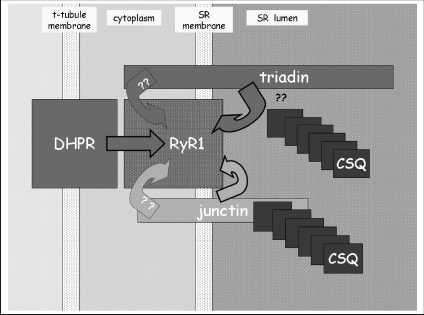
Recent advances in understanding skeletal and cardiac muscle function have evolved with recognition of the active role played by the intracellular sarcoplasmic reticulum (SR) Ca2+ store in contraction. The key proteins in this store are the Ca2+ binding protein calsequestrin (CSQ), the ryanodine receptor (RyR) Ca2+ release channel and triadin and junctin (Beard et al. 2004). The CSQ–triadin–junctin–RyR complex (Fig. 1) in the SR lumen forms a ‘Ca2+ transduction machine’ that is central to EC coupling and to normal muscle development. Other proteins in the lumen of the SR, including the histidine rich calcium binding protein (HRC) (Suk et al. 1999), JP-45 (Anderson et al. 2003) and SRP-27 (Bleunven et al. 2008), must also contribute to control of SR intraluminal Ca2+ load, but the precise nature of their role remains undetermined. JP-45 in particular is ideally placed to communicate store load to the excitation–contraction (EC) coupling process as it binds to both CSQ and the dihydropyridine receptor (DHPR) in the surface/transverse tubule membrane. The importance of the luminal proteins has been underlined by the recent discovery that changes in Ca2+ signalling due to mutations in CSQ or to lack of its expression can result in sudden cardiac death (Viatchenko-Karpinski et al. 2004). Furthermore, studies in animal models show that changes in CSQ, junctin, triadin and HRC expression can lead to defective Ca2+ signalling. The review by Pritchard & Kranius (2009) examines the roles of junctin and HRC in Ca2+ cycling and their potential significance in heart failure. This commentary focuses on protein-protein interactions between CSQ, triadin, junctin and RyR proteins that may underlie their roles in Ca2+ cycling.
Figure 1. Illustration of the possible interactions between triadin, junctin, CSQ1 and RyR1 in the lumen of the SR.
The question marks near CSQ and triadin indicate the uncertainty about simultaneous binding of CSQ1 and RyR1 to triadin. Question marks on the cytoplasmic side indicate the unknown nature of the cytoplasmic interaction between triadin and RyR1 and whether or not there is a cytoplasmic interaction between junctin and RyR1.
A brief background of the RyR, CSQ, HRC, junctin and triadin
Three RyR genes in mammals are the skeletal RyR1, the cardiac RyR2 and RyR3 first identified in brain. RyR1 in skeletal muscle has cleverly recruited the DHPR as a surface membrane voltage sensor and CSQ as an SR Ca2+ sensor as reviewed elsewhere (Dulhunty et al. 2002). Two CSQ genes encode type 1 CSQ (CSQ1), expressed in fast and slow twitch skeletal muscle, and type 2 CSQ (CSQ2) expressed in slow-twitch skeletal and cardiac muscle. The properties of isolated CSQ1 and CSQ2 differ and their opposite effects on RyR1 and RyR2 channels respectively in lipid bilayers suggest that CSQ1 may help conserve SR Ca2+ in skeletal muscle, but facilitate emptying of the SR in the heart (Wei et al. 2009b). HRC has been studied in less detail, however; although it has more acidic clusters than CSQ and a higher Ca2+ binding capacity, it constitutes only 1% of SR protein (Hofmann et al. 1989; Arvanitis et al. 2007). Like CSQ, HRC is associated with the RyR protein complex through triadin (Lee et al. 2001; Sacchetto et al. 2001; Arvanitis et al. 2007) and binds directly to sarco(endo)plasmic reticulum Ca2+-ATPase type 2 (SERCA2) (Arvanitis et al. 2007).
Junctin and triadin are found in a range of tissues and thus may have a ubiquitous role in Ca2+ signalling. One isoform of junctin is expressed in all tissues and is a splice variant of the aspartate β-hydroxylase gene. Several isoforms of triadin are splice variants of one gene; the 94 kDa isoform binds to RyR1 in skeletal muscle and the 32 kDa isofrom binds to RyR2 in the heart. Junctin and triadin both bind to CSQ and RyRs (Jones et al. 1995; Goonasekera et al. 2007) and are thought to anchor CSQ close to the RyR. Both proteins contain a short cytoplasmic tail, one trans-SR membrane domain and a long luminal tail in the SR. These structural similarities suggested that the proteins may be functionally redundant. However, recent evidence indicates that their functions are in fact vastly different and that junctin plays a distinct and important role in Ca2+ homeostasis (see below).
