Although calsequestrin (CASQ), a major sarcoplasmic reticulum (SR) Ca2+ binding protein, was discovered more than 30 years ago, its precise roles and modes of operation in both skeletal and cardiac muscle remain to be elucidated (Rios, 2009, this issue; Knollmann, 2009, this issue). Recent years witnessed a significant surge of interest in this Ca2+ binding protein after mutations in the gene encoding the cardiac isoform of calsequestrin (CASQ2) were linked to exercise-induced cardiac death due to catecholaminergic polymorphic ventricular tachycardia (CPVT) (Postma et al. 2002; Eldar et al. 2003). Surprisingly, humans and genetically altered mice devoid of CASQ2 preserve nearly normal cardiac structure and function but develop lethal arrhythmias under conditions of adrenergic stimulation (Postma et al. 2002; Knollmann et al. 2006; Song et al. 2007). Here we provide a brief overview of our current understanding of the functional role and mode of operation of CASQ2 in the heart, of how hearts missing CASQ2 cope in the absence of this protein and of the mechanisms of CPVT.
Role of luminal Ca2+ in controlling SR Ca2+ release
In cardiac myocytes, Ca2+-induced Ca2+ release (CICR) evoked by the action potential (AP) results in a rapid and temporary rise of the cytosolic Ca2+ concentration ([Ca2+]c) from ∼100 nm to ∼1 μm, i.e. the cardiac Ca2+ transient. Because of the limited size of the SR Ca2+ store, CICR in principle is a self-limiting process which must extinguish once the SR Ca2+ store is depleted. However, it is well known that during a regular Ca2+ transient only a portion of the total SR Ca2+ is released (40–60%; Bassani et al. 1993; Delbridge et al. 1997), leaving a sizable Ca2+ reserve in the SR. This implies that some mechanism is working to terminate the Ca2+ release process before the SR Ca2+ store is emptied. It is believed that such a restraining mechanism would also be required to stabilize the inherently unstable, self-regenerating CICR and to prevent it from spontaneous activation during diastole.
Growing evidence suggests that changes in Ca2+ concentration in the SR lumen ([Ca2+]SR) during the exodus of SR Ca2+ play an important role in controlling the release process by influencing the functional activity of the cardiac ryanodine receptor (RyR2) channels (Györke & Terentyev, 2008). RyR2 open probability changes as a monotonic function of luminal Ca2+ with an EC50∼1 mm (Györke & Györke, 1998). Since resting [Ca2+] in the SR is ∼1 mm, declining [Ca2+]SR reduces RyR2 activity forcing, upon reaching a critical level of [Ca2+]SR, CICR to terminate. This mechanism, termed luminal Ca2+-dependent deactivation (Terentyev et al. 2002) leaves the Ca2+ store in a temporarily unresponsive, refractory state preventing untimely SR Ca2+ release before the next AP. Mechanistically, luminal Ca2+ seems to act on RyR2s by allosterically affecting RyR2s’ sensitivity to cytosolic Ca2+, such that lowering luminal Ca2+ reduces the sensitivity of RyR2s to activation by cytosolic Ca2+ whereas increasing the SR Ca2+ load sensitizes the RyR2s (Endo, 1975; Fabiato & Fabiato, 1979; Györke & Györke, 1998; Qin et al. 2008; Stevens et al. 2009). Additionally, SR Ca2+ store size reportedly regulates RyR2 activity by influencing the access of luminal Ca2+ to the cytosolic activation sites of the channel (Laver, 2007). Thus, the functional status of RyR2s at any time is determined by combined inputs from cytosolic and luminal Ca2+. While the cytosolic Ca2+ activation site has been localized to certain residues of RyR2, the molecular nature of the luminal regulatory side, i.e. the Ca2+ sensor, is less clear.
