Abstract
Contractile dysfunction and ventricular arrhythmias associated with heart failure have been attributed to aberrant sarcoplasmic reticulum (SR) Ca2+ cycling. The study of junctin (JCN) and histidine-rich Ca2+ binding protein (HRC) becomes of particular importance since these proteins have been shown to be critical regulators of Ca2+ cycling. Specifically, JCN is a SR membrane protein, which is part of the SR Ca2+ release quaternary structure that also includes the ryanodine receptor, triadin and calsequestrin. Functionally, JCN serves as a bridge between calsequestrin and the Ca2+ release channel, ryanodine receptor. HRC is a SR luminal Ca2+ binding protein known to associate with both triadin and the sarcoplasmic reticulum Ca2+-ATPase, and may thus mediate the crosstalk between SR Ca2+ uptake and release. Indeed, evidence from genetic models of JCN and HRC indicate that they are important in cardiophysiology as alterations in these proteins affect SR Ca2+ handling and cardiac function. In addition, downregulation of JCN and HRC may contribute to Ca2+ cycling perturbations manifest in the failing heart, where their protein levels are significantly reduced. This review examines the roles of JCN and HRC in SR Ca2+ cycling and their potential significance in heart failure.
Heart failure affects more than 5 million Americans and more importantly, 550 000 new cases are diagnosed each year according to the American Heart Association. Heart disease continues to be the leading cause of death in the United States, and we can only expect these numbers to increase as the prevalence of coronary artery disease, diabetes, high blood pressure and other risk factors for heart failure continue to rise.
Heart failure is a disease that slowly progresses over time, although sudden death occurs in approximately 50% of patients with congestive heart failure (Janse, 2004; Pogwizd & Bers, 2004). This sudden death is primarily due to ventricular arrhythmias (Janse, 2004; Pogwizd & Bers, 2004). The propensity for sudden cardiac death is dependent on the severity of heart failure, as stage I and II heart failure patients are more susceptible to sudden cardiac death than patients with end-stage heart failure (Janse, 2004; Pogwizd & Bers, 2004). This observation appears counterintuitive, as patients with moderate heart failure are at greater risk, but it may be due to their sensitized β-adrenergic signalling (Pogwizd & Bers, 2004), compared to end-stage heart failure patients where β-adrenergic receptors are downregulated and/or signalling is blunted (Bristow et al. 1982). In fact, clinical trials demonstrate that administration of β-blockers can reduce the incidence of sudden cardiac death (Merit-HF Study Group, 1999; Poole-Wilson et al. 2003).
These deleterious effects are associated with altered Ca2+ handling often in the form of prolonged Ca2+ transients, altered diastolic and systolic function, and increased Ca2+ leak (Hasenfuss & Pieske, 2002). Prolonged action potentials are also characteristics of cardiomyocytes from heart failure patients (Weisser-Thomas et al. 2003). Impaired Ca2+ homeostasis in the failing heart is at least partly attributed to alterations in SR Ca2+ cycling.
Ca2+ cycling in heart failure
The SR is the major organelle responsible for proper Ca2+ cycling, which is normally tightly controlled on a beat-to-beat basis. In a healthy heart, membrane depolarization of the cardiomyocytes opens voltage-gated L-type Ca2+ channels (LTCCs), resulting in Ca2+ entering the cell, which subsequently activates opening of the ryanodine receptor permitting Ca2+ release (Ca2+ sparks) from the SR (Hasenfuss & Pieske, 2002). This Ca2+-induced-Ca2+-release (CICR) from the SR (Fabiato, 1983) results in a significant elevation in intracellular Ca2+ that mediates induction of contraction at the myofilaments via binding to troponin C (Bers, 2002). SR Ca2+-ATPase (SERCA), which is negatively regulated by phospholamban (PLN; Chu & Kranias, 2006), and the Na+–Ca2+ exchanger are the primary mechanisms responsible for clearing Ca2+ from the cytosol, promoting cardiomyocyte relaxation (Bers, 2002). SERCA is the main mediator of Ca2+ clearance in the cardiomyocyte (∼70% of Ca2+ clearance in humans; Bers, 2002). SERCA sequesters Ca2+ into the SR, so that sufficient Ca2+ is available for release for the next contraction, while Na+–Ca2+ exchanger extrudes most of the remaining cytosolic Ca2+ (Bers, 2002).
This tight regulation of Ca2+ handling is disrupted in heart failure. Specifically, failing cardiomyocytes have reduced SR Ca2+ uptake, as SERCA activity is depressed, due to decreased SERCA protein levels and an elevation of the PLN to SERCA ratio (Chu & Kranias, 2006). An additional insult is the elevated Ca2+ leak from the SR (George, 2008). Together, these impair both relaxation and contraction. Secondly, this reduced Ca2+ load in the face of elevated Ca2+ leak enhances Na+–Ca2+ exchange and makes cells susceptible to delayed afterdepolarizations (Pogwizd et al. 2001), which may explain why ∼50% of deaths of heart failure patients are sudden. Current treatments for heart failure patients include β-blockers, ACE inhibitors, diuretics and digitalis (Francis, 2001), which can improve survival but do not address the problem of sudden death. Therefore, there is a necessity to identify therapeutic targets, which may provide a more direct mechanism for improving Ca2+ cycling in failing hearts and prevent the incidence of deadly ventricular arrhythmias.
