Abstract
It has long been known that skeletal muscle contraction persists in the absence of extracellular Ca2+. Nevertheless, recent evidence indicates that multiple distinct Ca2+ entry pathways exist in skeletal muscle: one active at negative potentials that requires store depletion (store-operated calcium entry or SOCE) and a second that is independent of store depletion and is activated by depolarization (excitation-coupled calcium entry or ECCE). This review highlights recent findings regarding the molecular identity, subcellular localization, and inter-relationship between SOCE and ECCE in skeletal muscle. The respective roles of ryanodine receptors (RyRs), dihydropyridine receptors (DHPRs), inositol-1,4,5-trisphosphate receptors (IP3Rs), canonical transient receptor potential channels (TRPCs), STIM1 Ca2+ sensor proteins, and Orai1 Ca2+ permeable channels in mediating SOCE and ECCE in skeletal muscle are discussed. Differences between SOCE and ECCE in skeletal muscle with Ca2+ entry mechanisms in non-excitable cells are also reviewed. Finally, potential physiological roles for SOCE and ECCE in skeletal muscle development and function, as well as other currently unanswered questions and controversies in the field are also considered.
Skeletal muscle contraction and relaxation are controlled by the precise temporal delivery and removal of myoplasmic Ca2+. During excitation–contraction (EC) coupling in skeletal muscle, an action potential initiated at the neuromuscular junction rapidly propagates down the surface and transverse tubule (t-tubule) membranes and induces voltage-driven conformational changes in the t-tubule dihydropyridine receptor (DHPR) or voltage sensor that triggers the opening of ryanodine receptor (RYR1) channels to release Ca2+ stored in the terminal cisternae of the sarcoplasmic reticulum (SR) (Melzer et al. 1995). Muscle relaxation results from termination of release following membrane repolarization and subsequent resequestration of myoplasmic Ca2+ back into the SR via sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) pumps. As a result, skeletal muscle contraction is orchestrated by highly coordinated mechanisms that control SR Ca2+ release and reuptake (Melzer et al. 1995).
Ever since the original work of Armstrong et al. (1972) over three decades ago, it has become widely appreciated that twitch contractions of skeletal muscle persist in the complete absence of extracellular calcium. Subsequent work revealed that activation of SR Ca2+ release during skeletal muscle EC coupling arises from a direct mechanical ‘outside-in’ conformational coupling mechanism resulting from voltage-driven changes in DHPR voltage sensors that are transmitted to RyR1 Ca2+ release channels in the SR (Melzer et al. 1995). Importantly, unlike cardiac muscle, the DHPR–RyR1 conformational coupling mechanism in skeletal muscle is completely independent of the entry of extracellular Ca2+. Specifically, depolarization-induced SR Ca2+ release in muscle persists following removal of external Ca2+ (Armstrong et al. 1972) or blockade of Ca2+ influx with either diltiazem (Gonzalez-Serratos et al. 1982) or Cd2+ and La3+ (Nakai et al. 1996), and is unaffected by a mutation in the DHPR pore that abolishes Ca2+ permeation though the channel (Dirksen & Beam, 1999).
While clearly serving the essential function as the voltage sensor for mechanical activation of RyR1-mediated Ca2+ release during EC coupling, the skeletal muscle DHPR is also a slowly activating voltage-gated L-type Ca2+ channel (L-channel). Indeed, mechanical coupling between the DHPR and RyR1 in skeletal muscle represents a bi-directional interaction as both the DHPR controls RyR1-mediated SR Ca2+ release (orthograde coupling) and RyR1 channels in turn influence the Ca2+ conducting properties of the DHPR (retrograde coupling) (Nakai et al. 1996). However, as noted above, a physiological role for Ca2+ entry through the DHPR channel pore has largely been dismissed as Ca2+ entry is not required for skeletal muscle twitch contraction and the kinetics of L-channel activation (∼50–100 ms) is too slow to permit significant Ca2+ influx during a brief (2–5 ms) skeletal muscle action potential.