CASQ2 as a luminal Ca2+ sensor
Being Ca2+ dependent and strategically localized at the points of SR Ca2+ release, CASQ2 presents itself as a putative luminal Ca2+ sensor for the RyR2. As suggested by crosslinking studies (Froemming & Ohlendieck, 1998), CASQ2 exists in the junctional SR as a mixture of monomers, dimers and multimers. While the multimeric form of CASQ2 functions as a Ca2+ buffer (see below), the monomers appear to be responsible for the regulatory function of the protein (Qin et al. 2008) (although the role of multimers in RyR2 regulation cannot be excluded). Consistent with its regulatory role, CASQ2 inhibits RyR2 activity at low luminal Ca2+ and this inhibition is relieved at elevated luminal Ca2+ in reconstitution studies (Györke & Terentyev, 2008). Although direct effects of CASQ2 on RyR2 have been described, according to most reports Ca2+-dependent interactions of CASQ2 with RyR2 are mediated by the integral membrane proteins triadin (TRD) and/or junctin (JN). In further support of the role of CASQ2 as a Ca2+ sensor for the termination of SR Ca2+ release, expression of a dominant negative CASQ2 mutant (R33Q) has been shown to compromise SR Ca2+ release termination and shorten store refractoriness in cardiac myocytes (Terentyev et al. 2006). Based on these findings, CASQ2 is thought to modulate RyR2 function in the following manner (Györke & Terentyev, 2008): when [Ca2+]SR is low, CASQ2 is bound to TRD and/or JN and inhibits the activity of RyR2; with SR Ca2+ load restored, increased [Ca2+] inhibits binding of CASQ2 to TRD (JN), thereby relieving the inhibitory action of CASQ2 on the RyR2 channel activity. This Ca2+-dependent modulation of RyR2s by CASQ2 has been suggested as the molecular basis for deactivation of RyR2s and store refractoriness following SR Ca2+ release. However, modulation through CASQ2 may not be the only mechanism through which SR loading status influences RyR2 function.
CASQ2 as a Ca2+ storage reservoir
Since its discovery, calsequestrin has been presumed to play a major role as a Ca2+ storage site in the SR. This long-held view, however, is now being re-examined by several investigators (Royer & Ríos, 2009, this issue; Knollmann, 2009, this issue). The amount of Ca2+ bound to CASQ2 in cardiac SR has not been determined and is likely to vary with species and experimental conditions. Bers (2001) estimated that approximately 50% of Ca2+ taken up by the SR is bound to CASQ2 in cardiac muscle. We have shown that an acute, ∼3-fold reduction of CASQ2 in quiescent rat myocytes results in a ∼2-fold decrease in the SR Ca2+ content (Terentyev et al. 2003) which would imply that approximately 75% of Ca2+ was bound to CASQ2 in myocytes with a complete set of the protein. The reduction in SR Ca2+ content was not accompanied by changes in free intra-SR [Ca2+] (Kubalova et al. 2004) indicating that the reduced SR Ca2+ content indeed reflected a decrease in the amount of Ca2+ bound to CASQ2 rather than being caused by Ca2+ leaking out from the SR through RyR2s. However, the ∼75% of total SR Ca2+ bound to CASQ2 suggested by our study is likely to present an upper estimate because of the tendency of the SR to gain Ca2+ in rat myocytes at rest (Bers, 2001). Interestingly, ectopic expression of certain CPVT-causing CASQ2 mutants (e.g. CASQ2(G112+5X)) in cardiac myocytes diminishes the ability of the SR to store Ca2+ and disrupts myocyte Ca2+ handling by disrupting CASQ2 polymerization (Terentyev et al. 2008). These results are consistent with the notion that CASQ2 polymers shown to form at high Ca2+ levels in vitro (Park et al. 2004) and found in the SR as filamentous structures (Tijskens et al. 2003), represent the high Ca2+-binding capacity form of CASQ2 (Park et al. 2004).
In myocytes from genetically modified mice deficient of CASQ2, the total SR Ca2+ content was found to be either decreased 2-fold (Song et al. 2007) or preserved on the background of a compensatory increase in the SR volume (Knollmann et al. 2006). These results also seem to support the buffering role of CASQ2 in the heart. However, such a compensatory SR remodelling was not observed in mice with a 55% reduction of CASQ2 protein secondary to gene-targeted modification of CASQ2 (Casq2-R33Q) (Rizzi et al. 2008) or in TRD-deficient mice that exhibited an increase in the SR Ca2+ content despite a ∼60% reduction in CASQ2 (Knollmann, 2009, this issue). As noted by Knollmann (this issue), one possible interpretation of these findings is that CASQ2 may not play as important a role in storing Ca2+ as previously thought. Another possibility, however, is that the changes in expression of other SR proteins such as TRD and JN that occur in these genetically modified mice increase the SR Ca2+ content by affecting Ca2+ release through RyR2s (see below). Clearly, future studies are needed to define the exact Ca2+ buffering role of CASQ2 in the heart.