The distinct role of skeletal junctin
The activity of RyR1 and RyR2 channels is increased after addition of either junctin and/or triadin (purified from skeletal muscle) to the luminal side of purified RyR1 channels in lipid bilayers (Goonasekera et al. 2007; Wei et al. 2009a). However, the skeletal proteins behave quite differently in the presence of CSQ1 (Beard et al. 2008; Wei et al. 2009a). The junctin–RyR1 complex is inhibited when CSQ1 is added to the luminal solution (containing 1 mm Ca2+), while the triadin–RyR1 complex is unaffected by luminal CSQ1 addition (Wei et al. 2009a). Equivalent studies of HRC interactions with junctin or triadin and effects on RyR activity have yet to be performed. Different roles of junctin and triadin are also seen in intact skeletal myotubes where disruption of the triadin–RyR1 association depresses EC coupling, while junctin (which remains bound to RyR1) is unable to support EC coupling (Goonasekera et al. 2007).
These separate functions of junctin and triadin are reflected in changes in Ca2+ signalling in skeletal C2C12 myotubes after triadin and/or junctin knockdown, which indicate that junctin plays a major role in SR Ca2+ load, while triadin assists in depolarization-induced Ca2+ release (Wang et al. 2009). A role of triadin in skeletal EC coupling is similarly observed when over-expression of triadin reduces depolarisation-dependent Ca2+ transients (Fodor et al. 2008). Conversely, pan-triadin knockout does not appear to affect EC coupling (Shen et al. 2007), but numerous compensatory changes could mask effects of triadin depletion (Goonasekera et al. 2007).
The fact that junctin is more involved than triadin in mediating Ca2+-dependent signals between CSQ1 and RyR1 (Goonasekera et al. 2007; Wei et al. 2009a; Wang et al. 2009) may be explained if CSQ1 and RyR1 bind to a single KEKE motif between residues 200 and 232 in the C-terminal tail of triadin (Kobayashi et al. 2000; Lee et al. 2004). Although this site was determined for CSQ2 binding to cardiac triadin, and skeletal triadin binding to RyR1, the same sites are present in each of the isoforms of the proteins and are likely to have the same function. It is yet to be determined whether CSQ can also bind to the extended C tail of skeletal triadin which contains five additional KEKE motifs and several other clusters of acidic residues. If the binding site is unique, either CSQ cannot regulate the RyR through triadin or dynamic swapping between triadin binding to CSQ and the RyR would be required for CSQ to have an influence on RyR. In contrast to triadin, at least two of the KEKE-like motifs in the C-tail of junctin are involved in its binding to CSQ, so that it may bind to CSQ and the RyR simultaneously and transmit signals between the proteins.
Junctin in the heart
As with the skeletal proteins, junctin and triadin each increase the activity of RyR2 channels in the absence of CSQ2 (Gyorke et al. 2004). The in vivo roles of junctin and triadin in the heart have been explored in knockdown, knockout and over-expression models. The results are difficult to interpret in terms of protein–protein interactions because the expression of other proteins and the regulatory links between CSQ2 and RyR2 can be affected even after transient knockdown (Wang et al. 2009). However the results again support a hypothesis that junctin and triadin have separate roles. Junctin knockout and knockdown lead to an enhancement of Ca2+ release (Fan et al. 2008), suggesting that either junctin or junctin–CSQ2 inhibit RyR2. Similarly, the reduced Ca2+ release in response to β-adrenergic stimulation with triadin overexpression (Kirchhefer et al. 2007), suggests an inhibitory role for triadin. In contrast, enhanced EC coupling (Terentyev et al. 2005) and caffeine-induced Ca2+ release (Kirchhefer et al. 2007) with triadin overexpression indicate an excitatory action of triadin. The role of triadin in skeletal muscle is further addressed in this issue in the speakers review by Marty et al. (2009) and the perspective by Allen (2009).
Cytoplasmic RyR–junctin interactions
An unexplored possibility is that the cytoplasmic N-terminal tail of junctin interacts with the cytoplasmic domain of RyR1 or RyR2 to allow junctin to regulate cytoplasmic functions of the RyR. A curious observation is that the cytoplasmic tail of triadin can modulate RyR1 activity by binding to the cytoplasmic domain of RyR1 (Ohkura et al. 1998; Groh et al. 1999). It is possible that this cytoplasmic interaction is involved in the influence of triadin on EC coupling.
