Abstract
Malignant hyperthermia (MH) and exertional/environmental heat stroke (EHS) in humans present as similar life threatening crises triggered by volatile anaesthetics and strenuous exercise and/or high temperature, respectively. Many families (70–80%) diagnosed with MH susceptibility (MHS), and a few with EHS, are linked to mutations in the gene for the ryanodine receptor type-1 (RyR1), Ca2+ release channel of the sarcoplasmic reticulum (SR) of skeletal muscle and a key protein in excitation–contraction (EC) coupling. However, mutations in the RyR1 gene are not found in all MH families, suggesting that alternative genes remain to be identified. In our laboratory we have recently characterized a novel knockout model lacking skeletal muscle calsequestrin (CASQ1), a SR Ca2+-binding protein that modulates RyR1 function, and investigated whether these mice present a MH/EHS-like phenotype. Ablation of CASQ1 results in remodelling of the EC coupling apparatus and functional changes, which in male mice causes a striking increase in the rate of spontaneous mortality and susceptibility to trigger MH-like lethal episodes in response to halothane and heat stress. The demonstration that ablation of CASQ1 results in MH- and EHS-like lethal episodes validates CASQ1 as a viable candidate gene for linkage analysis in MH and EHS families where mutations in RyR1 are excluded.
Several skeletal muscle disorders result from abnormalities in proteins involved in excitation–contraction (EC) coupling, a highly coordinated process that controls myoplasmic Ca2+ dynamics during muscle activation (MacLennan, 2000). Malignant hyperthermia (MH), a pharmacogenetic disorder characterized by a hypermetabolic response to volatile anaesthetics, such as halothane and isofluorane (Denborough, 1998; Rosenberg et al. 2007), is life-threatening if not corrected immediately by suspension of triggering agent and treatment with dantrolene (Wedel et al. 1995). Mutations in the ryanodine receptor type 1 gene (RyR1), encoding the sarcoplasmic reticulum (SR) Ca2+ release channel of skeletal muscle account for MH susceptibility (MHS) in most MH families (70–80%) (Robinson et al. 2006). Interestingly, MH-like hyperthermic episodes, but unrelated to anaesthetic administration, have also been reported in humans during strenuous exercise conducted under conditions of excessive elevations in temperature, a condition referred to as exertional/environmental heat-stroke (EHS) (Pamukcoglu, 1988; Bouchama & Knochel, 2002). Interestingly, two of these cases have been reported in young, otherwise fit male athletes with documented family histories of MHS (Ryan & Tedeschi, 1997; Tobin et al. 2001), further validating a possible association between these two disorders (Hopkins et al. 1991; Muldoon et al. 2004).
While most MHS cases are linked to mutations in the RyR1 gene, about 20–30% of all MHS individuals do not possess an RyR1 gene mutation. In these cases, MH (and potentially EHS) could arise from mutations to genes for other EC coupling proteins, possibly those modulating directly RyR1 activity (Robinson et al. 1997). A mutation in the α1-subunit of the dihydropyridine receptor (DHPR), which controls directly RyR1 during EC coupling, has been reported (Monnier et al. 1997). One other possible candidate would be calsequestrin (CASQ)-1, a Ca2+ binding protein located in the SR terminal cisternae of striated muscle (MacLennan & Wong, 1971), which possibly acts as a luminal regulator of RyR1-mediated Ca2+ release (Beard et al. 2002). To date, no human disease has yet been associated with mutations in the CASQ1 gene. However, catecholaminergic polymorphic ventricular tachycardia (CPVT), a rare arrhythmogenic disorder characterized by physical/emotional stress-induced syncopal events and sudden cardiac death, is caused by both gain-of-function mutations in the cardiac RyR2 gene (Priori et al. 2001) and loss-of-function mutations in the cardiac CASQ2 gene (Lahat et al. 2001). By analogy to cardiac muscle, we hypothesized that MH and EHS may result from increased SR Ca2+ leak caused by either gain-of-function mutations in RyR1 (Chelu et al. 2006; Yang et al. 2006) or loss-of-function mutations in CASQ1. We have tested this hypothesis in CASQ1-knockout (null) mice (Paolini et al. 2007).