Life without CASQ2: the beat goes on albeit disturbed
Absence of CASQ2 in both humans (homozygous 62delA and 532+1 G/A) and mice CASQ2–/–) have been shown to be associated with CPVT in subjects with otherwise functionally and structurally normal hearts (Postma et al. 2002; Knollmann et al. 2006; Song et al. 2007; Knollmann, 2009, this issue). The finding that the absence of CASQ2 did not cause major alterations in cardiac function and structure is surprising given the presumed importance of CASQ2 to Ca2+ handling. Studies in CASQ2-deficient mice by Knollmann et al. (2006) and Song et al. (2007) provide some important answers in this regard. In the Knollmann et al. study, ablation of CASQ2 was accompanied by a striking expansion (∼50%) in SR volume and nearly complete loss of TRD and JN. The 50% increase in SR volume can to a large extent compensate for the loss of SR Ca2+ buffering by CASQ2 (Knollmann et al. 2006). Additionally, reduction in TRD and JN could contribute to the preserved SR Ca2+ content by reducing the leak through RyR2s (Yuan et al. 2007; Knollmann, 2009, this issue). In the Song et al. (2007) study, although wild-type (WT) and CASQ2-deficient mice had comparable myocyte shortening and Ca2+ transient amplitude, the SR Ca2+ content was significantly decreased (∼2-fold) in CASQ2-deficient myocytes with respect to WT myocytes. Whereas the SR volume was not assessed in this study, the expression of the SR/ER Ca2+-binding protein calreticulin and RyR2 were increased nearly 10-fold with no apparent change in TRD and JN in CASQ2-deficient hearts. The reason for the different secondary changes in CASQ2-deficient mice found in these two studies is not clear. They may point to the existence of different cellular programmes to compensate for the absence of CASQ2 in the heart. These changes may have helped CASQ2-deficient mice adapt to the loss of this protein; however, they proved inadequate to avert predisposition to CPVT.
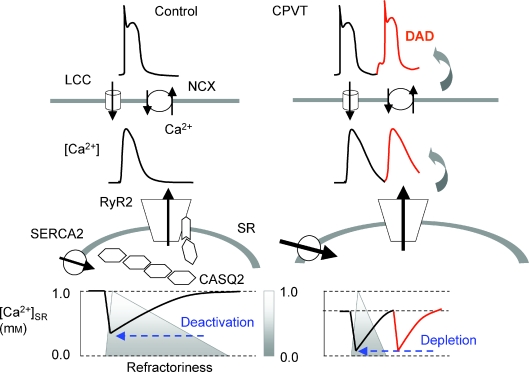
While these studies in CASQ2-deficient mice show how the storing function of the SR can be maintained in the absence of CASQ2 it is still difficult to understand how the hearts in these animals (or humans lacking CASQ2) can function without the regulatory influence of CASQ2 if this protein is indeed critical to the control of SR Ca2+ release. Recent results with simultaneous cytosolic and luminal Ca2+ measurements obtained in myocytes expressing CPVT CASQ2 mutants offer further insights as to how Ca2+ handling might be adjusted in the absence of CASQ2 and to the mechanisms of CPVT (Terentyev et al. 2008). See accompanying Fig. 1. As mentioned above, in control myocytes, SR Ca2+ release terminates at a certain threshold [Ca2+]SR leaving a substantial Ca2+ reserve in the SR consistent with the role of luminal Ca2+-dependent deactivation in termination of SR Ca2+ release. However, in myocytes expressing a CPVT CASQ2 mutant (R33Q) that disrupts RyR2 regulation by luminal Ca2+, SR Ca2+ release does not terminate until the SR is depleted of Ca2+. Since the baseline, diastolic [Ca2+]SR is significantly reduced due to an enhanced leak in the R33Q-expressing myocytes, the overall amount of Ca2+ released to the cytosol and hence the amplitudes of the cytosolic Ca2+ signals are similar between the two types of cells. Thus, although Ca2+ transients (or Ca2 sparks) exhibit overall similar amplitudes and shapes in normal and CASQ2-deficient myocytes, the causes of release termination are different: luminal-Ca2+-dependent deactivation in normal myocytes and depletion of Ca2+ from the SR in CASQ2-deficient myocytes. Consistent with the dominant role of SR Ca2+ depletion in the absence of the regulatory influence of CASQ2 in termination of SR Ca2+ release, the fractional SR Ca2+ release (portion of total Ca2+ released during a twitch) is significantly increased in myocytes from CASQ2-deficient mice (Knollmann et al. 2006; Song et al. 2007). Thus depletion may be an intrinsic fall-back condition that ensures release termination even in the absence of normal regulation by CASQ2. Although capable of effectively terminating SR Ca2+ release, CPVT myocytes lack a physiologically important SR Ca2+ reserve and become vulnerable to spontaneous bursts of release due to the absence of an effective refractory mechanism requiring the presence of CASQ2.