In spite of the well-accepted mechanism of bi-directional mechanical DHPR–RyR1 communication during skeletal muscle EC coupling, the recent identification of two apparently distinct trans-sarcolemmal Ca2+ entry mechanisms in skeletal muscle has served to reinvigorate the field by potentially challenging previously accepted paradigms. Kurebayashi & Ogawa (2001) identified a store-operated Ca2+ entry (SOCE) mechanism in adult rat EDL muscle fibres capable of replenishing previously depleted SR Ca2+ stores ∼2 min following the reintroduction of extracellular Ca2+. Three years later, Cherednichenko et al. (2004) identified a store-independent Ca2+ entry pathway that is activated be repetitive or prolonged depolarization (excitation-coupled Ca2+ entry or ECCE). The discovery of these two distinct Ca2+ entry pathways stimulated subsequent work designed to elucidate their respective molecular components and activation mechanisms. In addition, the identification of the SOCE and ECCE pathways in skeletal muscle also served to renew efforts to determine the potential role(s) of trans-sarcolemmal Ca2+ flux in skeletal muscle in ensuring store repletion (Kurebayashi & Ogawa, 2001), limiting fatigue resistance (Pan et al. 2002), stimulating NFAT transactivation (Rosenberg et al. 2004; Stiber et al. 2008), promoting muscle differentiation (Darbellay et al. 2009), and modifying muscle disease (Vandebrouck et al. 2002; Wang et al. 2005; Cherednichenko et al. 2008). This review provides a critical evaluation of recent work directed at elucidating the molecular identity, subcellular localization, and inter-relationship between SOCE and ECCE in skeletal muscle. In addition, several unanswered questions, gaps in knowledge, and areas of ongoing controversy are also discussed in the hope of stimulating further debate and future potential lines of investigation.
Proposed molecular models for SOCE in skeletal muscle
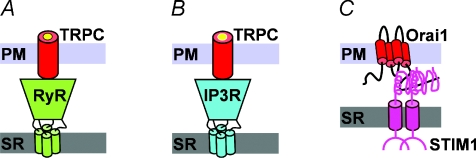
The identification of SOCE in adult mammalian muscle by Kurebayashi & Ogawa (2001) stimulated a series of studies designed to identify the physiological role and molecular components of SOCE in skeletal muscle. Prior results in non-muscle cells demonstrated that activation of store-operated Ca2+ channels (SOCCs) occurs following Ca2+ release from both IP3-sensitive (Kiselyov et al. 1998) and RyR-sensitive (Bennett et al. 1998) stores. Extracellular Ca2+ entry through SOCCs in HEK293 cells activated following caffeine-induced Ca2+ release is significantly increased following transient expression of RyR1 (Tong et al. 1999). Subsequently, Kiselyov et al. (2000) concluded that store-dependent human conical transient receptor potential type 3 (hTRPC3) channels are activated by conformational coupling to RyR channels. Together, these results suggested that SOCE in skeletal muscle might also be mediated by conformational coupling between RyR1 and TRPC channels in the t-tubule membrane (Fig. 1A). Indeed, the magnitude of SOCE is reduced in skeletal myotubes derived from RyR1/RyR3 deficient mice (Pan et al. 2002). Subsequent studies identified a potential role for conformational coupling between RyR1 and TRPC channels in skeletal muscle (Vandebrouck et al. 2002; Rosenberg et al. 2004) and that azumolene, an analog of dantrolene that binds RyR1, inhibits a component of SOCE that is coupled to RyR1 activation (Zhao et al. 2006). However, as a SOCE in myotubes persists even in the absence of RyRs (Pan et al. 2002; Lyfenko & Dirksen, 2008), the ryanodine receptor is clearly not a required or essential component of the SOCE pathway in muscle.
Figure 1. Proposed molecular models for SOCE in skeletal muscle.