The phenotype of CASQ1-null mice
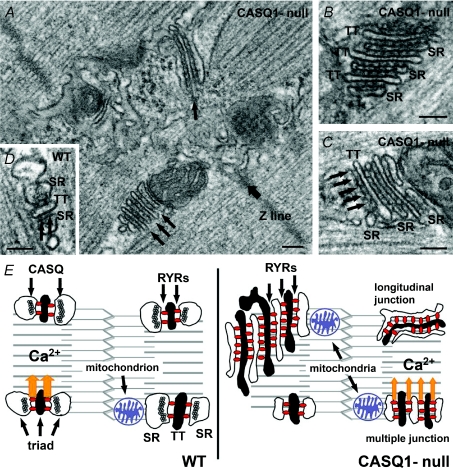
In 2007 we published in The Journal of Physiology the structural–functional characterization of the first knockout mouse lacking CASQ1 (Paolini et al. 2007). A year earlier Knollmann et al. (2006) had characterized the first CASQ2 knockout mouse. Lack of CASQ1, as the CASQ2 knockout, resulted in a non-lethal phenotype and, apparently, CASQ1-null mice did not present noticeable differences under standard housing conditions. The biochemical, structural and functional characterization of calcium release units (CRUs) – intracellular junctions between SR and transverse tubule (TT) – revealed significant remodelling possibly caused by compensatory postnatal adaptation. Figure 1 summarizes some of the findings of Paolini et al. 2007: (a) reduction in size of SR terminal cisternae, which in wild-type (WT) muscle contains CASQ; (b) proliferation of junctional SR–TT; (c) increased amount of RyRs. These results demonstrated the importance of CASQ1 for the correct assembling of CRUs and for the structural shaping of mature SR in fast twitch fibres. Also interesting was the increased frequency of mitochondria (Paolini et al. 2007), which have been shown to be functionally and structurally connected to the SR compartment (Rudolf et al. 2004; Boncompagni et al. 2009).
Figure 1. Remodelling of CRUs in EDL muscle following depletion of CASQ1.
A, in CASQ1-null fibres CRUs are often formed by multiple elements and more variably oriented (arrows). B and C, multilayered CRUs usually contain more than two rows of RyRs, or feet, between SR and TT (arrows). The lumen of the SR terminal cisternae is significantly narrower than in controls (compare with D). D, in WT fibres, CRUs are almost exclusively in the form of triads, SR has a wide profile, and feet usually form two rows between SR and TT (arrows). E, cartoon summarising the structural modifications in CASQ1-null CRUs: junctions are often multilayered; RyR-feet and mitochondria are increased in number; junctional SR width is smaller. See Paolini et al. 2007 for more detail. Bars: 0.1 μm.
One unexpected finding of our initial studies (Paolini et al. 2007) was the relatively small reduction in the size of electrically evoked Ca2+ transients and in the total amount of releasable Ca2+ from the SR (caffeine-induced responses). If, as commonly thought, CASQ1 is the main buffer inside the SR of skeletal muscle, one would have expected a far more dramatic effect following complete ablation of CASQ1 from fast twitch fibres. Results of others are also against the expectations (Wang et al. 2006; Knollmann et al. 2006) and in line with our results (Paolini et al. 2007), suggesting that means of Ca2+ storage unrelated to CASQ must play a key role in muscle, at least in the CASQ1-null animals.
