformationally sensitive, ongoing clinical trials, limited nation approval for head and neck cancer and glioma | [102] |
| 3. Monoclonal antibody derived from rats immunized with the EGFR overexpression breast cancer cell MDA-MB 468 | ||||
| ICR62 | Group “C”, also binds EGFRvIII [103] | Phase I trials | [104] | |
| 4. Fully human monoclonal antibodies derived from transgenic mice immunized with A431-derivered EGFR | ||||
| ABX-EGF | Domain III [67] | Panitumumab/Vectibix | Approved for colorectal cancer, in trials for NSCLC | [48] |
| HuMax-EGFr | Domain III [68] | Zalutumumab | FDA fast track status for head and neck cancer trials | [52] |
| 5. Fully human monoclonal antibody derived from library screening | ||||
| IMC-11F8 | Domain III [64] | Binds to cetuximab epitope, phase I trials | [42, 43] | |
| 6. Monoclonal antibody derived from mice immunized with fibroblasts expression EGFRvIII (also known as de2-7 EGFR) | ||||
| 806 | Domain II [83] | Binds to a fraction of wild-type receptor on EGFR overexpressing EGFR, chimerized to ch806, phase I trials | [56, 58] | |
| ErbB2/HER2 | ||||
| Monoclonal antibody derived from mice immunized with NIH 3T3 cells that had been transformed with human ErbB2 | ||||
| 4D5 | Domain IV [28] | Trastuzumab/Herceptin | Global approval from use in breast cancers | [61, 105] |
| 2C4 | Domain II [63] | Pertuzumab/Omnitarg | In phase II/III trials for a range of cancers | [61, 62] |
Antibody naming convention: ximab, chimeric; zumab, humanized; umab, fully human
References here are limited to original articles describing basic, preclinical properties of the antibody.
Structural studies in the past 6 years have led to a model for ligand-induced homo- and hetero-dimerization and activation of ErbB receptors, the details of which have been extensively reviewed [19–21]. Recent developments in understanding mechanisms of ErbB receptor activation are discussed in an accompanying review in this issue [22]. The salient points of this model for the discussion of antibody mediated inhibition of ErbB receptors are summarized in Figure 1. For three of the four members of the ErbB receptor family, EGFR/ErbB1, ErbB3/HER3 and ErbB4/HER4, the unliganded extracellular region adopts a “tethered” conformation in which domains II and IV interact [23–25]. The ligand bound form of the extracellular region has only been crystallographically observed for EGFR [26, 27] and, in this state, the extracellular region of the receptor adopts a very different conformation. Ligand binds between domains I and III of the receptor molecule, and drives exposure and remodeling of a dimerization interface on domain II. The fourth member of this family of receptors, ErbB2, has no known soluble ligand and is a structural outlier among this family of four receptors. The unliganded conformation of ErbB2 does not adopt the tethered conformation. Rather, ErbB2 adopts an extended arrangement of domains similar that of the ligand bound form of EGFR [28, 29]. Based on these structures a generalized mechanism of ligand induced homo- and hetero-dimerization has been proposed [19] and is shown schematically in Figure 1. This mechanism of activation of ErbB receptors has substantial implication for possible modes of binding by antibodies that could inhibit or modulate receptor activation.
Figure 1. Mechanism of homo- and hetero-dimerization of the extracellular regions of ErbB receptors [19].
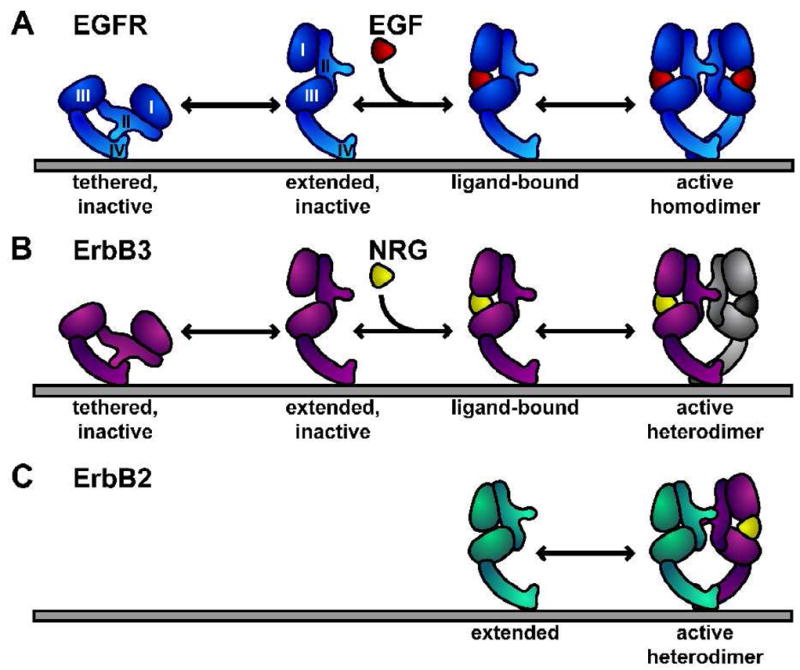
A The unliganded state of the EGFR extracellular region (sEGFR) adopts a tethered configuration of domains that is quite distinct from the domain arrangement in the ligand-induced dimer. Conceptual structural intermediates are shown: (i) an extended, unliganded monomer and (ii) an extended ligand-bound monomer. Ligand binding and dimerization are highly cooperative and such intermediates are not significantly populated in solution studies of sEGFR. Crystal structures have been observed of the tethered monomer [25] and ligand-induced dimers comprising domains I, II, III and the first disulfide-bonded module of domain IV [26, 27]. The remainder of domain IV is modeled as previously described [25].
B For ErbB3 and ErbB4 structures of tethered monomers have been determined that resemble that of EGFR [23, 24]. ErbB3 extracellular region does not homodimerize in solution [71] or in cells [72, 95]. Small angle X-ray scattering studies show that neuregulin does promotes the formation of an extended ligand-bound monomer [71]. The model predicts that ErbB4 will form homodimers as in A, and that both ErbB3 and ErbB4 can form heterodimers with at least a subset of other ligand-bound ErbB receptors.
C Unliganded ErbB2 adopts an extended structure with a domain configuration similar to that of extended (ligand-bound) sEGFR [28, 29]. ErbB2 is proposed to heterodimerize with other ligand-bound extended ErbB receptors using a similar, largely domain II-mediated, dimerization interface.
Antibodies against the extracellular region of ErbB receptors
In the following sections we briefly review a selection of the antibodies against the extracellular regions of ErbB receptors that are important inhibitors of receptor function, or that have provided opportunities to probe receptor activation mechanisms. Properties of key antibodies are also summarized in Table 1.
