Abstract
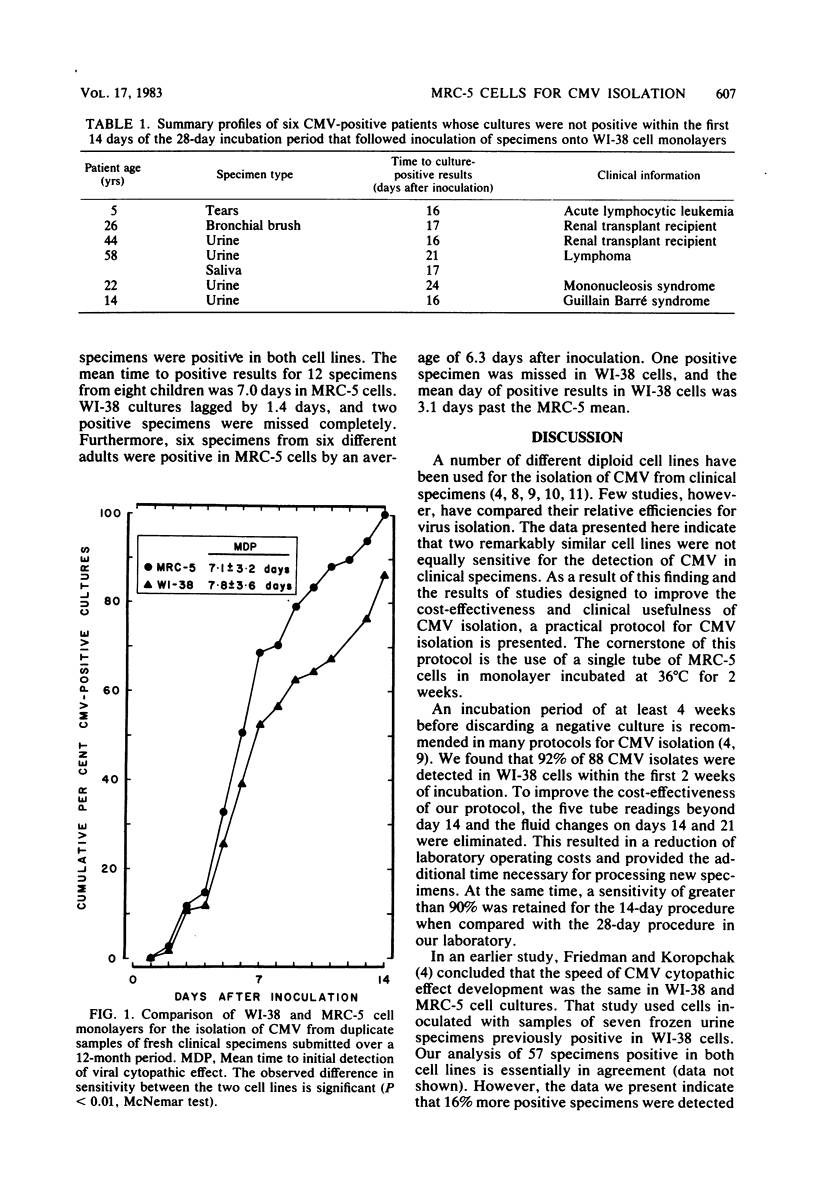
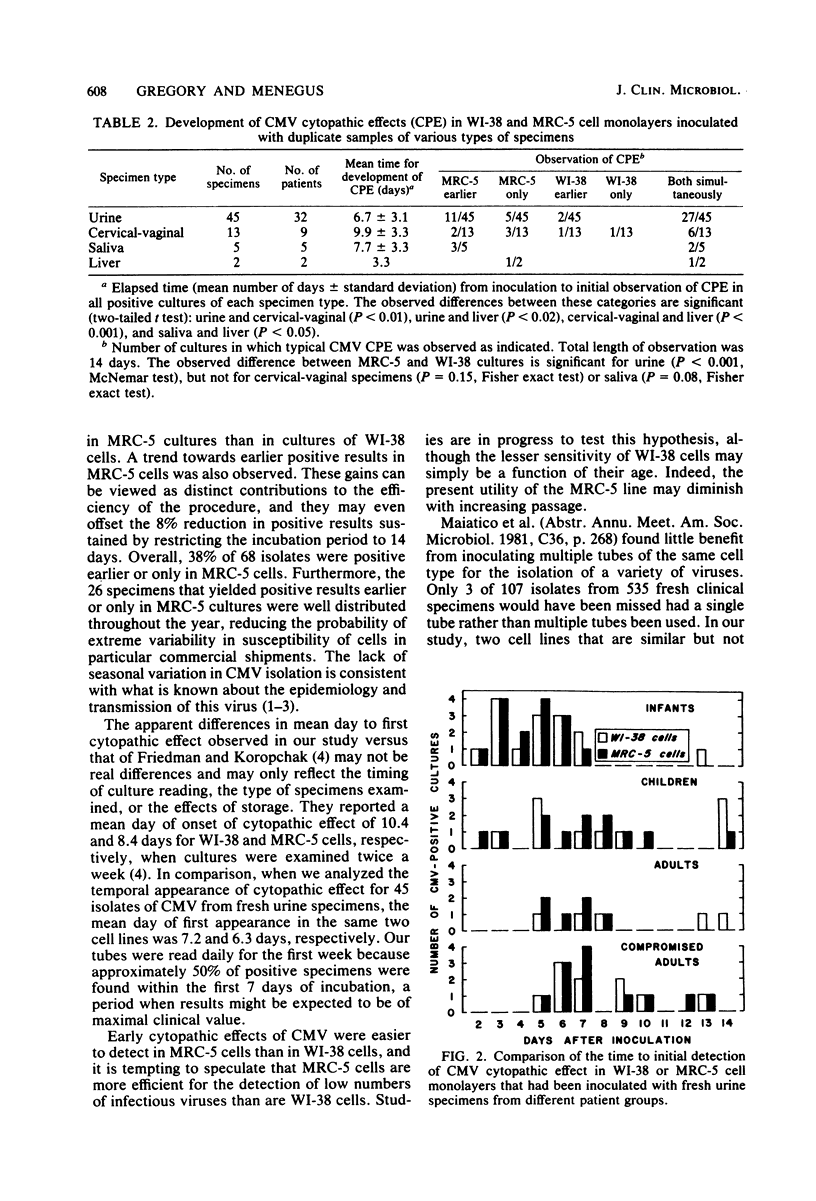
Isolation of cytomegalovirus (CMV) in tissue culture is presently the most reliable means of proving active CMV infection. To improve both the cost-effectiveness and clinical usefulness of procedures for the isolation of CMV from fresh clinical specimens, we analyzed results obtained with standard isolation procedures and compared them with results obtained under different conditions. Cell monolayers from commercial sources were inoculated with fresh specimens and then were observed for a cytopathic effect typical of CMV. Of 1,375 specimens submitted over a 12-month period, 6.4% were CMV positive in WI-38 monolayers within 28 days after inoculation. The mean day of CMV detection for 45 urine, 13 cervical-vaginal, and 5 saliva specimens was 6.7 +/- 3.1 (mean +/- standard deviation), 9.9 +/- 3.3, and 7.7 +/- 3.3 days, respectively, and 92% were positive within 14 days. When 1,058 subsequent specimens were inoculated in parallel onto WI-38 and MRC-5 cell monolayers, 8.7% were positive for CMV. MRC-5 cells were significantly more sensitive than WI-38 cells: 98% of all positive specimens appropriate for comparison were detected in MRC-5 cultures, but only 85% were detected in WI-38 cells. Although 1 specimen was positive in WI-38 cells only, 38% of all isolates were positive earlier (16 specimens) or only (10 specimens) in MRC-5 cultures. Based on these data, we have developed a practical 2-week protocol for CMV isolation from fresh clinical specimens that includes the use of MRC-5 cell monolayers incubated at 36 degrees C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford C. A., Pass R. F. Epidemiology of chronic congenital and perinatal infections of man. Clin Perinatol. 1981 Oct;8(3):397–414. [PubMed] [Google Scholar]
- Betts R. F., Freeman R. B., Douglas R. G., Jr, Talley T. E., Rundell B. Transmission of cytomegalovirus infection with renal allograft. Kidney Int. 1975 Dec;8(6):385–392. doi: 10.1038/ki.1975.131. [DOI] [PubMed] [Google Scholar]