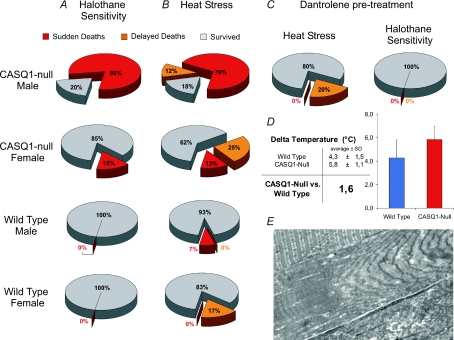
After the first study (Paolini et al. 2007), one puzzling observation prompted us to investigate the phenotype of CASQ1-null mice under conditions of increased stress: compared to their WT counterparts, we observed a significantly increased rate of spontaneous mortality in male CASQ1-null mice, particularly after 3 months of age (Dainese et al. 2009). Based on this initial observation, we hypothesized the presence of a pathological phenotype and decided to determine if CASQ1-null mice were susceptible to trigger MH-like episodes in response to halothane and heat. Protocols similar to those recently used to test the phenotype of RyR1-knockin mice expressing human MH mutations were employed (Chelu et al. 2006; Yang et al. 2006; Durham et al. 2008). Male CASQ1-null animals exhibited a very high mortality rate during exposure to either halothane (2%, 1 h) or heat (41°C, 30 min): lethal episodes were characterized by impaired movement, difficulty in breathing, whole-body contractions, arched back, followed by death (Dainese et al. 2009). Specifically, about 80% of male CASQ1-null mice died during both stress protocols (halothane: 16 out of 20; heat: 17 + 3 delayed out of 24). Female CASQ1-null mice displayed a higher survival rate following both procedures compared to male CASQ1-null mice, but still lower than WT mice (Fig. 2A and B, and Dainese et al. 2009 for more detail). Importantly, both halothane- and heat-induced crises could be completely prevented by prior intraperitoneal injection of dantrolene (Fig. 2C and Dainese et al. 2009 for more detail), the only pharmacological intervention for MH in humans (Wedel et al. 1995) and also been considered in the treatment of EHS (Hausfater, 2005).
Figure 2. CASQ1-null male mice exhibit enhanced sensitivity to halothane and heat exposure (prevented by dantrolene pre-treatment), hyperthermia and rhabdomyolysis.
A and B, incidence of sudden death in male/female WT and CASQ1-null mice as a result of halothane and heat exposure. C, sudden death may be prevented by prior administration of dantrolene. D, core temperature increases significantly (P < 0.05) more in CASQ1-null mice compared with WT animals. E, about 40% of EDL fibres appear severely damaged immediately after heat-induced sudden death. See Dainese et al. 2009 for more detail.
The parallel between MH and the phenotype of CASQ1-null mice was strengthened by assessing the presence of hyperthermia and rhabdomyolisis during heat stress (Fig. 2D and E) and by experiments that demonstrated the enhanced sensitivity of CASQ1-null vs. WT specimens (whole extensor digitorum longus (EDL) fibres, single flexor digitorum brevis (FDB) fibres, and myotubes) to increasing temperature and to caffeine (not shown, see Dainese et al. 2009 for detail).
Summary and conclusions
Mutations in the gene that encodes the skeletal muscle ryanodine receptor (RyR1) result in life-threatening MH and EHS in humans (Ryan & Tedeschi, 1997; Denborough, 1998; Tobin et al. 2001; Robinson et al. 2006; Rosenberg et al. 2007). However, since not all MH/EHS families are linked to RyR1 mutations, it is likely that other MH gene loci and different pathophysiological mechanisms remain to be identified. Our recent demonstration that loss-of-function mutations in CASQ1 strongly enhances MH and EHS susceptibility in mice (Dainese et al. 2009) validates CASQ1 as a powerful alternative candidate gene for linkage analysis in MH and EHS families where mutations in RyR1 are excluded.
The phenotype of CASQ1-knockout mice (Dainese et al. 2009) is strikingly similar to that resulting from gain-of-function mutations in RyR1 (Chelu et al. 2006; Yang et al. 2006; Durham et al. 2008) (Fig. 3). These findings create an important parallel with cardiac muscle, where loss-of-function mutations in cardiac CASQ2 and gain-of-function mutations in RyR2 result in analogous arrhythmogenic disorder, i.e. CPVT (Lahat et al. 2001; Priori et al. 2001). Thus, our study provides crucial evidences that disruption in RyR regulation by CASQ may represent an important novel pathogenic mechanism that underlies both cardiac (CPVT) and skeletal muscle (MH and EHS) diseases. We believe that these findings could be of clinical relevance to the fields of muscle disease, Ca2+ signalling, and medical genetics.