A, conformational coupling between the ryanodine receptor (RyR) and canonical transient receptor potential (TRPC) channels. B, conformational coupling between inositol-1,4,5-trisphosphate (IP3) receptors and TRPC channels. C, conformational coupling between ER/SR stromal interaction molecule 1 (STIM1) Ca2+ sensor proteins and Ca2+ permeable Orai1 channels. For clarity, only one Orai1 subunit is shown.
In an elegant study conducted in toad skeletal muscle fibres, Launikonis et al. (2003) provided evidence for conformational coupling between IP3 receptors and store-operated Ca2+ entry channels located in the T-tubule membrane (Fig. 1B). In this study, SOCE was quantified following store depletion by assessing a reduction in the fluorescence of a low affinity Ca2+ dye (Fluo-5N) which was trapped within the t-tubule system following mechanical skinning. Importantly, activation of SOCCs in this assay was markedly reduced following inhibition of IP3 receptors with heparin, a potent IP3 receptor competitive antagonist, which could also be reversed by IP3 (Launikonis et al. 2003). A similar conformational coupling between IP3 receptors and SOCCs (presumably TRPC3 channels) was subsequently suggested to be activated in skeletal myotubes during testosterone-induced Ca2+ oscillations (Estrada et al. 2005). However, the role of IP3 receptors in activation of SOCE remains uncertain given the relatively low level of IP3R expression and preferential localization to the nuclear envelope rather than the SR terminal cisternae. Similarly, TRPC3 channels are unlikely to function as the SOCC in muscle since SOCE is unaffected by either TRPC3 knockdown (Lee et al. 2006) or transient expression of dominant negative TRPC3 or TRPC6 constructs (data not shown).
The demonstration over the past several years that stromal interaction molecule 1 (STIM1) is an ER Ca2+ sensor protein (Roos et al. 2005) that orchestrates store-dependent activation of Ca2+ selective Orai1 channels (Feske et al. 2006; Vig et al. 2006) in the plasma membrane has single-handedly transformed the field by enabling detailed molecular and mechanistic investigations of SOCE for the first time. These studies revealed that store depletion triggers STIM1 oligimerization, translocation to ER contact sites with the plasma membrane, and the subsequent interaction, clustering and activation of transmembrane Ca2+ influx through Orai1-containing channels (see below for details). Several observations provide strong evidence that a similar STIM1/Orai1 activation mechanism also underlies SOCE in skeletal muscle. First of all, both STIM1 and Orai1 are expressed at high levels in skeletal muscle and deficiency of either protein in mice results in the loss of SOCE and the development of myopathy (Stiber et al. 2008; Vig et al. 2008). Second, severe combined immunodeficiency (SCID) patients possessing a loss-of-function Orai1 mutation (R91W) also present with a significant skeletal muscle myopathy (Feske et al. 2006). Finally, we demonstrated using both Ca2+ entry and Mn2+ quench assays that SOCE in skeletal muscle myotubes is abolished following either STIM1 knockdown or expression of dominant negative Orai1 (E106Q), and markedly reduced following expression of a Ca2+ permeation defective Orai1 mutant (E190Q) (Lyfenko & Dirksen, 2008). Together, these studies provide compelling evidence that a similar STIM1/Orai1 mechanism that underlies SOCE in T-lymphocytes is also operative in skeletal muscle (Fig. 1C).
CRAC activation via STIM1–Orai1 conformational coupling in T-lymphocytes
Following its initial landmark description by Putney (1986), remarkably limited progress was made in defining the molecular mechanism for activation of SOCE (or ‘capacitative Ca2+ entry’, as originally termed) over the next 20 years. However, the recent identification of STIM1 as the ER Ca2+ sensor and Orai1 as the Ca2+ release activated Ca2+ (CRAC) channel in T-lymphocytes provided the molecular tools required to begin to unravel this long-standing mystery.