EGFR antibodies
mAb 225 (chimerized to IMC-C225/cetuximab/Erbitux)
Monoclonal antibody 225 was one of several antibodies raised by inoculation of mice with A431 epidermoid carcinoma cells by a group led by Prof. John Mendelsohn (University of Texas M.D. Anderson Cancer Center). Three antibodies (mAbs 225, 528 and 579) were characterized that inhibited EGF binding to the receptor, blocked ligand-dependent receptor activation [11, 12, 30] and inhibited cellular proliferation in vitro and in vivo [31, 32]. Monoclonal antibody 225 was selected to generate a human/mouse chimeric molecule for clinical development [33]. The resulting chimeric antibody, IMC-C225/cetuximab, originally developed by ImClone Inc., was first approved for therapeutic application in 2004. It is marketed under the trade name Erbitux by Bristol Myers in the US and Merck KGaA elsewhere. Cetuximab is being investigated in multiple clinical trials to broaden its clinical uses. Clinical studies with this antibody have been extensively reviewed (see for example [34–36] and references therein).
mAb 425 (humanized to EMD 72000/matuzumab)
Independently, a group at the Wistar Institute (Philadelphia) also generated a mouse monoclonal antibody against the extracellular region of EGFR using A431 cells. Like cetuximab, mAb 425 blocks binding of EGF and TGFα to A431 cells, blocks EGFR activation [37] and inhibits tumor growth in mouse models [38]. A humanized version of mAb 425, matuzumab/EMD 72000 (Merck KGaA) has progressed to Phase II clinical trials to treat a range of cancers, both alone and in combination therapy [39, 40].
IMC-11F8
This fully human antibody was constructed using an isolate from a non-immunized human Fab phage display library [41, 42]. The Fab from this library was selected for high affinity binding to the EGFR on A431 cells, and for its ability to compete with cetuximab for binding to these cells [42]. IMC-11F8 inhibits EGFR activation in several cell-lines [42, 43], blocks tumor growth in xenograft models [44, 45], and has performed well in phase I clinical trials [46]. Now in Phase II clinical trials, IMC-11F8 holds promise as a next generation cetuximab.
ABX-EGF/Panitumumab/Vectibix and HuMax-EGFr/Zalutumumab
These two antibodies have been developed more recently from transgenic mice that express fully human antibodies [47]. ABX-EGF binds to EGFR with higher affinity than cetuximab, blocks ligand binding and receptor activation, and has potent anti-tumor activity in model systems [48]. It is the focus of multiple ongoing clinical trials and has been approved for use in colorectal cancer ([49] and references therein). Initially developed by Abgenix, ABX-EGF is now being developed and marketed by Amgen under the trade name Vectibix. ABX-EGF is an antibody of subtype IgG2 and does not stimulate robust antibody dependent cellular cytotoxicity (ADCC), an immune effector mechanism that contributes to the antitumor activity of many antibodies [50, 51]. HuMax-EGFr (originally named mAb 2F8) was developed by GenMab using a different transgenic mouse platform (generating IgG1 antibodies), and using both A431 cells and purified receptor as immunogen [52]. The preclinical characteristics of this antibody are similar to others that have shown clinical promise, with excellent anti-tumor activity at low dose. Zalutumumab is in accelerated clinical trials in a number of settings [53].
mAb 108 and mAb 2e9
These two mouse monoclonal antibodies have not been developed for clinical application. Rather these have provided interesting clues about the binding of ligand to cell surface EGFR. Monoclonal antibody 108 was raised using CHO cells that overexpress a human EGFR truncation variant lacking the intracellular domain [2]. These cells were used as an alternative to A431 cells as they lack certain highly antigenic carbohydrate groups. Mouse monoclonal antibody 108 selectively blocks binding of EGF to the high-affinity sub-population (5–10%) of EGF binding sites (with KD < 100 pM) observed on the surface of EGFR-expressing cultured cells, without affecting binding to the majority (90 – 95%) of lower affinity EGF binding sites (KD of 2–12 nM) [2]. Like cetuximab, mAb 108 is effective in inhibiting growth of human tumors in mouse xenografts, demonstrating that this antibody blocks proliferative EGFR signaling in vivo [54]. In vitro, early cellular responses to physiologically relevant concentrations of EGF, such as receptor phosphorylation and alterations in intracellular Ca2+ levels, are blocked by mAb 108 in a manner that coincides with the loss of higher affinity EGF-binding sites. Monoclonal antibody 2E9 was generated using A431 cell membranes as an immunogen [55]. By contrast with mAb 108, mAb 2E9 binding to cell surface EGFR blocks only the low affinity EGF binding sites, without influencing the high affinity sites [3, 55], and does not block cell proliferation. It should be noted that both mAbs 108 and 2E9 bind to all the receptors at the cell surface – they do not selectively bind to only “high” or “low” affinity receptors. Rather the binding of these antibodies to all receptors is able to modulate cell surface ligand binding.
mAb 806
This mouse monoclonal antibody was generated using cells expressing EGFRvIII, also known as de2-7 EGFR, as antigen [56]. EGFRvIII is the most common gene disruption in the region containing the exons for the extracellular region of EGFR, and is found in about 25 % of glioblastomas, as well as in a number of solid tumors [57]. Exons 2–7 are deleted resulting in the EGFRvIII protein that (i) lacks amino acids 6–273 of the mature protein, (ii) has a glycine following amino acid 5, and (iii) has an unpaired cysteine at amino acid 16 (amino acid 283 of mature EGFR). In addition to binding to cell surface EGFRvIII, mAb 806 also binds to a fraction (< 10%) of wild-type EGFR in cells expressing elevated levels of the receptor, but not to the wild-type EGFR in normal tissue or to tumor cells that express low levels of EGFR [58]. Consistent with this binding profile, this antibody shows antitumor activity against xenografts expressing EGFRvIII or high levels of EGFR. Treatment of cultured glioblastoma cell lines with mAb 806 leads to a decrease in EGFRvIII phosphorylation and reduced cellular proliferation [56]. A chimeric version of 806 (c806) has been engineered and has performed well in phase I trials [59]. Monoclonal antibody c806 appears to concentrate in the tumors of several patients in this trial, consistent with the argument that this antibody shows increase binding to tumors compared with normal tissue.
ErbB2 antibodies
4D5 (humanized to trastuzumab/Herceptin)
In the late 1980s the case for developing an antibody-based anti-ErbB2 therapeutic was strong. It had recently been identified that ErbB2 is overexpressed in almost 30 % of breast tumors, and that this correlates with aggressive disease progression [60]. Antibodies raised to mouse cells transformed with rat ErbB2 had been shown to reverse the transforming effects of the neu oncogene [4], and EGFR targeted antibodies with anti-tumor activity had been identified [32]. Researchers at Genentech generated a panel of antibodies against human ErbB2 [61]. These antibodies were tested against a range of cell lines and in xenograft studies. One antibody, mAb 4D5, was selected as the top candidate with therapeutic potential against ErbB2 overexpressing tumors ([18] and references therein). A human/mouse chimeric version of 4D5 was generated and, following rapid successful clinical trials (from Phase I to approval in 6 years), was FDA-approved for use against ErbB2 positive breast cancers in 1998. An excellent review detailing the progress of trastuzumab from concept to clinic has recently been published [18].
2C4 (humanized to pertuzumab/Omnitarg)
One of the antibodies from the panel generated by Genentech was found to have quite different properties form those of mAb 4D5 and to have a non-overlapping epitope [61, 62]. This antibody blocks ligand-dependent ErbB2 activation and has been the focus of particular attention for its therapeutic potential in breast and prostate cancers that do not express high levels of ErbB2. Monoclonal antibody 2C4 is a potent inhibitor of neuregulin-induced receptor phosphorylation and downstream signaling in cell lines with low levels of ErbB2, and has antitumor activity in xenograft models with this same receptor expression profile [62]. A humanized version of this antibody is in Phase II clinical trials [36].