This review will focus on two SR proteins, junctin (JCN) and histidine-rich Ca2+ binding protein (HRC), which are potential important mediators of cardiomyocyte Ca2+ cycling. The Ca2+ release unit in the SR is composed of a quaternary complex containing the ryanodine receptor (RyR), the anchoring proteins triadin and junctin, and calsequestrin (CSQ; a SR luminal Ca2+ binding protein) (Zhang et al. 1997). HRC has been shown to interact with both triadin (Lee et al. 2001; Sacchetto et al. 2001; Arvanitis et al. 2007) and SERCA2 (Arvanitis et al. 2007), suggesting that it may have important implications in both Ca2+ release and uptake. More importantly, experimental models and human studies have shown that alterations in these proteins may be associated with arrhythmias and impaired cardiac function, making them particularly interesting targets in the study of heart failure and sudden death.
Junctin structure and protein interactions
Junctin is a 210-amino-acid, 26 kDa protein found in skeletal and cardiac muscle (Jones et al. 1995; Dinchuk et al. 2000; Lim et al. 2000), which interacts with both calsequestrin (Zhang et al. 1997; Shin et al. 2000) and the ryanodine receptor (Zhang et al. 1997). JCN is an alternative splice product from the aspartyl-β-hydroxylase gene and shares exons 2 and 3 with this gene, as well as junctate and humbug genes, two other additional splice products from aspartyl-β-hydroxylase (Dinchuk et al. 2000; Treves et al. 2000; Feriotto et al. 2007). Two JCN isoforms exist via alternative splicing in human cardiac muscle, resulting in the inclusion of 15 amino acids at residue 55 (Lim et al. 2000). JCN is an integral protein of the SR membrane composed of a short cytosolic amino terminus (aa 1–22), a single transmembrane domain (aa 23–44), followed by an extensive clustering (aa 45–210) of lysine and glutamic acid residues, otherwise known as KEKE motifs (Jones et al. 1995).
Structurally similar to triadin (Jones et al. 1995), KEKE motifs within JCN are important for interacting with the aspartyl-rich region of CSQ in a Ca2+-dependent manner (Zhang et al. 1997; Shin et al. 2000). Whereas a specific KEKE motif in triadin is central to binding to CSQ, elimination of any of the KEKE motifs in JCN disrupts CSQ binding (Kobayashi et al. 2000). Similar to triadin, JCN associates with the RyR in a Ca2+-independent manner (Zhang et al. 1997). JCN also binds to triadin in a Ca2+-independent fashion (Zhang et al. 1997). In cardiac SR microsomes fused into planar lipid bilayers, findings indicate that ryanodine receptor open probability may be regulated by (1) JCN alone in a Ca2+-independent manner and (2) CSQ, JCN and/or triadin in a Ca2+-dependent manner (Györke et al. 2004). To date, no JCN polymorphisms have been identified in cardiomyopathies; however, JCN is almost undetectable in human failing hearts (Gergs et al. 2007), suggesting that its expression levels may be important in Ca2+ cycling changes, which occur in heart failure. Transgenic mice expressing the β1-adrenergic receptor that exhibit heart failure also have reduced JCN levels (Engelhardt et al. 2001). Given the interaction of JCN with both CSQ and the RyR, it is hypothesized that JCN is important in SR Ca2+ release, and experimental models of JCN ablation and overexpression have begun to reveal its functional role.
Junctin ablation
Acute downregulation of JCN by 40% in adult rat cardiomyocytes by antisense mRNA resulted in increased contractility and improved Ca2+ kinetics in the absence of changes in the levels of other Ca2+ handling proteins (Fan et al. 2007). Our group showed that cardiomyocytes, isolated from JCN-null mice, displayed enhanced contractility, SR Ca2+ load, and Ca2+ kinetics (Yuan et al. 2007). In vivo cardiac function in JCN-deficient mice was also enhanced as assessed by echocardiography (Yuan et al. 2007). Remarkably, there were no changes in the levels of other SR proteins (triadin, RyR, FKBP12.6, HRC, SERCA, PLN) involved in Ca2+ handling of the JCN-null mice. However, the Na+–Ca2+ exchanger was altered with a 70% increase in protein accompanied by a similar increase in current density (Yuan et al. 2007).