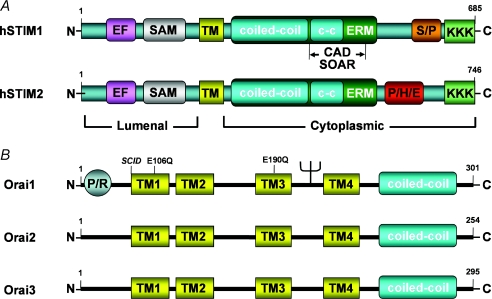
There are two human STIM homologues (STIM1 and STIM2), both of which contain a single transmembrane (TM) domain with an N-terminal luminal domain and a C-terminal cytosolic domain (Fig. 2A). While STIM1 is predominantly localized within the ER, it has also been reported to be expressed at lower levels in the plasma membrane (Zhang et al. 2005). The N-terminal region of STIM1/2 contains single Ca2+ binding EF hand (kd∼400 μm; Stathopulos et al. 2006) and sterile-α-motif (SAM) domains. With Ca2+ bound, the EF hand and SAM domains are folded together in a conformation that inhibits STIM aggregation (Stathopulos et al. 2006). Ca2+ dissociation from the EF hand domain results in a conformational change in the protein that unfolds the EF hand and SAM domains, thus permitting STIM aggregation (Stathopulos et al. 2006, 2008). The larger C-terminal region of STIM1 contains two adjacent coiled-coil (c-c) domains within an ezrin–radixin moesin (ERM) domain, as well as serine-proline (S/P)- and lysine-rich (KKK) domains. A similar structure is also observed for STIM2 except the S/P domain is replaced with a histidine-proline-glutamate-rich (P/H/E) domain. There are three human Orai homologues (Orai1–3), each exhibiting four conserved transmembrane domains, as well as cytosolic N- and C-termini (Fig. 2B). Orai1 also contains an N-terminal proline-arginine-rich (PR) domain, a glyosylation site between TM2 and TM3, and a C-terminal coiled-coil protein interaction domain that is required for interaction with STIM1, formation of ORAI1–STIM1 puncta and channel activation (Muik et al. 2009). While debate persists with regard to whether the CRAC channel at rest exists as a dimer (Penna et al. 2008) or tetramer (Ji et al. 2008), the activated CRAC channel is a functional tetramer of four Orai1 subunits (Mignen et al. 2008) in which conserved glutamate residues in TM1 (E106) and TM3 (E190) are required for Ca2+ permeation through the channel (Prakriya et al. 2006).
Figure 2. Structural features of human STIM and Orai proteins.
A, structural features of human STIM proteins. EF, Ca2+ binding EF hand domain; SAM, sterile-α-motif; TM, transmembrane domain; c-c, coiled coil domain; ERM, ezrin–radixin moesin domain; S/P serine-proline-rich domain; KKK, lysine-rich domain; CAD, channel activation domain; SOAR, STIM1–Orai activation region; P/H/E, proline-histidine-glutimate-rich domain. B, structural features of human Orai proteins. P/R, proline-arginine-rich domain; TM, transmembrane domain; c-c, coiled coil domain; SCID, severe combined immunodeficiency.