Mechanisms of inhibition of ErbB receptor activation by therapeutic antibodies
The mechanism of activation of ErbB receptors, illustrated in Figure 1, suggests several modes of binding by an antibody that could inhibit ligand-dependent activation of these receptors. These mechanisms can be conceptually broken down: (i) stabilization of the tethered conformation, (ii) block of the domain rearrangement required to attain the extended state, (iii) direct block of ligand binding (iv) direct block of receptor dimerization. Co-crystal structures have been determined of the Fab fragments from five different clinically relevant antibodies in complex with the extracellular regions of EGFR and of ErbB2 (Figure 2) [28, 63–66]. As summarized in Figure 3 and discussed below, these structures illustrate aspects of these different mechanisms of ErbB receptor inhibition.
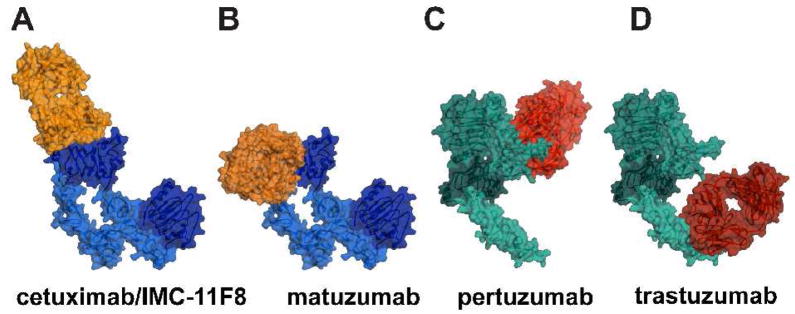
Figure 2. Co-crystal structures of ErbB extracellular regions with Fab fragments from inhibitory antibodies.
A molecular surface representations of the Fab fragment from each antibody in complex with the entire extracellular region of EGFR (blue) or ErbB2 (green) are shown.
A. Cetuximab (orange) binds to domain III of EGFR [65] (pdb id. 1yy9).
B. Matuzumab (orange) binds to a different epitope on domain III. The co-crystal structure contained only domain III [66] (pdb id. 3c09), the other domains of EGFR are modeled using pdb id. 1yy9.
C. Pertuzumab (red) binds to the domain II dimerization arm of ErbB2 [63] (pdb id. 1s78).
D. Trastuzumab (red) binds to domain IV of ErbB2 [28] (pdb id. 1n8z).
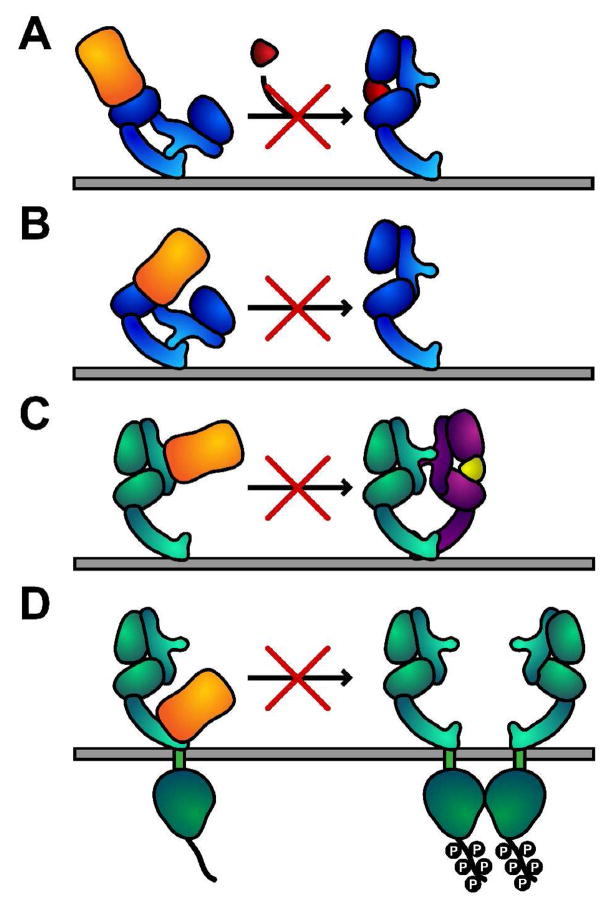
Figure 3. Mechanisms of inhibition of ErbB receptor activation by antibodies that bind to the extracellular region.
A. Antibody binding directly occludes the ligand-binding site – this mode of inhibition is important for cetuximab, IMC-11F8, panitumumab and zalutumumab.
B. Antibody sterically prevents the receptor from adopting the conformation required for high-affinity ligand binding and dimerization, without directly occluding a ligand-binding site. This mode is observed for matuzumab.
C. Antibody binding directly prevents receptor dimerization. Observed for pertuzumab.
D. For trastuzumab binding to domain IV prevents constitutive activation of ErbB2 via a number of mechanisms. Trastuzumab may also block domain IV contacts that are important for formation of heterodimers with some ErbB receptors (not shown).
Direct competition for ligand binding
The X-ray crystal structure of the Fab fragment from cetuximab in complex with sEGFR shows that the epitope for this antibody lies on domain III of EGFR and overlaps substantially with the EGF binding site on that domain [65] (Figure 2A & 4A). Direct occlusion of the ligand-binding site is the primary mechanism of inhibition by this antibody (Figure 3A). The epitope for the fully human inhibitory antibody mAb IMC-11F8 has also been crystallographically defined and is almost identical to that of cetuximab, although the sequences of the complementarity determining regions (CDRs) for these two antibodies are quite different [64].
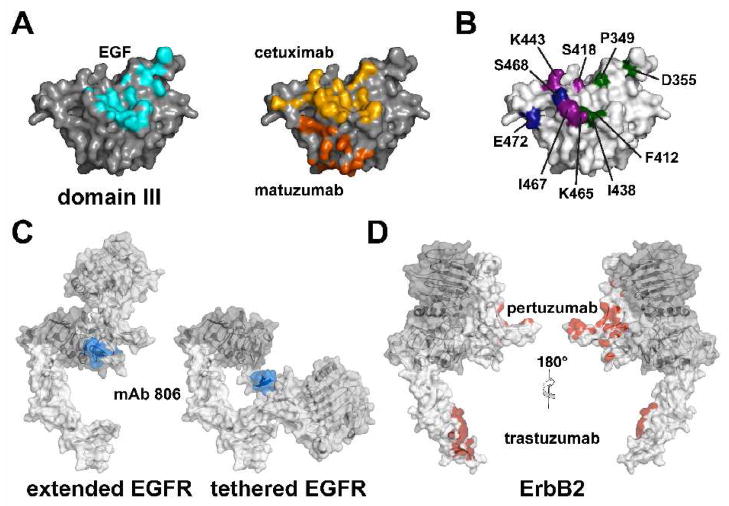
Figure 4. Epitopes for EGFR and ErbB2 antibodies.
A. A molecular surface is shown of isolated domain III of EGFR looking down onto the ligand-binding site. Amino acids within 4 Å of the EGF (pdb id. 1ivo) are shaded in blue. The same surface is also shown with the cetuximab and matuzumab epitopes shaded in yellow and orange respectively.
B. The same projection of the domain III molecular is shown. Mutation of the highlighted amino acids affects binding of (i) panitumumab (green) [67], (ii) zalutumumab (magenta) [68] and (iii) mAb 13A9 (blue) [84].
C. The epitope of mAb 806 (blue) lies on a loop between C287 and C302 in domain II [83]. In both the extended ligand-bound, and the tethered structures of EGFR this epitope is partially occluded and would not be accessible for antibody binding.
D. The binding footprints (atoms within 4 Å of the bound Fab) of trastuzumab and pertuzumab are highlighted in red on a molecular surface representation of ErbB2.