In conclusion, despite the numerous gaps that remain in the CSQ–junctin–RyR story, the unfolding picture is one of a complex integration of signals arising from these proteins that regulates Ca2+ homeostasis in skeletal muscle and the heart. Yet to be addressed are the functional consequences of molecular interactions between HRC, triadin, junctin, CSQ and the RyR and the SR Ca2+ pump, which suggest that the protein may facilitate communication between the Ca2+ release and uptake function in the SR (Arvanitis et al. 2007).
References
- Allen PD. Triadin, not essential, but useful. J Physiol. 2009;587:3123–3124. doi: 10.1113/jphysiol.2009.172015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AA, Treves S, Biral D, Betto R, Sandona D, Ronjat M, Zorzato F. The novel skeletal muscle sarcoplasmic reticulum JP-45 proteinMolecular cloning, tissue distribution, developmental expression, and interaction with α1.1 subunit of the voltage-gated calcium channel. J Biol Chem. 2003;278:39987–39992. doi: 10.1074/jbc.M305016200. [DOI] [PubMed] [Google Scholar]
- Arvanitis DA, Vafiadaki E, Fan GC, Mitton BA, Gregory KN, Del Monte F, Kontrogianni-Konstantopoulos A, Sanoudou D, Kranias EG. Histidine-rich Ca-binding protein interacts with sarcoplasmic reticulum Ca-ATPase. Am J Physiol Heart Circ Physiol. 2007;293:H1581–1589. doi: 10.1152/ajpheart.00278.2007. [DOI] [PubMed] [Google Scholar]
- Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004;85:33–69. doi: 10.1016/j.pbiomolbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Beard NA, Wei L, Cheung SN, Kimura T, Varsanyi M, Dulhunty AF. Phosphorylation of skeletal muscle calsequestrin enhances its Ca2+ binding capacity and promotes its association with junctin. Cell Calcium. 2008;44:363–373. doi: 10.1016/j.ceca.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bleunven C, Treves S, Jinyu X, Leo E, Ronjat M, De Waard M, Kern G, Flucher BE, Zorzato F. SRP-27 is a novel component of the supramolecular signalling complex involved in skeletal muscle excitation-contraction coupling. Biochem J. 2008;411:343–349. doi: 10.1042/BJ20070906. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Haarmann CS, Green D, Laver DR, Board PG, Casarotto MG. Interactions between dihydropyridine receptors and ryanodine receptors in striated muscle. Prog Biophys Mol Biol. 2002;79:45–75. doi: 10.1016/s0079-6107(02)00013-5. [DOI] [PubMed] [Google Scholar]
- Fan GC, Yuan Q, Kranias EG. Regulatory roles of junctin in sarcoplasmic reticulum calcium cycling and myocardial function. Trends Cardiovasc Med. 2008;18:1–5. doi: 10.1016/j.tcm.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor J, Gonczi M, Sztretye M, Dienes B, Olah T, Szabo L, Csoma E, Szentesi P, Szigeti GP, Marty I, Csernoch L. Altered expression of triadin 95 causes parallel changes in localized Ca2+ release events and global Ca2+ signals in skeletal muscle cells in culture. J Physiol. 2008;586:5803–5818. doi: 10.1113/jphysiol.2008.160457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonasekera SA, Beard NA, Groom L, Kimura T, Lyfenko AD, Rosenfeld A, Marty I, Dulhunty AF, Dirksen RT. Triadin binding to the C-terminal luminal loop of the ryanodine receptor is important for skeletal muscle excitation contraction coupling. J Gen Physiol. 2007;130:365–378. doi: 10.1085/jgp.200709790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh S, Marty I, Ottolia M, Prestipino G, Chapel A, Villaz M, Ronjat M. Functional interaction of the cytoplasmic domain of triadin with the skeletal ryanodine receptor. J Biol Chem. 1999;274:12278–12283. doi: 10.1074/jbc.274.18.12278. [DOI] [PubMed] [Google Scholar]
- Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SL, Goldstein JL, Orth K, Moomaw CR, Slaughter CA, Brown MS. Molecular cloning of a histidine-rich Ca2+-binding protein of sarcoplasmic reticulum that contains highly conserved repeated elements. J Biol Chem. 1989;264:18083–18090. [PubMed] [Google Scholar]
- Jones LR, Zhang L, Sanborn K, Jorgensen AO, Kelley J. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J Biol Chem. 1995;270:30787–30796. doi: 10.1074/jbc.270.51.30787. [DOI] [PubMed] [Google Scholar]