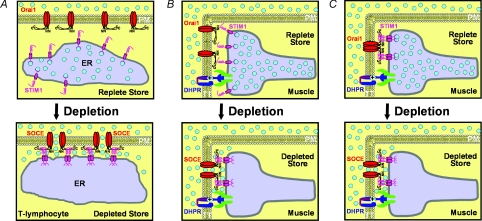
The discoveries of STIM1 as the ER Ca2+ sensor and Orai1 as the Ca2+ permeable CRAC channel has stimulated a rapidly evolving and remarkably detailed elucidation of the molecular mechanism for CRAC channel activation in non-excitable cells (Fig. 3A). Under resting conditions, when intracellular stores are replete with Ca2+, STIM1 proteins are diffusely distributed throughout the ER as STIM1 monomers are stabilized by Ca2+ binding to the EF hand motif. Store depletion results in Ca2+ unloading from the EF hand and subsequent oligomerization of STIM1 (Stathopulos et al. 2006; Liou et al. 2007; Muik et al. 2009). STIM1 oligomers then aggregate and redistribute into discrete puncta located in ER junctions 10–25 nm from the plasma membrane (Wu et al. 2006). Association with the plasma membrane within these puncta appears to involve an interaction between membrane phospholipids and the C-terminal polybasic domains of oligomerized STIM1 (Liou et al. 2007; Park et al. 2009). Orai1 proteins are then recruited into STIM1-containing puncta (Luik et al. 2006; Wu et al. 2006), where CRAC channel activation results from an interaction between a highly conserved C-terminal domain of STIM1 (termed the CRAC activation domain, CAD (Park et al. 2009) or STIM1 Orai activating region, SOAR (Yuan et al. 2009)) and the C-terminal coiled-coil domain of Orai1 (Muik et al. 2009). An incompletely resolved issue is the number of STIM1 proteins required for CRAC channel activation, though indirect evidence suggests that two to four STIM1 proteins are required for CRAC channel activation (Ji et al. 2008; Luik et al. 2008). The proposed role for CRAC activation resulting from a Ca2+ influx factor (CIF) released during store depletion (Bolotina, 2008) is inconsistent with the observations that both rapamycin-induced heterodimerization of STIM1 oligomers (Luik et al. 2008) and intracellular application CAD/SOAR peptides are sufficient alone to activate CRAC channels (Park et al. 2009) even in the absence of store depletion. Together, these results provide strong support for an ‘inside-out’ STIM1–Orai1 conformational coupling mechanism for CRAC channel activation.
Figure 3. Proposed models for activation of SOCE in T-lymphocytes and skeletal muscle.
A, model for activation of SOCE in T-lymphocytes. At rest (upper), Ca2+-bound STIM1 proteins are diffusely distributed in the ER and Orai1 proteins are randomly located in the plasma membrane (shown as non-functional dimer; Penna et al. 2008). Following store depletion (lower), Ca2+ unbinding from the STIM1 EF hand results in STIM1 oligomerization and redistribution into discrete puncta under the plasma membrane. Orai1 channels are recruited into these puncta and conformational coupling between STIM1 oligomers and tetrameric Orai1 channels results in activation of SOCE. B, proposed model for rapid activation of SOCE in skeletal muscle. At rest (upper), Ca2+-bound STIM1 proteins are pre-localized to the SR terminal cisternae and Orai1 proteins are located in the t-tubule membrane. Following store depletion (lower), Ca2+ unbinding from the STIM1 EF hand results in STIM1 oligomerization, recruitment of nearby Orai1 subunit, and conformational activation of SOCE. C, alternate proposed model for ultra-rapid activation of SOCE in skeletal muscle. At rest (upper), Ca2+-bound STIM1 proteins in the SR terminal cisternae are pre-bound to inactive tetrameric Orai1 channels located in the t-tubule membrane. Following store depletion (lower), Ca2+ unbinding from the STIM1 EF hand results in an immediate conformational change in the STIM1–Orai1 interaction that rapidly activates SOCE.
SOCE activation via STIM1–Orai1 conformational coupling in skeletal muscle?
The elucidation of STIM1–Orai1 conformational coupling as the mechanism for CRAC channel activation in T-lymphocytes and demonstration that STIM1 and Orai1 are required for SOCE in skeletal muscle (Stiber et al. 2008; Lyfenko & Dirksen, 2008) suggest that SOCE in muscle also involves conformational STIM1–Orai1 coupling. In support of this idea, both STIM1 (Stiber et al. 2008) and Orai1 (Vig et al. 2008) are expressed at high levels in skeletal muscle and STIM1 is pre-localized to SR junctions with the surface membrane in myotubes and in the SR terminal cisternae in adult skeletal muscle (Stiber et al. 2008). Thus, in contrast to T-lymphocytes and other non-excitable cells, STIM1 in skeletal muscle is pre-segregated to junctional sites of potential SOCE under resting conditions (i.e. with a full SR Ca2+ store). Important questions to be addressed in future work will be to determine the mechanism(s) responsible for the pre-clustering of STIM1 to SR-sarcolemmal junctions in resting muscle and whether junctional STIM1 proteins exist as monomers or oligomers, and/or are already located in a preformed complex with Orai1.