Surprisingly, 25% of JCN-null mice died prematurely by 3 months of age in the absence of any cardiac remodelling, as indicated by histology and assessment of hypertrophic signalling cascades (Yuan et al. 2007). Short-term and long-term isoproterenol administration to JCN-null mice elicited arrhythmias, which included premature ventricular contractions and A-V heart block (Fig. 1; Yuan et al. 2007). Furthermore, JCN-null cardiomyocytes exhibited delayed afterdepolarizations as well as ryanodine-sensitive aftercontractions (Fig. 2; Yuan et al. 2007). Junctin-null cardiomyocytes also displayed an increased frequency of Ca2+ sparks, indicative of increased SR leak (Yuan et al. 2007). These phenotypes can be associated with arrhythmias (Janse, 2004), and thus the loss of JCN function appears to at least in part mediate the propensity for arrhythmias. Whether the absence of JCN is directly responsible for this, via destabilization of the RyR resulting in increased SR leak, or if enhanced Na+–Ca2+ exchange as a result of altered Ca2+ handling (Bers 2002) is the prevailing mechanism underlying these arrhythmias remains to be seen.
Figure 1. Cardiac arrhythmias in junctin-null mice.
A, top, representative ECG recordings of WT mice after injection of isoproterenol (0.25 μg g−1i.p.); bottom: ventricular tachycardia in junctin-KO mice after injection of isoproterenol (0.25 μg g−1i.p.; n= 3). B, top, representative ECG tracings in WT mice after isoproterenol pump implantation; Middle and lower panels, premature ventricular contractions in 2 junctin-null mice during chronic isoproterenol stimulation. Modified with permission from Yuan et al. (2007).
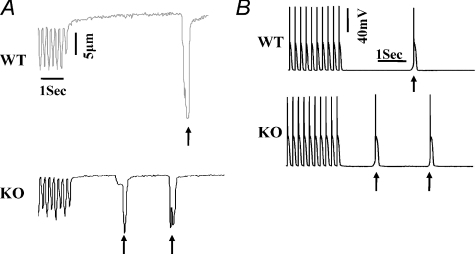
Figure 2. Altered Ca2+ handling in junctin-null mice.
A, representative traces of aftercontractions (signified by arrows) in WT and junctin KO myocytes at 5 Hz and 1 μmol l−1 isoproterenol stimulation. B, representative traces of action potentials in WT and junctin KO myocytes at 5 Hz and isoproterenol stimulation. Delayed afterdepolarization is marked with arrows. Modified with permission from Yuan et al. 2007.
Junctin overexpression
Acute JCN overexpression in adenovirally infected adult rat cardiomyocytes resulted in depressed contractility, Ca2+ transients, and SR load (Fan et al. 2007; Gergs et al. 2007). Similar to impaired function measured in vitro, 10-fold cardiac overexpression of canine JCN in mice resulted in decreased contractility, impaired relaxation and hypertrophy (Kirchhefer et al. 2003). In contrast to our JCN ablated model, JCN overexpression mice displayed reduced frequency of Ca2+ sparks and SR Ca2+ load (Kirchhefer et al. 2006). These mice also exhibited compensatory changes in other Ca2+ handling proteins, as triadin and RyR levels were decreased (Kirchhefer et al. 2003) and Na+–Ca2+ exchange was diminished (Kirchhefer et al. 2006), which may contribute in part to the phenotypes observed. However, our studies with acute infection of JCN in the absence of changes in other Ca2+ handling proteins suggest that JCN can have a direct effect on contractility, SR Ca2+ load and release (Fan et al. 2007).
Overexpression of JCN in mice displayed a gene dosage effect on function as mice expressing canine JCN by 29-fold exhibited cardiac hypertrophy, increased L-type Ca2+ current density, and prolonged action potential duration accompanied by heart failure and bradycardia (Hong et al. 2002). Interestingly, atrial fibrillation was observed in these mice, which may be attributed to prolonged action potentials as well as other Ca2+ perturbations that occurred with increasing JCN levels (Hong et al. 2002).
Junctin perspective
Data from isolated cardiomyocytes suggest that JCN is important in Ca2+ handling, and in vivo models show that JCN ablation may be associated with cardiac arrhythmias. Junctin is hypothesized to be associated with the RyR, and its interaction with CSQ is subjected to SR Ca2+ levels. When SR Ca2+ is low, CSQ and JCN interact. As SR Ca2+ levels increase and more Ca2+ binds to CSQ during loading of the SR, JCN dissociates from CSQ. This may be due to the fact that CSQ is a ‘flexible molecule’ that can change conformation upon Ca2+ binding (Mitchell et al. 1988). When JCN is overexpressed, JCN-CSQ interactions may increase, possibly leading to reduced SR load and reduced spontaneous SR Ca2+ release. On the other hand, upon JCN ablation, the ‘bridge’ between CSQ and the RyR may be abolished, reducing CSQ–RyR interaction, which may result in desynchronized SR Ca2+-release (i.e. Ca2+ leak/sparks). The regulation of Ca2+ release by JCN is an important area of investigation, since both increases and decreases in JCN levels may be associated with arrhythmogenesis. Elucidation of the exact mechanism by which JCN regulates RyR activity will be important in our understanding of the underlying causes of such arrhythmias.