Based on these co-crystal structures, and on the structures of sEGFR in complex with EGF [27] and TGFα [26], alterations were made in the domain III binding site of EGFR that disrupt ligand binding, cetuximab/IMC-11F8 binding, or both [64, 65]. This information was used to glean information about the epitopes of several other antibodies that are performing well as EGFR targeted drugs [67, 68] (Figure 4B), and these epitope mapping data have been corroborated with cross-competition between different antibodies for binding to cell surface EGFR [69]. The epitopes for panitumumab and zalutumumab both overlap substantially with that of cetuximab, and with the EGF binding site on domain III. It is highly likely that the mechanism of inhibition of ligand dependent EGFR activation by these two antibodies is similar to that of cetuximab, although the precise orientation of these two Fab fragments on sEGFR has not been crystallographically defined.
Steric block of the conformation change required for high affinity ligand binding and dimerization
As discussed above the primary mechanism for cetuximab-mediated inhibition of ligand induced EGFR activation is the direct block of ligand binding. However the structure suggests an additional mechanism that may also play a role in the antitumor activity of cetuximab. The orientation of the Fab when bound to its epitope on domain III sterically blocks the receptor from adopting the extended conformation required for dimerization [65]. We speculated that this represents a meaningful component of the inhibitory mechanism in tumors that express aberrantly high levels of EGFR, where ligand-independent dimerization and activation of the receptor could contribute to tumorigenesis [16].
Structural studies of the Fab fragment from another clinically significant antibody, matuzumab, show that steric block of receptor conformational change can be the sole mechanism of inhibition of ligand induced EGFR dimerization and activation [66] (Figure 2B). The epitope for matuzumab also lies on domain III of EGFR, but matuzumab binding does not occlude the ligand-binding site on the receptor. In this sense, matuzumab is quite different from cetuximab, IMC-11F8, panitumumab and zalutumumab (Figure 4A & B). When bound to EGFR, the matuzumab Fab is oriented such that it sterically prevents domain II from adopting the conformation required for high affinity ligand-binding and receptor dimerization [66]. Notably, matuzumab binding prevents interactions between domains II and III that are known to be critical for receptor activation [27, 70]. It is highly likely that this “conformational-restriction” mechanism will be exploited by antibodies that bind to other epitopes either on domain III or elsewhere on the receptor.
Stabilization of the inactive tethered conformation
From the structure-based mechanism presented in Figure 1, it is clear that an agent that locks the receptor in the tethered conformation would be an effective inhibitor. The identification of such a mode of binding based on studies of the soluble extracellular region of the receptor has not been possible since no direct assay for the stability of the tethered conformation has been established. Disrupting all structurally defined interactions that characterize the tethered conformation does not perturb the solution conformation of the protein [71]. The electron microscopy based technique of Protein Tomography has been used to study the conformation of cell surface EGFR [68]. These studies show evidence of different conformations of unliganded cell surface EGFR, including some that are consistent with the crystallographically defined tethered state, and others that appear to be more extended [68]. Significantly, in the presence of zalutumumab only the more compact, tethered conformation of EGFR is observed. These data suggest that binding of zalutumumab to domain III may stabilize the tethered conformation, although the precise molecular basis for this stabilization is not yet clear.
Direct block of receptor dimerization
It is clear that an agent that directly blocks formation of the receptor mediated contacts in the activated dimer would be an effective inhibitor. Precisely this mechanism is suggested by the structure of pertuzumab in complex with the extracellular region of ErbB2 [63]. Pertuzumab binds to an epitope on the domain II dimerization arm of ErbB2 (Figure 3C), effectively blocking the ability of ErbB2 to form heterodimers with other ErbB receptors (Figure 2C). The identification of the epitope for pertuzumab on the dimerization arm of ErbB2 is convincing corroboration of the model for heterodimerization presented in Figure 1C. The soluble extracellular regions of ErbB receptors do not form stable heterodimers [72] and the structure of an ErbB receptor heterodimer has yet to be determined. Pertuzumab blocks formation of cell surface heterodimers of ErbB2 with both ErbB3 and with EGFR [62, 73], implying that the domain II dimerization arm of ErbB2 is required for formation of these heterodimers. By contrast, pertuzumab does not inhibit the ligand-independent activation of overexpressed ErbB2 [62], suggesting that in this context the activation of the kinase domain of ErbB2 is not triggered by formation of a domain II mediated homodimer akin to the homodimer observed structurally for ligand-bound EGFR.
The binding of an inhibitory agent to the domain II dimerization arm of EGFR has not yet been described, however such an agent would clearly be a potent inhibitor of ligand induced homodimerization of EGFR. It is possible that the lack of such an inhibitor may result from the relatively limited selection procedures that have been used to generate the current repertoire of inhibitory antibodies. A431 cells have been the primary immunogen or selection agent used for almost all antibodies to the extracellular region of EGFR. This may bias the array of conformations of the receptor presented for antibody selection.
Insights from the binding of trastuzumab to ErbB2
Trastuzumab prevents proliferation of cells that overexpress ErbB2 [18]. In these cells ErbB2 is constitutively active, and there is no requirement for an activating ErbB ligand. The antitumor activity of trastuzumab is multifaceted in animals, with critical roles arising from receptor downregulation and ADCC (antibody dependent cellular cytotoxicity) [18, 36]. Here we consider the insights that come from the structural definition of the trastuzumab-epitope on domain IV of ErbB2 [28].
Domain IV has been suggested to play a direct role in the stabilization of the ligand-induced dimers of EGFR [19, 25], although the energetic contribution of this interaction is not large [70]. If domain IV of ErbB2 plays a role in stabilizing heterodimers involving ErbB2, then it might be anticipated that trastuzumab would block their formation. In cells expressing modest levels of ErbB2, trastuzumab does not block the formation of neuregulin-induced heterodimers with ErbB3 [62, 73], but the formation of ErbB2/EGFR heterodimers appears to be inhibited [73]. One interpretation of these observations is that the conformations of the heterodimers formed with ErbB3 and EGFR differ such that only ErbB2/EGFR heterodimers are blocked by trastuzumab binding to ErbB2. Domain IV interactions might be more important in one case than the other. This indirect evidence supports the model that heterodimers of ErbB receptors - at least between ErbB2 and EGFR - will indeed resemble the structure of the EGFR homodimer. Confirmation of this model awaits a detailed structural description of an ErbB receptor heterodimer.
As mentioned above, activation of overexpressed ErbB2 is unlikely to occur through formation of domain II mediated ErbB2 homodimers. One mechanism of inhibition of ErbB2 activation that is clearly explained by binding of trastuzumab to domain IV of ErbB2 is prevention of ectodomain shedding [74]. Cells that overexpress ErbB2 exhibit increased ectodomain shedding and this has been linked to oncogenesis [74]. Trastuzumab occludes the proteolytic cleavage site in the juxtamembrane region of the ErbB2 [28] and would thus block activation of the intracellular kinase domains that follows cleavage.