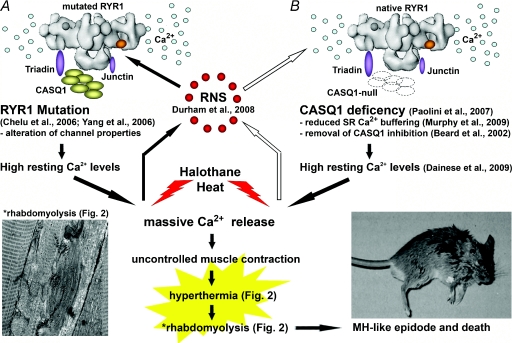
Figure 3. MH/EHS susceptibility may result from either gain-of-function mutations in RyR1 or loss-of-function mutations in CASQ1.
A, gain of function mutations in RyR1 changes the properties of the SR Ca2+ release channels. RyR1 channels carrying human MH mutations have been expressed in knock-in mice causing high resting Ca2+ levels and MH susceptibility (Chelu et al. 2006; Yang et al. 2006). B, CASQ1 deficiency in knockout mice also results in high resting Ca2+ levels (Dainese et al. 2009). Lack of CASQ1 causes a reduction in the SR Ca2+ buffering (Murphy et al. 2009) and may remove the inhibitory influence on RyR1-mediated Ca2+ release (Beard et al. 2002). The precise mechanism(s) underlying the abnormal intracellular Ca2+ levels in CASQ1-null fibres and myotubes has not yet been elucidated (see Discussion). High resting Ca2+ leak resulting from either RyR1 mutations or CASQ1-deficiency results in massive anaesthetic- and heat-induced Ca2+ release, uncontrolled muscle contraction, hyperthermia, and rhabdomyolysis followed by sudden death. Durham et al. (2008) have recently proved that overproduction of reactive nitrogen species (RNS) due to abnormally high resting Ca2+ levels generates a feed-forward mechanism by nitrosilating RyR1. This mechanism remains to be tested in CASQ1-null mice (open arrows).
In addition, our studies reinforce a parallel between MH and EHS (Hopkins et al. 1991; Muldoon et al. 2004), and support the notion that the two syndromes may in some cases arise from a common pathogenic mechanism, i.e. lack of proper regulation of SR Ca2+ release. Indeed, MH and EHS are becoming more and more relevant, since global warming is affecting many world regions potentially rendering a greater number of susceptible humans exposed to environmental temperatures sufficient to trigger EHS with higher frequency (Semenza et al. 1996; Bouchama & Knochel, 2002).
Our study leaves, though, some important questions that will need to be addressed in future work to fully understand some of the observations of Dainese et al. 2009.
(1) What is the molecular mechanism leading to high resting Ca2+ concentrations and to MH/EHS episodes in CASQ1-knockout mice? The mechanism(s) underlying abnormal Ca2+ levels in CASQ1-null fibres and myotubes (Dainese et al. 2009) has not yet been elucidated. Lack of CASQ1 causes a reduction in the SR Ca2+ buffering (Murphy et al. 2009) and may remove the inhibitory influence on RyR1-mediated Ca2+ release (Beard et al. 2002) (see Fig. 3). However, since the final steady value of free intracellular [Ca2+] cannot be directly determined by intracellular organelles, one possible explanation is that these changes are the result of a net Ca2+ entry through the plasmalemma. This would demands some kind of link between the primary effect (lack of CASQ1 in the SR) and the fibre's surface membrane. As is well known, the mechanism that allows such a communication has been identified as store-operated Ca2+ entry (SOCE). Recent data presented at the Biophysical Society Meeting 2009 support this hypothesis: in skeletal fibres with knockdown of CASQ1 SOCE is significantly increased with respect to control fibres (Kee Min et al. 2009).