- Fiala M., Payne J. E., Berne T. V., Moore T. C., Henle W., Montgomerie J. Z., Chatterjee S. N., Guze L. B. Epidemiology of cytomegalovirus infection after transplantation and immunosuppression. J Infect Dis. 1975 Oct;132(4):421–433. doi: 10.1093/infdis/132.4.421. [DOI] [PubMed] [Google Scholar]
- Friedman H. M., Koropchak C. Comparison of WI-38, MRC-5, and IMR-90 cell strains for isolation of viruses from clinical specimens. J Clin Microbiol. 1978 Apr;7(4):368–371. doi: 10.1128/jcm.7.4.368-371.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. Cytomegalovirus infections following renal transplantation. Rev Infect Dis. 1981 Nov-Dec;3(6):1151–1178. doi: 10.1093/clinids/3.6.1151. [DOI] [PubMed] [Google Scholar]
- Jacobs J. P., Jones C. M., Baille J. P. Characteristics of a human diploid cell designated MRC-5. Nature. 1970 Jul 11;227(5254):168–170. doi: 10.1038/227168a0. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Balfour H. H., Jr Optimal method for recovery of cytomegalovirus from urine of renal transplant patients. Transplantation. 1977 Sep;24(3):228–230. doi: 10.1097/00007890-197709000-00010. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., WATERMAN S., TURNER H. C., HUEBNER R. J. Cytopathogenic agent resembling human salivary gland virus recovered from tissue cultures of human adenoids. Proc Soc Exp Biol Med. 1956 Jun;92(2):418–424. [PubMed] [Google Scholar]
- SMITH M. G. Propagation in tissue cultures of a cytopathogenic virus from human salivary gland virus (SGV) disease. Proc Soc Exp Biol Med. 1956 Jun;92(2):424–430. doi: 10.3181/00379727-92-22498. [DOI] [PubMed] [Google Scholar]
- Stagno S., Pass R. F., Dworsky M. E., Henderson R. E., Moore E. G., Walton P. D., Alford C. A. Congenital cytomegalovirus infection: The relative importance of primary and recurrent maternal infection. N Engl J Med. 1982 Apr 22;306(16):945–949. doi: 10.1056/NEJM198204223061601. [DOI] [PubMed] [Google Scholar]
- Stagno S., Pass R. F., Reynolds D. W., Moore M. A., Nahmias A. J., Alford C. A. Comparative study of diagnostic procedures for congenital cytomegalovirus infection. Pediatrics. 1980 Feb;65(2):251–257. [PubMed] [Google Scholar]
- Wade N. Hayflick's Tragedy: The Rise and Fall of a Human Cell Line. Science. 1976 Apr 9;192(4235):125–127. doi: 10.1126/science.192.4235.125. [DOI] [PubMed] [Google Scholar]