Insights into the activation of ErbB receptors at the cell surface from studies with antibodies
High and low affinity binding sites for growth factor at the cell surface
The binding of EGF to cell surface EGFR produces curvilinear Scatchard plots, the molecular explanation of which remains a matter of debate, and considerable interest. The predominant view is that there are two classes of ligand binding site at the cell surface [75, 76] – a low population of high affinity sites (KD < 100 pM) and a majority lower affinity class of sites (KD ≈ 10 nM) [2], although alternative explanations, such as negative cooperativity in ligand binding, have also been suggested [77, 78]. Adding weight to the argument for two populations of receptors are mAb 108 and mAb 2E9. While mAb 108 selectively blocks the high-affinity class of binding sites, mAb 2E9 blocks only the low affinity receptors (leaving the high-affinity sites intact). The nature of the difference between high affinity and low affinity EGF binding sites is not at all clear [79]. However, it appears that the high-affinity class of EGF receptors is the most important in cellular EGFR signaling [2, 3]. Several suggestions have been made as to what defines the high- and low-affinity sites, including the possibility that high-affinity sites represent pre-formed receptor dimers, and low-affinity sites represent monomers [79]. Attempts to reconcile the two affinity classes with the structure-based model [19] have led to conflicting conclusions [80–82]. Structural definition of the epitopes and modes of binding to EGFR of mAb 108 and 2E9 would likely shed substantial light not only on how these antibodies achieve these specific inhibitory effects, but also on the origin of these effects on ligand binding to cell surface EGFR.
Conformation of EGFR at the cell surface
It is clear that the preferred solution conformation of the extracellular region of EGFR, ErbB3 and ErbB4 is the tethered configuration, and that of ErbB2 is extended [71]. The conformation of these receptors at the cell surface is less clear. All antibodies discussed in this review bind to EGFR or to ErbB2 at the cell surface, or at least to a sub-population of these receptors. Can the definition of the epitopes and modes of binding for these antibodies shed light on conformations of ErbB receptors at the cell surface? Binding of the Fab fragments from cetuximab, trastuzumab and pertuzumab to the entire extracellular regions of EGFR and ErbB2 does not perturb the conformation of these receptors. These epitopes must be exposed at the cell surface as they are in the solution and crystal conformations of these receptors. Further, recent Protein Tomography studies of binding of zalutumumab to EGFR at the surface of A431 cells are consistent with its binding to a tethered EGFR [68].
Monoclonal antibody 806 has been suggested to offer a unique insight into the conformational transition from tethered to extended receptor at the cell surface [82, 83]. The epitope for this antibody has been mapped by deletion analysis to a short loop between amino acids C287 and C302, and amino acids in this loop that are critical for binding have been defined using yeast phage display [83, 84]. This epitope is occluded both in the tethered and extended, dimeric, conformations of EGFR. This explains satisfyingly why this antibody does not react with wild-type EGFR in normal tissue or on cells that express low levels of this receptor. Monoclonal antibody 806 does bind to a fraction (< 10%) of EGFR at the surface of cells expression high levels of this receptor [85] – clearly this fraction of the receptor does not adopt either of the crystallographically defined receptor conformations. It has been suggested that the population of EGFR to which mAb 806 binds represents an intermediate between tethered monomer and extended ligand-bound dimer [82, 83], and a model of such a structure has been proposed [86]. Since monoclonal antibody 806 is not conformationally sensitive - it binds to denatured EGFR [85] - this population may instead be misfolded. It remains to be confirmed whether the mAb 806 reactive population of receptors truly represents an intermediate conformation of wild-type EGFR, or simply a population of partially denatured receptors that accumulates on the cell surface under conditions of aberrantly high EGFR expression. Irrespective of the precise nature of the EGFR to which mAb 806 binds, this population of mAb 806 reactive EGFR serves as a tumor marker and lends mAb 806 a distinctive therapeutic potential.
Conclusion
The current repertoire of antibodies to the extracellular region of ErbB receptors has provided valuable information on the function of these receptors in both normal and cancerous cells. The structures of the Fab fragments of five different antibodies in complex with ErbB receptor extracellular regions highlight four distinct modes of binding that can inhibit receptor activation. It is likely that other inhibitory modes of binding are possible, but different strategies may be needed to isolate antibodies with alternate binding properties. ErbB3 and possible ErbB4 may also play a role in development and progression of human cancers [87–89] - only a few antibodies to the extracellular regions of these receptors have been investigated [90, 91]. Combinations of different ErbB targeted antibodies can have synergistic effects [69, 92, 93], and the engineering of bispecific antibodies with dual epitope recognition is another promising direction [36, 94]. Whether new antibodies against ErbB receptors that have different modes of binding will prove to be of therapeutic value remains to be evaluated. Certainly such antibodies will be exploited to test and extend the current understanding of ErbB receptor activation.
Acknowledgments
We thank Mark Lemmon and members of the Ferguson laboratory for critical comments on the manuscript. K.M.F. is supported by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund, and is the Dennis and Marsha Dammerman Scholar of the Damon Runyon Cancer Research Foundation (DRS-52-06). KRS is supported in part by a Predoctoral Fellowship (BC051591) from the U.S. Army Breast Cancer Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yarden Y, Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987;26:1443–1451. doi: 10.1021/bi00379a035. [DOI] [PubMed] [Google Scholar]
- 2.Bellot F, Moolenaar W, Kris R, Mirakhur B, Verlaan I, Ullrich A, Schlessinger J, Felder S. High-affinity epidermal growth factor binding is specifically reduced by a monoclonal antibody, and appears necessary for early responses. J Cell Biol. 1990;110:491–502. doi: 10.1083/jcb.110.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Defize LH, Boonstra J, Meisenhelder J, Kruijer W, Tertoolen LG, Tilly BC, Hunter T, van Bergen en Henegouwen PM, Moolenaar WH, de Laat SW. Signal transduction by epidermal growth factor occurs through the subclass of high affinity receptors. J Cell Biol. 1989;109:2495–2507. doi: 10.1083/jcb.109.5.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drebin JA, Stern DF, Link VC, Weinberg RA, Greene MI. Monoclonal antibodies identify a cell-surface antigen associated with an activated cellular oncogene. Nature. 1984;312:545–548. doi: 10.1038/312545a0. [DOI] [PubMed] [Google Scholar]