- Kirchhefer U, Klimas J, Baba HA, Buchwalow IB, Fabritz L, Huls M, Matus M, Muller FU, Schmitz W, Neumann J. Triadin is a critical determinant of cellular Ca cycling and contractility in the heart. Am J Physiol Heart Circ Physiol. 2007;293:H3165–3174. doi: 10.1152/ajpheart.00799.2007. [DOI] [PubMed] [Google Scholar]
- Kobayashi YM, Alseikhan BA, Jones LR. Localization and characterization of the calsequestrin-binding domain of triadin 1. Evidence for a charged β-strand in mediating the protein-protein interaction. J Biol Chem. 2000;275:17639–17646. doi: 10.1074/jbc.M002091200. [DOI] [PubMed] [Google Scholar]
- Lee HG, Kang H, Kim DH, Park WJ. Interaction of HRC (histidine-rich Ca2+-binding protein) and triadin in the lumen of sarcoplasmic reticulum. J Biol Chem. 2001;276:39533–39538. doi: 10.1074/jbc.M010664200. [DOI] [PubMed] [Google Scholar]
- Lee JM, Rho SH, Shin DW, Cho C, Park WJ, Eom SH, Ma J, Kim DH. Negatively charged amino acids within the intraluminal loop of ryanodine receptor are involved in the interaction with triadin. J Biol Chem. 2004;279:6994–7000. doi: 10.1074/jbc.M312446200. [DOI] [PubMed] [Google Scholar]
- Marty I, Fauré J, Fourest-Lieuvin A, Vassilopoulos S, Oddoux S, Brocard J. Triadin: what possible function 20 years later? J Physiol. 2009;587:3117–3121. doi: 10.1113/jphysiol.2009.171892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura M, Furukawa K, Fujimori H, Kuruma A, Kawano S, Hiraoka M, Kuniyasu A, Nakayama H, Ohizumi Y. Dual regulation of the skeletal muscle ryanodine receptor by triadin and calsequestrin. Biochemistry. 1998;37:12987–12993. doi: 10.1021/bi972803d. [DOI] [PubMed] [Google Scholar]
- Pritchard TJ, Kranias EG. Junctin and the histidine-rich Ca2+ binding protein: potential roles in heart failure and arrhythmogenesis. J Physiol. 2009;587:3125–3133. doi: 10.1113/jphysiol.2009.172171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetto R, Damiani E, Turcato F, Nori A, Margreth A. Ca2+-dependent interaction of triadin with histidine-rich Ca2+-binding protein carboxyl-terminal region. Biochem Biophys Res Commun. 2001;289:1125–1134. doi: 10.1006/bbrc.2001.6126. [DOI] [PubMed] [Google Scholar]
- Shen X, Franzini-Armstrong C, Lopez JR, Jones LR, Kobayashi YM, Wang Y, Kerrick WG, Caswell AH, Potter JD, Miller T, Allen PD, Perez CF. Triadins modulate intracellular Ca2+ homeostasis but are not essential for excitation-contraction coupling in skeletal muscle. J Biol Chem. 2007;282:37864–37874. doi: 10.1074/jbc.M705702200. [DOI] [PubMed] [Google Scholar]
- Suk JY, Kim YS, Park WJ. HRC (histidine-rich Ca2+ binding protein) resides in the lumen of sarcoplasmic reticulum as a multimer. Biochem Biophys Res Commun. 1999;263:667–671. doi: 10.1006/bbrc.1999.1432. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Cala SE, Houle TD, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, Williams SC, Gyorke S. Triadin overexpression stimulates excitation-contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes. Circ Res. 2005;96:651–658. doi: 10.1161/01.RES.0000160609.98948.25. [DOI] [PubMed] [Google Scholar]
- Viatchenko-Karpinski S, Terentyev D, Gyorke I, Terentyeva R, Volpe P, Priori SG, Napolitano C, Nori A, Williams SC, Gyorke S. Abnormal calcium signalling and sudden cardiac death associated with mutation of calsequestrin. Circ Res. 2004;94:471–477. doi: 10.1161/01.RES.0000115944.10681.EB. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li X, Duan H, Fulton TR, Eu JP, Meissner G. Altered stored calcium release in skeletal myotubes deficient of triadin and junctin. Cell Calcium. 2009;45:29–37. doi: 10.1016/j.ceca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Gallant EM, Dulhunty AF, Beard NA. Junctin and triadin activate skeletal ryanodine receptors; junctin alone mediates functional interactions with calsequestrin. Int J Biochem Cell Biol. 2009a doi: 10.1016/j.biocel.2009.04.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Hanna AD, Beard NA, Dulhunty AF. Unique isoform-specifc properties of calsequestrin in the heart and skeletal muscle. Cell Calcium. 2009b doi: 10.1016/j.ceca.2009.03.006. in press DOI 10.1016/j.ceca.2009.1003.1006. [DOI] [PubMed] [Google Scholar]