(2) What is the contribution of adaptative changes (i.e. increase in RyR and mitochondria, see Fig. 1) and of oxidative stress (Durham et al. 2008) in the MH/EHS-like phenotype of knockout mice? Increased mitochondrial frequency could indeed be considered an expected consequence of increased intracellular Ca2+ level, given that Murphy et al. (2009) have pointed out that this would inevitably mean higher resting consumption of ATP, which might readily predict an adaptive increase in mitochondrial density in fast-twitch muscle fibres.
(3) What are the reasons for the striking difference in spontaneous and stress-induced mortality between male and female CASQ1-null mice (Fig. 2)? Possible explanations may be found in hormonal differences between the two sexes, but also other possibilities (possible differences in muscle mass, different capacity to handle oxidative stress) need to be taken into consideration. To date, the precise mechanism underlying this sex difference remains unclear and will require further investigation.
Acknowledgments
We thank C. Reggiani, R. T. Dirksen, and P. Volpe and their collaborators for crucial experiments, which have contributed to characterize the phenotype of CASQ1-null mice (Paolini et al. 2007; Dainese et al. 2009). This study was supported by Research Grant no. GGP080153 from the Italian Telethon Foundation.
Glossary
Abbreviations
- Ca2+
calcium ions
- CASQ
calsequestrin
- CVPT
catecholamine-induced polymorphic ventricular tachycardia
- CRUs
calcium release units
- EDL
extensor digitorum longus
- EM
electron microscopy
- EHS
environmental heat stroke
- IVCT
in vitro contracture test
- MH
malignant hyperthermia
- MHS
MH susceptibility
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
- TT
transverse tubule
References
- Beard NA, Sakowska MM, Dulhunty AF, Laver DR. Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys J. 2002;82:310–320. doi: 10.1016/S0006-3495(02)75396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncompagni S, Rossi AE, Micaroni M, Beznoussenko GV, Polishchuk RS, Dirksen RT, Protasi F. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol Biol Cell. 2009;20:1058–1067. doi: 10.1091/mbc.E08-07-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- Chelu MG, Goonasekera SA, Durham WJ, Tang W, Lueck JD, Riehl J, Pessah IN, Zhang P, Bhattacharjee MB, Dirksen RT, Hamilton SL. Heat- and anaesthesia-induced malignant hyperthermia in an RyR1 knock-in mouse. FASEB J. 2006;20:329–330. doi: 10.1096/fj.05-4497fje. [DOI] [PubMed] [Google Scholar]
- Dainese M, Quarta M, Lyfenko AD, Paolini C, Canato M, Reggiani C, Dirksen RT, Protasi F. Anaesthetic- and heat-induced sudden death in calsequestrin-1-knockout mice. FASEB J. 2009 doi: 10.1096/fj.08-121335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denborough M. Malignant hyperthermia. Lancet. 1998;352:1131–1136. doi: 10.1016/S0140-6736(98)03078-5. [DOI] [PubMed] [Google Scholar]
- Durham WJ, Aracena-Parks P, Long C, Rossi AE, Goonasekera SA, Boncompagni S, Galvan DL, Gilman CP, Baker MR, Shirokova N, Protasi F, Dirksen R, Hamilton SL. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133:53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausfater P. Dantrolene and heatstroke: a good molecule applied in an unsuitable situation. Crit Care. 2005;9:23–24. doi: 10.1186/cc2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins PM, Ellis FR, Halsall PJ. Evidence for related myopathies in exertional heat stroke and malignant hyperthermia. Lancet. 1991;338:1491–1492. doi: 10.1016/0140-6736(91)92304-k. [DOI] [PubMed] [Google Scholar]
- Kee Min C, Zhao X, KO J-K, Pan Z, Parness J, Kim DH, Weisleder N, Ma J. Increased store-operated Ca2+ entry in skeletal muscle with knockdown of calsequestrin. Biophys J. 2009;96:A115. doi: 10.1016/j.bpj.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan DH. Ca2+ signalling and muscle disease. Eur J Biochem. 2000;267:5291–5297. doi: 10.1046/j.1432-1327.2000.01566.x. [DOI] [PubMed] [Google Scholar]