- 5.Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, Weinberg RA. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312:513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 6.Drebin JA, Link VC, Stern DF, Weinberg RA, Greene MI. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985;41:697–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, Greene MI. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117:2051–2058. doi: 10.1172/JCI32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waterfield MD, Mayes EL, Stroobant P, Bennet PL, Young S, Goodfellow PN, Banting GS, Ozanne B. A monoclonal antibody to the human epidermal growth factor receptor. J Cell Biochem. 1982;20:149–161. doi: 10.1002/jcb.240200207. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber AB, Lax I, Yarden Y, Eshhar Z, Schlessinger J. Monoclonal antibodies against receptor for epidermal growth factor induce early and delayed effects of epidermal growth factor. Proc Natl Acad Sci USA. 1981;78:7535–7539. doi: 10.1073/pnas.78.12.7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schreiber AB, Libermann TA, Lax I, Yarden Y, Schlessinger J. Biological role of epidermal growth factor-receptor clustering. Investigation with monoclonal anti-receptor antibodies. J Biol Chem. 1983;258:846–853. [PubMed] [Google Scholar]
- 11.Gill GN, Kawamoto T, Cochet C, Le A, Sato JD, Masui H, McLeod C, Mendelsohn J. Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984;259:7755–7760. [PubMed] [Google Scholar]
- 12.Sato JD, Kawamoto T, Le AD, Mendelsohn J, Polikoff J, Sato GH. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med. 1983;1:511–529. [PubMed] [Google Scholar]
- 13.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, Pinto A, Normanno N. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214:559–567. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 14.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 15.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–2459. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- 16.Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33:369–385. doi: 10.1053/j.seminoncol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Shepard HM, Jin P, Slamon DJ, Pirot Z, Maneval DC. Herceptin. Handb Exp Pharmacol. 2008:183–219. doi: 10.1007/978-3-540-73259-4_9. [DOI] [PubMed] [Google Scholar]
- 19.Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, Lemmon MA, Sliwkowski MX, Ward CW, Yokoyama S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson KM. A structure-based view of Epidermal Growth Factor Receptor regulation. Ann Rev Biophys. 2008;37:353–373. doi: 10.1146/annurev.biophys.37.032807.125829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leahy DJ. Structure and function of the epidermal growth factor (EGF/ErbB) family of receptors. Adv Protein Chem. 2004;68:1–27. doi: 10.1016/S0065-3233(04)68001-6. [DOI] [PubMed] [Google Scholar]
- 22.Lemmon MA. Exp Cell Res. 2008 in press. [Google Scholar]
- 23.Bouyain S, Longo PA, Li S, Ferguson KM, Leahy DJ. The extracellular region of ErbB4 adopts a tethered conformation in the absence of ligand. Proc Natl Acad Sci USA. 2005;102:15024–15029. doi: 10.1073/pnas.0507591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science. 2002;297:1330–1333. doi: 10.1126/science.1074611. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson KM, Berger MB, Mendrola JM, Cho HS, Leahy DJ, Lemmon MA. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell. 2003;11:507–517. doi: 10.1016/s1097-2765(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 26.Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Zhu HJ, Walker F, Frenkel MJ, Hoyne PA, Jorissen RN, Nice EC, Burgess AW, Ward CW. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell. 2002;110:763–773. doi: 10.1016/s0092-8674(02)00940-6. [DOI] [PubMed] [Google Scholar]
- 27.Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, Saito K, Sakamoto A, Inoue M, Shirouzu M, Yokoyama S. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–787. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 28.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 29.Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Kofler M, Jorissen RN, Nice EC, Burgess AW, Ward CW. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell. 2003;11:495–505. doi: 10.1016/s1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 30.Kawamoto T, Sato JD, Le A, Polikoff J, Sato GH, Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci USA. 1983;80:1337–1341. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masui H, Kawamoto T, Sato JD, Wolf B, Sato G, Mendelsohn J. Growth inhibition of human tumor cells in athymic mice by anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1984;44:1002–1007. [PubMed] [Google Scholar]
- 32.Mendelsohn J. Anti-EGF receptor monoclonal antibodies: biological studies and potential clinical applications. Trans Am Clin Climatol Assoc. 1988;100:31–38. [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- 34.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 35.Blick SK, Scott LJ. Cetuximab: a review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal cancer. Drugs. 2007;67:2585–2607. doi: 10.2165/00003495-200767170-00008. [DOI] [PubMed] [Google Scholar]
- 36.Friedlander E, Barok M, Szollosi J, Vereb G. ErbB-directed immunotherapy: antibodies in current practice and promising new agents. Immunol Lett. 2008;116:126–140. doi: 10.1016/j.imlet.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Murthy U, Basu A, Rodeck U, Herlyn M, Ross AH, Das M. Binding of an antagonistic monoclonal antibody to an intact and fragmented EGF-receptor polypeptide. Arch Biochem Biophys. 1987;252:549–560. doi: 10.1016/0003-9861(87)90062-2. [DOI] [PubMed] [Google Scholar]
- 38.Rodeck U, Williams N, Murthy U, Herlyn M. Monoclonal antibody 425 inhibits growth stimulation of carcinoma cells by exogenous EGF and tumor-derived EGF/TGF-alpha. J Cell Biochem. 1990;44:69–79. doi: 10.1002/jcb.240440202. [DOI] [PubMed] [Google Scholar]
- 39.Schiller JH. Developments in epidermal growth factor receptor-targeting therapy for solid tumors: focus on matuzumab (EMD 72000) Cancer Invest. 2008;26:81–95. doi: 10.1080/07357900701511847. [DOI] [PubMed] [Google Scholar]
- 40.Seiden MV, Burris HA, Matulonis U, Hall JB, Armstrong DK, Speyer J, Weber JD, Muggia F. A phase II trial of EMD72000 (matuzumab), a humanized anti-EGFR monoclonal antibody, in patients with platinum-resistant ovarian and primary peritoneal malignancies. Gynecol Oncol. 2007;104:727–731. doi: 10.1016/j.ygyno.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 41.de Haard HJ, van Neer N, Reurs A, Hufton SE, Roovers RC, Henderikx P, de Bruine AP, Arends JW, Hoogenboom HR. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem. 1999;274:18218–18230. doi: 10.1074/jbc.274.26.18218. [DOI] [PubMed] [Google Scholar]
- 42.Lu D, Zhang H, Ludwig D, Persaud A, Jimenez X, Burtrum D, Balderes P, Liu M, Bohlen P, Witte L, Zhu Z. Simultaneous blockade of both the epidermal growth factor receptor and the insulin-like growth factor receptor signaling pathways in cancer cells with a fully human recombinant bispecific antibody. J Biol Chem. 2004;279:2856–2865. doi: 10.1074/jbc.M310132200. [DOI] [PubMed] [Google Scholar]
- 43.Liu M, Zhang H, Jimenez X, Ludwig DL, Witte L, Bohlen P, Hicklin DJ, Zhu Z. Identification and characterization of a fully human antibody directed against epidermal growth factor receptor for cancer therapy. AACR Meeting Abstracts. 2004;2004:163-c. [Google Scholar]