Histidine-rich Ca2+ binding protein structure and protein interactions
HRC is a 170 kDa protein found primarily in striated and arteriolar smooth muscle (Hofmann et al. 1989a; Pathak et al. 1992). HRC, originally identified in skeletal muscle, is a charged molecule with more than 30% of the protein composed of acidic residues and 13% of histidine (Hofmann et al. 1989b). There is no EF hand Ca2+ binding motif in HRC, and it is presumed to bind Ca2+ via its acid repeats (Hofmann et al. 1989b). HRC is a low-affinity, high capacity Ca2+ binding protein (Picello et al. 1992) that contains an enormous ability for binding Ca2+, given that it has more acidic clusters than CSQ (Hofmann et al. 1989b; Yano & Zarain-Herzberg, 1994; Arvanitis et al. 2007). CSQ, though, serves as the major Ca2+ binding protein, as HRC comprises only about 1% of protein of the skeletal muscle SR (Damiani et al. 1997). HRC has been shown to be located in the SR lumen in cardiac muscle (Arvanitis et al. 2007). Unlike cardiac muscle, there is some discrepancy in skeletal muscle as to whether HRC resides in the SR lumen (Hofmann et al. 1989a) or is just associated with the SR membrane (Damiani & Margreth, 1991; Sacchetto et al. 1999, 2001). However, strong evidence suggests it is a luminal protein in skeletal muscle as well based on immunofluorescence, immunoelectron microscopy and coimmunoprecipitation studies (Hofmann et al. 1989a; Suk et al. 1999; Lee et al. 2001).
HRC is associated with the RyR complex by binding to triadin, and several potential interactions between triadin and HRC have been identified in both skeletal and cardiac muscle (Lee et al. 2001; Sacchetto et al. 2001; Arvanitis et al. 2007). HRC binds to triadin in a Ca2+-dependent manner in both cardiac and skeletal preparations (Lee et al. 2001; Sacchetto et al. 2001; Arvanitis et al. 2007) where binding increases with increasing Ca2+ levels (Sacchetto et al. 2001; Arvanitis et al. 2007). HRC has also been shown to interact with SERCA2 in both mouse and human hearts (Arvanitis et al. 2007). This interaction, which has been localized to amino acid residues 320–460 of HRC and residues 74–90 of SERCA2, is also Ca2+ dependent (Arvanitis et al. 2007). Increases in Ca2+ result in diminished interaction between HRC and SERCA2 (Arvanitis et al. 2007).
HRC interacts with SERCA2 and triadin via different domains as the triadin interaction domain of HRC is localized to residues 609–699 (Sacchetto et al. 2001; Arvanitis et al. 2007). This dual interaction makes HRC an attractive molecule for understanding the balance between SR Ca2+ uptake and Ca2+ release in cardiomyocytes. Thus, it is hypothesized that HRC interacts with SERCA2, when Ca2+ load is decreased, affecting SERCA2 activity. When HRC becomes saturated with Ca2+, it dissociates from SERCA2 and exhibits increased binding to triadin, modulating Ca2+ release.
Several polymorphisms have been identified in the HRC gene, and one of these variants, Ser96Ala, correlates with ventricular arrhythmias in patients with idiopathic dilated cardiomyopathy (Fig. 3; Arvanitis et al. 2008). The functional significance of this particular mutation is unclear; however, Ser96 represents a potential phosphorylation site for casein kinase II (Arvanitis et al. 2008). Casein kinase II has been demonstrated to phosphorylate HRC (Shoshan-Barmatz et al. 1996; Hadad et al. 1999), although the effects of this phosphorylation are not currently known. In addition, HRC is decreased in heart failure patients as well as animal models of heart failure (Fan et al. 2004). Therefore, understanding the role of HRC in Ca2+ cycling and its dual interaction with RyR and SERCA2 may provide further insights into the occurrence of ventricular arrhythmias in dilated cardiomyopathy individuals.
Figure 3. Kaplan–Meier plots for the probability of survival of patients with idiopathic dilated cardiomyopathy according to HRC genotype.
Kaplan–Meier plots of life-threatening ventricular arrhythmic events including sudden cardiac death and episodes of unstable VT (>180 b.p.m.) or ventricular fibrillation (A) and cardiac death from any cause, including pump failure, transplantation, sudden cardiac death, and episodes of unstable VT (>180 b.p.m.) or ventricular fibrillation (B). Arrhythmic events and cardiac death events were recorded by an implantable cardioverter-defibrillator device. Each trace signifies the HRC genotype at amino acid 96 for that patient group, and each event (life-threatening arrhythmia (A) or cardiac death (B)) is depicted as a step down. Each censored case (patient withdrawal due to other causes except sudden cardiac death, heart transplantation, and study termination (A) and death from non-cardiac etiology and study termination (B)) is marked with a cross. The table at the bottom of the plots indicates the number of dilated cardiomyopathy patients at risk for each year of follow-up study. The Ala/Ala homozygotes for the Ser96Ala polymorphism were statistically more susceptible to ventricular arrhythmic events, compared with Ser/Ala heterozygotes and Ser/Ser homozygotes. Modified from Arvanitis et al. 2008).