Figure 1. Ca2+ handling in myocytes from normal hearts and hearts lacking CASQ2.
In a normal myocyte, Ca2+ release from the SR evoked by Ca2+ entry through voltage-dependent Ca2+ channels (LCCs) results in a decline of [Ca2+] in the SR. As [Ca2+]SR declines, Ca2+ dissociates from CASQ2 and the Ca2+-free CASQ2 monomers bind to and inhibit RyR2s causing Ca2+ release to terminate at a certain threshold [Ca2+]SR. The SR Ca2+ store stays refractory for some time after luminal Ca2+ is recovered by the SERCA2 pump and Ca2+-bound CASQ2 dissociates from TRD. This prevents spontaneous Ca2+ release during diastole. In myocytes lacking CASQ2, reduced SR Ca2+ binding capacity is compensated by increased SR Ca2+ volume. In the absence of the inhibitory influence of CASQ2 increased leak through the RyR2 results in reduced basal [Ca2+]SR. In addition, the RyR2s lack the capacity to deactivate until nearly complete depletion stops CICR and store refractoriness is substantially shortened predisposing the SR to premature SR Ca2+ release. Extrasystolic elevation of [Ca2+]c evokes pro-arrhythmic delayed afterdepolarizations (DADs) by stimulating the Na+/Ca2+ exchanger (NCX).
Summary
In recent years substantial progress has been made in understanding the roles of the molecular components of the Ca2+ release machinery, including CASQ2, in normal cardiac function and disease. In addition to serving as a Ca2+ buffer, CASQ2 plays an important part as a dynamic regulator of SR Ca2+ release. Ca2+-dependent regulation of the RyR2 channel by CASQ2 contributes to Ca2+ release deactivation and Ca2+ store refractoriness, and alterations of this mechanism caused by genetic mutations in CASQ2 contribute to the genesis of arrhythmia in CPVT. Despite this substantial progress several important questions remain to be resolved. How do functionally different mutations in CASQ2 and RyR2 result in similar disease phenotypes and what is the role of different compensatory mechanisms and of genetic background in the manifestation of CPVT?
References
- Bassani JW, Bassani RA, Bers DM. Twitch-dependent SR Ca accumulation and release in rabbit ventricular myocytes. Am J Physiol Cell Physiol. 1993;265:C533–C540. doi: 10.1152/ajpcell.1993.265.2.C533. [DOI] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. The Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- Delbridge LM, Satoh H, Yuan W, Bassani JW, Qi M, Ginsburg KS, Samarel AM, Bers DM. Cardiac myocyte volume, Ca2+ fluxes, and sarcoplasmic reticulum loading in pressure-overload hypertrophy. Am J Physiol Heart Circ Physiol. 1997;272:H2425–H2435. doi: 10.1152/ajpheart.1997.272.5.H2425. [DOI] [PubMed] [Google Scholar]
- Eldar M, Pras E, Lahat H. A missense mutation in the CASQ2 gene is associated with autosomal-recessive catecholamine-induced polymorphic ventricular tachycardia. Trends Cardiovasc Med. 2003;13:148–151. doi: 10.1016/s1050-1738(03)00025-2. [DOI] [PubMed] [Google Scholar]
- Endo M. Conditions required for calcium-induced release of calcium from the sarcoplasmic reticulum. Proc Jpn Acad. 1975;51:467–472. [Google Scholar]
- Fabiato A, Fabiato F. Use of chlorotetracycline fluorescence to demonstrate Ca2+-induced release of Ca2+ from the sarcoplasmic reticulum of skinned cardiac cells. Nature. 1979;281:146–148. doi: 10.1038/281146a0. [DOI] [PubMed] [Google Scholar]
- Froemming GR, Ohlendieck K. Oligomerisation of Ca2+-regulatory membrane components involved in the excitation-contraction-relaxation cycle during postnatal development of rabbit skeletal muscle. Biochim Biophys Acta. 1998;1387:226–238. doi: 10.1016/s0167-4838(98)00126-5. [DOI] [PubMed] [Google Scholar]