- 44.Lu D, Zhang H, Koo H, Tonra J, Balderes P, Prewett M, Corcoran E, Mangalampalli V, Bassi R, Anselma D, Patel D, Kang X, Ludwig DL, Hicklin DJ, Bohlen P, Witte L, Zhu Z. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem. 2005;280:19665–19672. doi: 10.1074/jbc.M500815200. [DOI] [PubMed] [Google Scholar]
- 45.Prewett M, Tonra JR, Rajiv B, Hooper AT, Makhoul G, Finnerty B, Witte L, Bohlen P, Zhu Z, Hicklin DJ. Antitumor activity of a novel, human anti-epidermal growth factor receptor (EGFR) monoclonal antibody (IMC-11F8) in human tumor xenograft models. AACR Meeting Abstracts. 2004;2004:1235. [Google Scholar]
- 46.Kuenen B, Witteveen E, Ruijter R, Ervin-Haynes A, Tjin-A-ton M, Fox F, Ding C, Giaccone G, Voest EE. A phase I study of IMC-11F8, a fully human anti-epidermal growth factor receptor (EGFR) IgG1 monoclonal antibody in patients with solid tumors. Interim results. J Clin Oncol. 2006;24:3024. [Google Scholar]
- 47.Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol. 2005;23:1117–1125. doi: 10.1038/nbt1135. [DOI] [PubMed] [Google Scholar]
- 48.Yang XD, Jia XC, Corvalan JR, Wang P, Davis CG. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol. 2001;38:17–23. doi: 10.1016/s1040-8428(00)00134-7. [DOI] [PubMed] [Google Scholar]
- 49.Wu M, Rivkin A, Pham T. Panitumumab: Human monoclonal antibody against epidermal growth factor receptors for the treatment of metastatic colorectal cancer. Clin Ther. 2008;30:14–30. doi: 10.1016/j.clinthera.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 50.Iannello A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 2005;24:487–499. doi: 10.1007/s10555-005-6192-2. [DOI] [PubMed] [Google Scholar]
- 51.Peipp M, Dechant M, Valerius T. Effector mechanisms of therapeutic antibodies against ErbB receptors. Curr Opin Immunol. 2008;20:436–443. doi: 10.1016/j.coi.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 52.Bleeker WK, Lammerts van Bueren JJ, van Ojik HH, Gerritsen AF, Pluyter M, Houtkamp M, Halk E, Goldstein J, Schuurman J, van Dijk MA, van de Winkel JG, Parren PW. Dual mode of action of a human anti-epidermal growth factor receptor monoclonal antibody for cancer therapy. J Immunol. 2004;173:4699–4707. doi: 10.4049/jimmunol.173.7.4699. [DOI] [PubMed] [Google Scholar]
- 53.Ruuls SR, Lammerts van Bueren JJ, van de Winkel JG, Parren PW. Novel human antibody therapeutics: The age of the Umabs. Biotechnol J. 2008 doi: 10.1002/biot.200800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aboud-Pirak E, Hurwitz E, Pirak ME, Bellot F, Schlessinger J, Sela M. Efficacy of antibodies to epidermal growth factor receptor against KB carcinoma in vitro and in nude mice. J Natl Cancer Inst. 1988;80:1605–1611. doi: 10.1093/jnci/80.20.1605. [DOI] [PubMed] [Google Scholar]
- 55.Defize LH, Moolenaar WH, van der Saag PT, de Laat SW. Dissociation of cellular responses to epidermal growth factor using anti-receptor monoclonal antibodies. EMBO J. 1986;5:1187–1192. doi: 10.1002/j.1460-2075.1986.tb04345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mishima K, Johns TG, Luwor RB, Scott AM, Stockert E, Jungbluth AA, Ji XD, Suvarna P, Voland JR, Old LJ, Huang HJ, Cavenee WK. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res. 2001;61:5349–5354. [PubMed] [Google Scholar]
- 57.Kuan CT, Wikstrand CJ, Bigner DD. EGF mutant receptor vIII as a molecular target in cancer therapy. Endocr Relat Cancer. 2001;8:83–96. doi: 10.1677/erc.0.0080083. [DOI] [PubMed] [Google Scholar]
- 58.Jungbluth AA, Stockert E, Huang HJ, Collins VP, Coplan K, Iversen K, Kolb D, Johns TJ, Scott AM, Gullick WJ, Ritter G, Cohen L, Scanlan MJ, Cavenee WK, Old LJ. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc Natl Acad Sci USA. 2003;100:639–644. doi: 10.1073/pnas.232686499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott AM, Lee FT, Tebbutt N, Herbertson R, Gill SS, Liu Z, Skrinos E, Murone C, Saunder TH, Chappell B, Papenfuss AT, Poon AM, Hopkins W, Smyth FE, MacGregor D, Cher LM, Jungbluth AA, Ritter G, Brechbiel MW, Murphy R, Burgess AW, Hoffman EW, Johns TG, Old LJ. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci USA. 2007;104:4071–4076. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 61.Fendly BM, Winget M, Hudziak RM, Lipari MT, Napier MA, Ullrich A. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res. 1990;50:1550–1558. [PubMed] [Google Scholar]
- 62.Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, Scher HI, Sliwkowski MX. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 63.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 64.Li S, Kussie P, Ferguson KM. Structural basis for EGF receptor inhibition by the therapeutic antibody IMC-11F8. Structure. 2008;16:216–227. doi: 10.1016/j.str.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Schmiedel J, Blaukat A, Li S, Knochel T, Ferguson KM. Matuzumab binding to EGFR prevents the conformational rearrangement required for dimerization. Cancer Cell. 2008;13:365–373. doi: 10.1016/j.ccr.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freeman D, Sun J, Bass R, Jung K, Ogbagabriel S, Elliot G, Radinsky R. Panitumumab and cetuximab epitope mapping and in vitro activity. Clin Oncol. 2008;26:A14536. [Google Scholar]
- 68.Lammerts van Bueren JJ, Bleeker WK, Brannstrom A, von Euler A, Jansson M, Peipp M, Schneider-Merck T, Valerius T, van de Winkel JG, Parren PW. The antibody zalutumumab inhibits epidermal growth factor receptor signaling by limiting intra- and intermolecular flexibility. Proc Natl Acad Sci USA. 2008;105:6109–6014. doi: 10.1073/pnas.0709477105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Dechant M, Weisner W, Berger S, Peipp M, Beyer T, Schneider-Merck T, Lammerts van Bueren JJ, Bleeker WK, Parren PW, van de Winkel JG, Valerius T. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res. 2008;68:4998–5003. doi: 10.1158/0008-5472.CAN-07-6226. [DOI] [PubMed] [Google Scholar]
- 70.Dawson JP, Berger MB, Lin CC, Schlessinger J, Lemmon MA, Ferguson KM. Epidermal growth factor receptor dimerization and activation require ligand-induced conformational changes in the dimer interface. Mol Cell Biol. 2005;25:7734–7742. doi: 10.1128/MCB.25.17.7734-7742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dawson JP, Bu Z, Lemmon MA. Ligand-induced structural transitions in ErbB receptor extracellular domains. Structure. 2007;15:942–954. doi: 10.1016/j.str.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 72.Ferguson KM, Darling PJ, Mohan MJ, Macatee TL, Lemmon MA. Extracellular domains drive homo- but not hetero-dimerization of erbB receptors. EMBO J. 2000;19:4632–4643. doi: 10.1093/emboj/19.17.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wehrman TS, Raab WJ, Casipit CL, Doyonnas R, Pomerantz JH, Blau HM. A system for quantifying dynamic protein interactions defines a role for Herceptin in modulating ErbB2 interactions. Proc Natl Acad Sci USA. 2006;103:19063–19068. doi: 10.1073/pnas.0605218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]
- 75.Greenebaum E, Nicolaides M, Eisinger M, Vogel RH, Weinstein IB. Binding of phorbol dibutyrate and epidermal growth factor to cultured human epidermal cells. J Natl Cancer Inst. 1983;70:435–441. [PubMed] [Google Scholar]
- 76.Gullick WJ, Downward DJ, Marsden JJ, Waterfield MD. A radioimmunoassay for human epidermal growth factor receptor. Anal Biochem. 1984;141:253–261. doi: 10.1016/0003-2697(84)90454-8. [DOI] [PubMed] [Google Scholar]