Genetic models of HRC
Little is known about the effects of HRC ablation on Ca2+ cycling either in vitro or in vivo. Mice lacking HRC had normal basal cardiac function, but they displayed elevated hypertrophy in response to isoproterenol relative to WTs (Jaehnig et al. 2006). HRC knockout mice also displayed a compensatory upregulation of triadin (Jaehnig et al. 2006). Of note, HRC knockout mice had reduced skeletal and fat mass, beginning at approximately 1 year of age (Jaehnig et al. 2006). The relation of loss of HRC to impaired skeletal weight gain is not known, although it may be linked to alterations in cell metabolism (Jaehnig et al. 2006). Future studies using this mouse model may further characterize the effects of HRC on SR Ca2+ cycling.
There is more literature available investigating the effects of elevated HRC on cardiac function. Acute overexpression of HRC in adult rat cardiomyocytes increased SR Ca2+ load, but cells exhibited diminished Ca2+ release (Fan et al. 2004). This increase in Ca2+ load was also reported in neonatal rat cardiomyocytes (Kim et al. 2003). HRC overexpression in vitro translated to impaired cardiomyocyte contractility and Ca2+ kinetics both under basal conditions and upon isoproterenol stimulation (Fan et al. 2004). Both triadin and junctin were increased, while other Ca2+ handling proteins (SERCA, PLN, RyR, CSQ) were unaltered (Fan et al. 2004). These findings suggest that HRC is a player in SR Ca2+ cycling and even acute overexpression may elicit compensatory changes in the expression of other SR proteins.
In contrast to in vitro studies, cardiac specific overexpression of HRC in mice had no effect on SR Ca2+ content, but it reduced Ca2+ uptake and slowed Ca2+ decay (Gregory et al. 2006). Triadin and Na+–Ca2+ exchanger protein levels were elevated; however, Na+–Ca2+ exchange activity was decreased possibly to maintain SR Ca2+ load in the face of reduced SERCA activity (Gregory et al. 2006). LTCC current density was increased, while protein levels were maintained (Gregory et al. 2006). The slowed Ca2+ decay was most likely a result of the compromised SERCA activity and reduced Na+–Ca2+ exchange. This translated to depressed in vivo cardiac function and hypertrophy in HRC transgenic mice, which progressed to heart failure upon ageing or pressure overload stress (Gregory et al. 2006).
While overexpression of HRC appeared to compromise cardiac function, it seemed to be protective against ischaemic stress both in vivo and ex vivo, associated with reduced infarct size and improved contractility after reperfusion (Zhou et al. 2007). The ratio of Bcl-2/Bax was elevated upon reperfusion, and caspase activation was reduced (Zhou et al. 2007). This cardioprotection could be conferred by reduced apoptosis and necrosis, which, due to attenuated SR Ca2+ uptake, decreased oscillations in SR Ca2+ release and reduced mitochondrial Ca2+ load (Zhou et al. 2007).
HRC perspective
Genetically engineered animal models show that HRC may have a significant effect on cardiac function and the development of heart failure. The incidence of arrhythmias has not been investigated in these mouse models, but this is of interest, given the genetic linkage of Ser96Ala polymorphism in HRC to human ventricular arrhythmias. HRC provides a unique component to the regulation of Ca2+ cycling due to its interaction with the RyR via triadin and SERCA; however, its effect on the activity of each protein is unclear. Studies in HRC overexpression mice by Gregory et al. (2006) suggest that the ratio of HRC to SERCA may be important for SERCA function, as HRC can bind near the cation transporter domain (Arvanitis et al. 2007). In heart failure, the ratio of HRC to SERCA is increased (Dash et al. 2001; Fan et al. 2004), and may partially explain depressed Ca2+ cycling as supported by HRC transgenic mice (Gregory et al. 2006). The effect of HRC on RyR activity is not known and should be examined in future studies utilizing HRC mouse models. Because of its potential dual regulation, HRC is an attractive molecule yet a difficult target to assess due to the number of other players involved. Elucidating the exact role of the HRC interactions with SERCA and triadin will be central in our understanding of the mechanisms by which HRC regulates SR Ca2+-uptake and release under physiological and pathophysiological conditions.
Summary
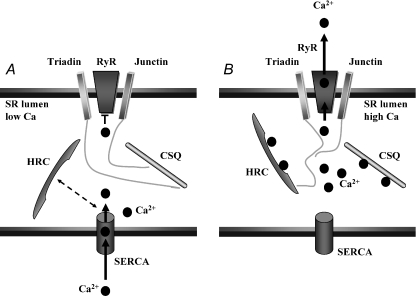
Junctin and HRC have been identified to be mediators of SR Ca2+ cycling. Figure 4 illustrates how these proteins may influence Ca2+ cycling under low and high Ca2+ levels in the SR lumen. When the SR Ca2+ levels are low, HRC interacts with SERCA (Arvanitis et al. 2007) and may limit the rate at which Ca2+ is sequestered into the SR, as HRC overexpression mice displayed depressed SR Ca2+ uptake (Gregory et al. 2006). Under these conditions, JCN and CSQ can interact (Zhang et al. 1997; Shin et al. 2000), limiting the open probability of the RyR (Györke et al. 2004). When SR Ca2+ levels are high, HRC can bind triadin (Arvanitis et al. 2007) and potentially regulate RyR activity. Likewise, the association of JCN and CSQ will be reduced, possibly facilitating open probability of the RyR.