STIM1 oligomerization and redistribution into puncta at ER junctions with the plasma membrane in T-lymphocytes results in a significant ∼1 min time delay between store depletion and CRAC channel activation. However, the presence of extensive pre-formed SR contacts with the t-tubule system (i.e. triads) containing pre-localized STIM1 and Orai1 proteins could provide an ideal system for rapid and local activation of SOCE in skeletal muscle. Indeed, Launikonis & Rios (2007) found that SOCE across the t-tubule membrane is graded in nature and activated <1 s following store depletion. Two hypothetical mechanisms to account for such rapid and local activation of SOCE in skeletal muscle are illustrated in Fig. 3B and C. In resting muscle with a full complement SR Ca2+ store (Fig. 3B, upper), STIM1 monomers are shown to be pre-localized within the triad junction, directly across from inactive Orai1 channels. Given the high local concentration of STIM1 and Orai1 in the junction, STIM1 oligomer formation and subsequent SOCE channel activation would occur relatively rapidly following Ca2+ dissociation from the EF hand of STIM1 during depletion of Ca2+ within the terminal cisternae (Fig. 3B, lower). Alternatively, preferential localization of STIM1 proteins to the SR terminal cisternae in resting muscle might reflect pre-association of STIM1 to the C-terminal region of inactive Orai1 channels in the adjacent t-tubule membrane (Fig. 3C, upper). This possibility is supported by the recent demonstration that the CAD domain of STIM1 can directly bind to Orai1 (Park et al. 2009) and the fact that the likelihood of Orai1 binding to STIM1 will be greatly increased when the local concentration of the two interacting domains is increased. For the model presented in Fig. 3C, extremely rapid activation of SOCE would result from a direct conformational change in the preformed STIM1–Orai1 interaction following Ca2+ unloading from the STIM1 EF hand during local store depletion (Fig. 3C, lower). Though highly speculative, the provocative mechanism proposed in Fig. 3C would enable an exquisitely fast means for activating SOCCs in muscle as it would bypass time delays required for STIM1 oligomerization, redistribution and Orai1 recruitment/binding observed in non-excitable cells. Such an efficient and high fidelity ‘inside-out’ STIM1–Orai1 conformational coupling mechanism for SOCE activation in muscle might be required to keep pace with the rapid Ca2+ release dynamics characteristic of ‘outside-in’ DHPR-RyR1 conformational coupling that dictates Ca2+ release during muscle excitation.
Molecular identity of the ECCE pathway in skeletal muscle
In 2004, Pessah and colleagues identified a novel mechanism for depolarization-induced Ca2+ influx in skeletal muscle myotubes (Cherednichenko et al. 2004). This trans-sarcolemmal Ca2+ influx pathway was termed ‘excitation-coupled Ca2+ entry’ (ECCE) because it is activated by KCl depolarization and high frequency stimulation and requires functional DHPR–RyR1 coupling (ECCE is absent in myotubes derived from RyR1-null and α1S-null mice). This study also concluded that ECCE was not mediated by Ca2+ permeation through the DHPR L-type Ca2+ channel pore during depolarization because ECCE activity (assessed from the maximum rate of KCl-induced Mn2+ quench of fura-2 fluorescence) was normal in α1S-null myotubes expressing a Ca2+ impermeable α1S pore mutant (SkEIIIK; (Dirksen et al. 1999)). ECCE was subsequently shown to also be operable in adult skeletal muscle fibres (Cherednichenko et al. 2008), potentiated by ryanodine (Cherednichenko et al. 2004, 2008), insensitive to 1 μm nifedipine (Yang et al. 2007), abolished by siRNA-mediated knockdown of the DHPR α2-δ subunit (Gach et al. 2008), inhibited by La3+, Gd3+, SKF-96356, 2-APB and dantrolene (Hurne et al. 2005; Yang et al. 2007; Cherednichenko et al. 2008), and augmented by mutations in RyR1 that cause malignant hyperthermia (Yang et al. 2007; Cherednichenko et al. 2008).