- 77.Wofsy C, Goldstein B, Lund K, Wiley HS. Implications of epidermal growth factor (EGF) induced egf receptor aggregation. Biophys J. 1992;63:98–110. doi: 10.1016/S0006-3495(92)81572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Macdonald JL, Pike LJ. Heterogeneity in EGF-binding affinities arises from negative cooperativity in an aggregating system. Proc Natl Acad Sci USA. 2008;105:112–117. doi: 10.1073/pnas.0707080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 80.Klein P, Mattoon D, Lemmon MA, Schlessinger J. A structure-based model for ligand binding and dimerization of EGF receptors. Proc Natl Acad Sci USA. 2004;101:929–934. doi: 10.1073/pnas.0307285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mattoon D, Klein P, Lemmon MA, Lax I, Schlessinger J. The tethered configuration of the EGF receptor extracellular domain exerts only a limited control of receptor function. Proc Natl Acad Sci USA. 2004;101:923–928. doi: 10.1073/pnas.0307286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walker F, Orchard SG, Jorissen RN, Hall NE, Zhang HH, Hoyne PA, Adams TE, Johns TG, Ward C, Garrett TP, Zhu HJ, Nerrie M, Scott AM, Nice EC, Burgess AW. CR1/CR2 interactions modulate the functions of the cell surface epidermal growth factor receptor. J Biol Chem. 2004;279:22387–22398. doi: 10.1074/jbc.M401244200. [DOI] [PubMed] [Google Scholar]
- 83.Johns TG, Adams TE, Cochran JR, Hall NE, Hoyne PA, Olsen MJ, Kim YS, Rothacker J, Nice EC, Walker F, Ritter G, Jungbluth AA, Old LJ, Ward CW, Burgess AW, Wittrup KD, Scott AM. Identification of the epitope for the epidermal growth factor receptor-specific monoclonal antibody 806 reveals that it preferentially recognizes an untethered form of the receptor. J Biol Chem. 2004;279:30375–30384. doi: 10.1074/jbc.M401218200. [DOI] [PubMed] [Google Scholar]
- 84.Chao G, Cochran JR, Wittrup KD. Fine epitope mapping of anti-epidermal growth factor receptor antibodies through random mutagenesis and yeast surface display. J Mol Biol. 2004;342:539–550. doi: 10.1016/j.jmb.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 85.Johns TG, Stockert E, Ritter G, Jungbluth AA, Huang HJ, Cavenee WK, Smyth FE, Hall CM, Watson N, Nice EC, Gullick WJ, Old LJ, Burgess AW, Scott AM. Novel monoclonal antibody specific for the de2-7 epidermal growth factor receptor (EGFR) that also recognizes the EGFR expressed in cells containing amplification of the EGFR gene. Int J Cancer. 2002;98:398–408. doi: 10.1002/ijc.10189. [DOI] [PubMed] [Google Scholar]
- 86.Sivasubramanian A, Chao G, Pressler HM, Wittrup KD, Gray JJ. Structural model of the mAb 806-EGFR complex using computational docking followed by computational and experimental mutagenesis. Structure. 2006;14:401–414. doi: 10.1016/j.str.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 87.Karamouzis MV, Badra FA, Papavassiliou AG. Breast cancer: the upgraded role of HER-3 and HER-4. Int J Biochem Cell Biol. 2007;39:851–856. doi: 10.1016/j.biocel.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 88.Wheeler DL, Rangnekar VM, Schwarze SR. ErbB4 targeting approaches for prostate cancer treatment. Cancer Biol Ther. 2008;7:1095–1097. doi: 10.4161/cbt.7.7.6537. [DOI] [PubMed] [Google Scholar]
- 89.Sithanandam G, Anderson LM. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther. 2008;15:413–448. doi: 10.1038/cgt.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vexler A, Lidawi G, Loew V, Barnea I, Karaush V, Lev-Ari S, Shtabsky A, Ben-Yosef R. Anti-ERBb4 targeted therapy combined with radiation therapy in prostate cancer. Results of in vitro and invivo studies. Cancer Biol Ther. 2008;7:1090–1094. doi: 10.4161/cbt.7.7.6167. [DOI] [PubMed] [Google Scholar]
- 91.Gilmour LM, Macleod KG, McCaig A, Sewell JM, Gullick WJ, Smyth JF, Langdon SP. Neuregulin expression, function, and signaling in human ovarian cancer cells. Clin Cancer Res. 2002;8:3933–3942. [PubMed] [Google Scholar]
- 92.Friedman LM, Rinon A, Schechter B, Lyass L, Lavi S, Bacus SS, Sela M, Yarden Y. Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy. Proc Natl Acad Sci USA. 2005;102:1915–1920. doi: 10.1073/pnas.0409610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kamat V, Donaldson JM, Kari C, Quadros MR, Lelkes PI, Chaiken I, Cocklin S, Williams JC, Papazoglou E, Rodeck U. Enhanced EGFR inhibition and distinct epitope recognition by EGFR antagonistic mAbs C225 and 425. Cancer Biol Ther. 2008;7:726–733. doi: 10.4161/cbt.7.5.6097. [DOI] [PubMed] [Google Scholar]
- 94.Lu D, Zhang H, Koo H, Tonra J, Balderes P, Prewett M, Corcoran E, Mangalampalli V, Bassi R, Anselma D, Patel D, Kang X, Ludwig DL, Hicklin DJ, Bohlen P, Witte L, Zhu Z. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem. 2005;280:19665–19672. doi: 10.1074/jbc.M500815200. [DOI] [PubMed] [Google Scholar]
- 95.Berger MB, Mendrola JM, Lemmon MA. ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett. 2004;569:332–326. doi: 10.1016/j.febslet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 96.Lax I, Fischer R, Ng C, Segre J, Ullrich A, Givol D, Schlessinger J. Noncontiguous regions in the extracellular domain of EGF receptor define ligand-binding specificity. Cell Regul. 1991;2:337–345. doi: 10.1091/mbc.2.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Winkler ME, O’Connor L, Winget M, Fendly B. Epidermal growth factor and transforming growth factor alpha bind differently to the epidermal growth factor receptor. Biochemistry. 1989;28:6373–6378. doi: 10.1021/bi00441a033. [DOI] [PubMed] [Google Scholar]
- 98.Gooi HC, Hounsell EF, Lax I, Kris RM, Libermann TA, Schlessinger J, Sato JD, Kawamoto T, Mendelsohn J, Feizi T. The carbohydrate specificities of the monoclonal antibodies 29.1, 455 and 3C1B12 to the epidermal growth factor receptor of A431 cells. Biosci Rep. 1985;5:83–94. doi: 10.1007/BF01117444. [DOI] [PubMed] [Google Scholar]
- 99.Schlessinger J, Lax I, Yarden Y, Kanety H, Libermann TA. Monoclonal Antibodies to Receptors. In: Greaves MF, editor. Receptors and Recognition. Vol. 17. Chapman and Hall; London: 1984. pp. 279–303. [Google Scholar]
- 100.Yarden Y, Harari I, Schlessinger J. Purification of an active EGF receptor kinase with monoclonal antireceptor antibodies. J Biol Chem. 1985;260:315–319. [PubMed] [Google Scholar]
- 101.Reuter CW, Morgan MA, Eckardt A. Targeting EGF-receptor-signalling in squamous cell carcinomas of the head and neck. Br J Cancer. 2007;96:408–416. doi: 10.1038/sj.bjc.6603566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fernandez A, Spitzer E, Perez R, Boehmer FD, Eckert K, Zschiesche W, Grosse R. A new monoclonal antibody for detection of EGF-receptors in western blots and paraffin-embedded tissue sections. J Cell Biochem. 1992;49:157–165. doi: 10.1002/jcb.240490208. [DOI] [PubMed] [Google Scholar]
- 103.Modjtahedi H, Moscatello DK, Box G, Green M, Shotton C, Lamb DJ, Reynolds LJ, Wong AJ, Dean C, Thomas H, Eccles S. Targeting of cells expressing wild-type EGFR and type-III mutant EGFR (EGFRvIII) by anti-EGFR MAb ICR62: a two-pronged attack for tumour therapy. Int J Cancer. 2003;105:273–280. doi: 10.1002/ijc.11055. [DOI] [PubMed] [Google Scholar]
- 104.Modjtahedi H, Styles JM, Dean CJ. The human EGF receptor as a target for cancer therapy: six new rat mAbs against the receptor on the breast carcinoma MDA-MB 468. Br J Cancer. 1993;67:247–253. doi: 10.1038/bjc.1993.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9:1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]