- Györke I, Györke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- Knollmann BC. New roles of calsequestrin and triadin in cardiac muscle. J Physiol. 2009;587:3081–3087. doi: 10.1113/jphysiol.2009.172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubalova Z, Györke I, Terentyeva R, Viatchenko-Karpinski S, Terentyev D, Williams SC, Györke S. Modulation of cytosolic and intra-sarcoplasmic reticulum calcium waves by calsequestrin in rat cardiac myocytes. J Physiol. 2004;561:515–524. doi: 10.1113/jphysiol.2004.073940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Clin Exp Pharmacol Physiol. 2007;34:889–896. doi: 10.1111/j.1440-1681.2007.04708.x. [DOI] [PubMed] [Google Scholar]
- Park H, Park IY, Kim E, Youn B, Fields K, Dunker AK, Kang C. Comparing skeletal and cardiac calsequestrin structures and their calcium binding: a proposed mechanism for coupled calcium binding and protein polymerization. J Biol Chem. 2004;279:18026–18033. doi: 10.1074/jbc.M311553200. [DOI] [PubMed] [Google Scholar]
- Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, Mannens MM, Wilde AA, Guicheney P. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91:e21–e26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- Qin J, Valle G, Nani A, Nori A, Rizzi N, Priori SG, Volpe P, Fill M. Luminal Ca2+ regulation of single cardiac ryanodine receptors: Insights provided by calsequestrin and its mutants. J Gen Physiol. 2008;131:325–334. doi: 10.1085/jgp.200709907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi N, Liu N, Napolitano C, Nori A, Turcato F, Colombi B, Bicciato S, Arcelli D, Spedito A, Scelsi M, Villani L, Esposito G, Boncompagni S, Protasi F, Volpe P, Priori SG. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circ Res. 2008;103:298–306. doi: 10.1161/CIRCRESAHA.108.171660. [DOI] [PubMed] [Google Scholar]
- Royer L, Ríos R. Deconstructing calsequestrin. Complex buffering in the calcium store of skeletal muscle. J Physiol. 2009;587:3101–3111. doi: 10.1113/jphysiol.2009.171934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, Berul CI, Eldar M, Seidman CE, Seidman JG. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2007;117:1814–1823. doi: 10.1172/JCI31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SC, Terentyev D, Györke S. Ca-dependency of cardiac SR Ca release reveals no sign of Ca-dependent inactivation and points to luminal Ca as a principal regulator of release. 2009 Biophysical Society Meeting Abstracts. Biophys J. 2009;275a(Suppl.) 1404-Pos. [Google Scholar]
- Terentyev D, Kubalova Z, Valle G, Nori A, Vedamoorthyrao S, Terentyeva R, Viatchenko-Karpinski S, Bers DM, Williams SC, Volpe P, Györke S. Modulation of SR Ca release by luminal Ca and calsequestrin in cardiac myocytes: Effects of CASQ2 mutations linked to sudden cardiac death. Biophys J. 2008;95:2037–2048. doi: 10.1529/biophysj.107.128249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Györke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, Napolitano C, Williams SC, Volpe P, Priori SG, Györke S. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res. 2006;98:1151–1158. doi: 10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Györke I, Volpe P, Williams SC, Györke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc Natl Acad Sci U S A. 2003;100:11759–11764. doi: 10.1073/pnas.1932318100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Györke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- Tijskens P, Jones LR, Franzini-Armstrong C. Junctin and calsequestrin overexpression in cardiac muscle: The role of junctin and the synthetic and delivery pathways for the two proteins. J Mol Cell Cardiol. 2003;35:961–974. doi: 10.1016/s0022-2828(03)00181-0. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Fan GC, Dong M, Altschafl B, Diwan A, Ren X, Hahn HH, Zhao W, Waggoner JR, Jones LR, Jones WK, Bers DM, Dorn GW, 2nd, Wang HS, Valdivia HH, Chu G, Kranias EG. Sarcoplasmic reticulum calcium overloading in junctin deficiency enhances cardiac contractility but increases ventricular automaticity. Circulation. 2007;115:300–309. doi: 10.1161/CIRCULATIONAHA.106.654699. [DOI] [PubMed] [Google Scholar]