- MacLennan DH, Wong PT. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1971;68:1231–1235. doi: 10.1073/pnas.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier N, Procaccio V, Stieglitz P, Lunardi J. Malignant-hyperthermia susceptibility is associated with a mutation of the α1-subunit of the human dihydropyridine-sensitive L-type voltage-dependent calcium-channel receptor in skeletal muscle. Am J Hum Genet. 1997;60:1316–1325. doi: 10.1086/515454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon S, Deuster P, Brandom B, Bunger R. Is there a link between malignant hyperthermia and exertional heat illness? Exerc Sport Sci Rev. 2004;32:174–179. doi: 10.1097/00003677-200410000-00009. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J Physiol. 2009;587:443–460. doi: 10.1113/jphysiol.2008.163162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamukcoglu T. Sudden death due to malignant hyperthermia. Am J Forensic Med Pathol. 1988;9:161–162. doi: 10.1097/00000433-198806000-00015. [DOI] [PubMed] [Google Scholar]
- Paolini C, Quarta M, Nori A, Boncompagni S, Canato M, Volpe P, Allen PD, Reggiani C, Protasi F. Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J Physiol. 2007;583:767–784. doi: 10.1113/jphysiol.2007.138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- Robinson R, Carpenter D, Shaw MA, Halsall J, Hopkins P. Mutations in RyR1 in malignant hyperthermia and central core disease. Hum Mutat. 2006;27:977–989. doi: 10.1002/humu.20356. [DOI] [PubMed] [Google Scholar]
- Robinson RL, Monnier N, Wolz W, Jung M, Reis A, Nuernberg G, Curran JL, Monsieurs K, Stieglitz P, Heytens L, Fricker R, van Broeckhoven C, Deufel T, Hopkins PM, Lunardi J, Mueller CR. A genome wide search for susceptibility loci in three European malignant hyperthermia pedigrees. Hum Mol Genet. 1997;6:953–961. doi: 10.1093/hmg/6.6.953. [DOI] [PubMed] [Google Scholar]
- Rosenberg H, Davis M, James D, Pollock N, Stowell K. Malignant hyperthermia. Orphanet J Rare Dis. 2007;2:21. doi: 10.1186/1750-1172-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf R, Mongillo M, Magalhaes PJ, Pozzan T. In vivo monitoring of Ca2+ uptake into mitochondria of mouse skeletal muscle during contraction. J Cell Biol. 2004;166:527–536. doi: 10.1083/jcb.200403102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JF, Tedeschi LG. Sudden unexplained death in a patient with a family history of malignant hyperthermia. J Clin Anaesth. 1997;9:66–68. doi: 10.1016/S0952-8180(96)00207-3. [DOI] [PubMed] [Google Scholar]
- Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, Wilhelm JL. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med. 1996;335:84–90. doi: 10.1056/NEJM199607113350203. [DOI] [PubMed] [Google Scholar]
- Tobin JR, Jason DR, Challa VR, Nelson TE, Sambuughin N. Malignant hyperthermia and apparent heat stroke. JAMA. 2001;286:168–169. doi: 10.1001/jama.286.2.168. [DOI] [PubMed] [Google Scholar]
- Wedel DJ, Quinlan JG, Iaizzo PA. Clinical effects of intravenously administered dantrolene. Mayo Clin Proc. 1995;70:241–246. doi: 10.4065/70.3.241. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu L, Duan H, Pasek DA, Eu JP, Meissner G. Knocking down type 2 but not type 1 calsequestrin reduces calcium sequestration and release in C2C12 skeletal muscle myotubes. J Biol Chem. 2006;281:15572–15581. doi: 10.1074/jbc.M600090200. [DOI] [PubMed] [Google Scholar]
- Yang T, Riehl J, Esteve E, Matthaei KI, Goth S, Allen PD, Pessah IN, Lopez JR. Pharmacologic and functional characterization of malignant hyperthermia in the R163C RyR1 knock-in mouse. Anaesthesiology. 2006;105:1164–1175. doi: 10.1097/00000542-200612000-00016. [DOI] [PubMed] [Google Scholar]