Figure 4. Proposed protein interactions in the SR lumen under low and high SR Ca2+.
A, when the SR Ca2+ levels are low, maximal interaction may occur between HRC and SERCA thereby mediating the rate of SR Ca2+ uptake, while interaction between JCN and CSQ reduces Ca2+ leak through the RyR. B, when the SR Ca2+ content is high, HRC dissociates from SERCA and promotes interaction with triadin, which may simultaneously affect Ca2+ uptake and RyR activity. Junctin interaction with CSQ is reduced, possibly facilitating Ca2+ leak through the RyR.
Experimental findings to date emphasize the importance for understanding the functional roles that these proteins play in Ca2+ cycling and their association to heart failure. Further biochemical and physiological assessment of these proteins in the normal cardiomyocyte and under pathophysiological conditions may lead to additional and improved treatments for heart failure and in particular the associated ventricular arrhythmias.
Acknowledgments
This work was supported by NIH grants HL 26057, HL 64018, HL 77101 (to E.G.K.), NIH training grant T32 HL 007382, and the Leducq Foundation.
Glossary
Abbreviations
- ACE
angiotensin-converting enzyme
- CSQ
calsequestrin
- HRC
histidine-rich Ca2+ binding protein
- JCN
junctin
- LTCC
L-type Ca2+ channel
- PLN
phospholamban
- RyR
ryanodine receptor
- SERCA
sarcoplasmic reticulum Ca2+-ATPase
- SR
sarcoplasmic reticulum
References
- Arvanitis DA, Sanoudou D, Kolokathis F, Vafiadaki E, Papalouka V, Kontrogianni-Konstantopoulos A, Theodorakis GN, Paraskevaidis IA, Adamopoulos S, Dorn GW, 2nd, Kremastinos DT, Kranias EG. The Ser96Ala variant in histidine-rich calcium-binding protein is associated with life-threatening ventricular arrhythmias in idiopathic dilated cardiomyopathy. Eur Heart J. 2008;29:2514–2525. doi: 10.1093/eurheartj/ehn328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis DA, Vafiadaki E, Fan GC, Mitton BA, Gregory KN, Del Monte F, Kontrogianni-Konstantopoulos A, Sanoudou D, Kranias EG. Histidine-rich Ca-binding protein interacts with sarcoplasmic reticulum Ca-ATPase. Am J Physiol Heart Circ Physiol. 2007;293:H1581–H1589. doi: 10.1152/ajpheart.00278.2007. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and β-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- Chu G, Kranias EG. Phospholamban as a therapeutic modality in heart failure. Novartis Found Symp. 2006;274:156–171. [PubMed] [Google Scholar]
- Damiani E, Margreth A. Subcellular fractionation to junctional sarcoplasmic reticulum and biochemical characterization of 170 kDa Ca2+- and low-density-lipoprotein-binding protein in rdefinitionpairit skeletal muscle. Biochem J. 1991;277:825–832. doi: 10.1042/bj2770825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E, Tobaldin G, Bortoloso E, Margreth A. Functional behaviour of the ryanodine receptor/Ca2+-release channel in vesiculated derivatives of the junctional membrane of terminal cisternae of rdefinitionpairit fast muscle sarcoplasmic reticulum. Cell Calcium. 1997;22:129–150. doi: 10.1016/s0143-4160(97)90113-5. [DOI] [PubMed] [Google Scholar]
- Dash R, Frank KF, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium. J Mol Cell Cardiol. 2001;33:1345–1353. doi: 10.1006/jmcc.2001.1394. [DOI] [PubMed] [Google Scholar]
- Dinchuk JE, Henderson NL, Burn TC, Huber R, Peng Ho SP, Link J, O’Neil KT, Focht RJ, Scully MS, Hollis JM, Hollis GF, Friedman PA. Aspartyl β-hydroxylase (Asph) and an evolutionary conserved isoform of Asph missing the catalytic domain share exons with junctin. J Biol Chem. 2000;275:39543–39554. doi: 10.1074/jbc.M006753200. [DOI] [PubMed] [Google Scholar]
- Engelhardt S, Boknik P, Keller U, Neumann J, Lohse MJ, Hein L. Early impairment of calcium handling and altered expression of junctin in hearts of mice overexpressing the β1-adrenergic receptor. FASEB J. 2001;15:2718–2720. doi: 10.1096/fj.01-0107fje. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol Cell Physiol. 1983;14:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fan GC, Gregory KN, Zhao W, Park WJ, Kranias EG. Regulation of myocardial function by histidine-rich calcium-binding protein. Am J Physiol Heart Circ Physiol. 2004;287:H1705–H1711. doi: 10.1152/ajpheart.01211.2003. [DOI] [PubMed] [Google Scholar]
- Fan GC, Yuan Q, Zhao W, Chu G, Kranias EG. Junctin is a prominent regulator of contractility in cardiomyocytes. Biochem Biophys Res Commun. 2007;352:617–622. doi: 10.1016/j.bbrc.2006.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feriotto G, Finotti A, Breveglieri G, Treves S, Zorzato F, Gambari R. Transcriptional activity and Sp 1/3 transcription factor binding to the P1 promoter sequences of the human AβH-J-J locus. FEBS J. 2007;274:4476–4490. doi: 10.1111/j.1742-4658.2007.05976.x. [DOI] [PubMed] [Google Scholar]
- Francis GS. Pathophysiology of chronic heart failure. Am J Med. 2001;110:37S–46S. doi: 10.1016/s0002-9343(98)00385-4. [DOI] [PubMed] [Google Scholar]
- George CH. Sarcoplasmic reticulum Ca2+ leak in heart failure: mere observation or functional relevance? Cardiovasc Res. 2008;77:302–314. doi: 10.1093/cvr/cvm006. [DOI] [PubMed] [Google Scholar]
- Gergs U, Berndt T, Buskase J, Jones LR, Kirchhefer U, Müller FU, Schlüter KD, Schmitz W, Neumann J. On the role of junctin in cardiac Ca2+ handling, contractilty, and heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H728–H734. doi: 10.1152/ajpheart.01187.2006. [DOI] [PubMed] [Google Scholar]
- Gregory KN, Ginsburg KS, Bodi I, Hahn H, Marreez YM, Song Q, Padmanabhan PA, Mitton BA, Waggoner JR, Del Monte F, Jin Park W, Dorn GW, 2nd, Bers DM, Kranias EG. Histidine-rich Ca binding protein: a regulator of sarcoplasmic reticulum calcium sequestration and cardiac function. J Mol Cell Cardiol. 2006;40:653–665. doi: 10.1016/j.yjmcc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Györke I, Hester N, Jones LR, Györke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadad N, Meyer HE, Varsanyi M, Fleischer S, Shoshan-Barmatz V. Cardiac sarcalumenin: phosphorylation, comparison with the skeletal muscle sarcalumenin and modulation of ryanodine receptor. J Membr Biol. 1999;170:39–49. doi: 10.1007/s002329900536. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002;34:951–969. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- Hofmann SL, Brown MS, Lee E, Pathak RK, Anderson RG, Goldstein JL. Purification of a sarcoplasmic reticulum protein that binds Ca2+ and plasma lipoproteins. J Biol Chem. 1989a;264:8260–8270. [PubMed] [Google Scholar]
- Hofmann SL, Goldstein JL, Orth K, Moomaw CR, Slaughter CA, Brown MS. Molecular cloning of a histidine-rich Ca2+-binding protein of sarcoplasmic reticulum that contains highly conserved repeated elements. J Biol Chem. 1989b;264:18083–18090. [PubMed] [Google Scholar]
- Hong CS, Cho MC, Kwak YG, Song CH, Lee YH, Lim JS, Kwon YK, Chae SW, Kim DH. Cardiac remodelling and atrial fibrillation in transgenic mice overexpressing junctin. FASEB J. 2002;16:1310–1312. doi: 10.1096/fj.01-0908fje. [DOI] [PubMed] [Google Scholar]
- Jaehnig EJ, Heidt AB, Greene SB, Cornelissen I, Black BL. Increased susceptibility to isoproterenol-induced cardiac hypertrophy and impaired weight gain in mice lacking the histidine-rich calcium-binding protein. Mol Cell Biol. 2006;26:9315–9326. doi: 10.1128/MCB.00482-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse MJ. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res. 2004;61:208–217. doi: 10.1016/j.cardiores.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Jones LR, Zhang L, Sanborn K, Jorgensen AO, Kelley J. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J Biol Chem. 1995;270:30787–30796. doi: 10.1074/jbc.270.51.30787. [DOI] [PubMed] [Google Scholar]
- Kim E, Shin DW, Hong CS, Jeong D, Kim DH, Park WJ. Increased Ca2+ storage capacity in the sarcoplasmic reticulum by overexpression of HRC (histidine-rich Ca2+ binding protein) Biochem Biophys Res Commun. 2003;300:192–196. doi: 10.1016/s0006-291x(02)02829-2. [DOI] [PubMed] [Google Scholar]
- Kirchhefer U, Hanske G, Jones LR, Justus I, Kaestner L, Lipp P, Schmitz W, Neumann J. Overexpression of junctin causes adaptive changes in cardiac myocyte Ca2+ signalling. Cell Calcium. 2006;39:131–142. doi: 10.1016/j.ceca.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Kirchhefer U, Neumann J, Bers DM, Buchwalow IB, Fabritz L, Hanske G, Justus I, Riemann B, Schmitz W, Jones LR. Impaired relaxation in transgenic mice overexpressing junctin. Cardiovasc Res. 2003;59:369–379. doi: 10.1016/s0008-6363(03)00432-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi YM, Alseikhan BA, Jones LR. Localization and characterization of the calsequestrin-binding domain of triadin 1Evidence for a charged β-strand in mediating the protein-protein interaction. J Biol Chem. 2000;275:17639–17646. doi: 10.1074/jbc.M002091200. [DOI] [PubMed] [Google Scholar]
- Lee HG, Kang H, Kim DH, Park WJ. Interaction of HRC (histidine-rich Ca2+ binding protein) and triadin in the lumen of sarcoplasmic reticulum. J Biol Chem. 2001;276:39533–39538. doi: 10.1074/jbc.M010664200. [DOI] [PubMed] [Google Scholar]
- Lim KY, Song CS, Kim DH. cDNA cloning and characterization of human cardiac junctin. Gene. 2000;255:35–42. doi: 10.1016/s0378-1119(00)00299-7. [DOI] [PubMed] [Google Scholar]
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- Mitchell RD, Simmerman HK, Jones LR. Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J Biol Chem. 1988;263:1376–1381. [PubMed] [Google Scholar]
- Pathak RK, Anderson RG, Hofmann SL. Histidine-rich calcium binding protein, a sarcoplasmic reticulum protein of striated muscle, is also abundant in arteriolar smooth muscle cells. J Muscle Res Cell Motil. 1992;13:366–76. doi: 10.1007/BF01766464. [DOI] [PubMed] [Google Scholar]
- Picello E, Damiani E, Margreth A. Low-affinity Ca2+-binding sites versus Zn2+-binding sites in histidine-rich Ca2+-binding protein of skeletal muscle sarcoplasmic reticulum. Biochem Biophys Res Commun. 1992;186:659–667. doi: 10.1016/0006-291x(92)90797-o. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual β-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A, for the COMET investigators Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- Sacchetto R, Damiani E, Turcato F, Nori A, Margreth A. Ca2+-dependent interaction of triadin with histidine-rich Ca2+-binding protein carboxyl-terminal region. Biochem Biophys Res Commun. 2001;289:1125–1134. doi: 10.1006/bbrc.2001.6126. [DOI] [PubMed] [Google Scholar]
- Sacchetto R, Turcato F, Damiani E, Margreth A. Interaction of triadin with histidine-rich Ca2+-binding protein at the triadic junction in skeletal muscle fibres. J Muscle Res Cell Motil. 1999;20:403–415. doi: 10.1023/a:1005580609414. [DOI] [PubMed] [Google Scholar]
- Shin DW, Ma J, Kim DH. The asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin. FEBS Lett. 2000;486:178–182. doi: 10.1016/s0014-5793(00)02246-8. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Orr I, Weil S, Meyer H, Varsanyi M, Heilmeyer LM. The identification of the phosphorylated 150/160-kDa proteins of sarcoplasmic reticulum, their kinase and their association with the ryanodine receptor. Biochim Biophys Acta. 1996;1283:89–100. doi: 10.1016/0005-2736(96)00079-x. [DOI] [PubMed] [Google Scholar]
- Suk JY, Kim YS, Park WJ. HRC (histidine-rich Ca2+ binding protein) resides in the lumen of sarcoplasmic reticulum as a multimer. Biochem Biophys Res Commun. 1999;263:667–671. doi: 10.1006/bbrc.1999.1432. [DOI] [PubMed] [Google Scholar]
- Treves S, Feriotto G, Moccagatta L, Gambari R, Zorzato F. Molecular cloning, expression, functional characterization, chromosomal localization, and gene structure of junctate, a novel integral calcium binding protein of sarco(endo)plasmic reticulum membrane. J Biol Chem. 2000;275:39555–39568. doi: 10.1074/jbc.M005473200. [DOI] [PubMed] [Google Scholar]
- Weisser-Thomas J, Piacentino V, 3rd, Gaughan JP, Margulies K, Houser SR. Calcium entry via Na/Ca exchange during the action potential directly contributes to contraction of failing human ventricular myocytes. Cardiovasc Res. 2003;57:974–985. doi: 10.1016/s0008-6363(02)00732-0. [DOI] [PubMed] [Google Scholar]
- Yano K, Zarain-Herzberg A. Sarcoplasmic reticulum calsequestrins: structural and functional properties. Mol Cell Biochem. 1994;135:61–70. doi: 10.1007/BF00925961. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Fan GC, Dong M, Altschafl B, Diwan A, Ren X, Hahn HH, Zhao W, Waggoner JR, Jones LR, Jones WK, Bers DM, Dorn GW, Wang HS, Valdivia HH, Chu G, Kranias EG. Sarcoplasmic reticulum calcium overloading in junctin deficiency enhances cardiac contractility but increases ventricular automaticity. Circulation. 2007;115:300–309. doi: 10.1161/CIRCULATIONAHA.106.654699. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]
- Zhou X, Fan G, Ren X, Waggoner JR, Gregory KN, Chen G, Jones WK, Kranias EG. Overexpression of histidine-rich Ca-binding protein protects against ischemia/reperfusion-induced cardiac injury. Cardiovasc Res. 2007;75:487–497. doi: 10.1016/j.cardiores.2007.04.005. [DOI] [PubMed] [Google Scholar]