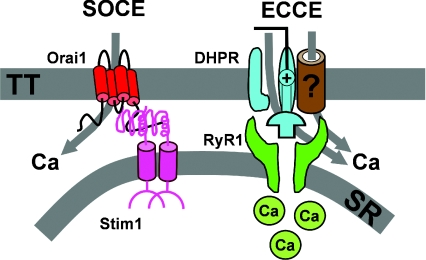
Several lines of evidence demonstrate that Ca2+ influx through the ECCE pathway is fundamentally distinct from store-operated Ca2+ entry. First of all, activation of the ECCE pathway does not require store depletion (Cherednichenko et al. 2004; Hurne et al. 2005). Second, both DHPR and RyR1 are required for ECCE, but not for SOCE (Cherednichenko et al. 2004; Lyfenko & Dirksen, 2008). Third, while depolarization activates ECCE, the strong inwardly rectifying voltage dependence of the SOCC current (Stiber et al. 2008) results in depolarization inhibiting Ca2+ entry through this mechanism (Kurebayashi & Ogawa, 2001). However, conceivably ECCE could either reflect DHPR–RyR1 conformational coupling activating a distinct Ca2+ permeation pathway or providing a unique store-independent mechanism for opening Ca2+ permeable Orai1 channels. Such a scenario is plausible considering the fact that both the SOCE (STIM1–Orai1) and EC coupling (DHPR–RyR1) machineries co-localize within pre-formed SR–sarcolemmal junctions. To distinguish between these two possibilities, we assessed effects on ECCE of molecular interventions that abolish SOCE. We found that ECCE was unaffected by both siRNA-mediated STIM1 knockdown and expression of dominant-negative Orai1 (E106Q), interventions sufficient to eliminate SOCE (Lyfenko & Dirksen, 2008). Together, these results demonstrate that store-operated and excitation-coupled Ca2+ entry arise from two distinct molecular pathways of Ca2+ influx across the t-tubule membrane (Fig. 4).
Figure 4. SOCE and ECCE muscle are determined by distinct molecular complexes within SR–sarcolemmal junctions.
Left, SOCE involves ‘inside-out’ conformational coupling between SR STIM1 Ca2+ sensor proteins and tetrameric Ca2+ permeable Orai1 channels located in the t-tubule membrane. For clarity, only one Orai1 subunit is shown. Right, ECCE involves ‘outside-in’ conformational coupling between the DHPR voltage sensor and the SR Ca2+ release channel. The Ca2+ permeation pathway for ECCE involves Ca2+ flux through the L-type Ca2+ channel pore and/or an unidentified associated channel. Figure modified with permission from Lyfenko & Dirksen (2008).
Identification of the ECCE permeation pathway
The identity of the Ca2+ permeation pathway(s) for ECCE is still being debated. Cherednichenko et al. (2004) proposed that DHPR–RyR1 conformational coupling activated an undefined Ca2+ permeable channel. As noted above, this report excluded the potential role of Ca2+ permeation through the DHPR pore since Mn2+ flux in α1S-null myotubes was fully restored following expression of SkEIIIK. ECCE also does not involve Ca2+ influx through either Orai1 (Lyfenko & Dirksen, 2008) or TRPC3 (Lee et al. 2006) channels. However, the contribution of the L-type Ca2+ channel conductance to ECCE was recently re-evaluated by Bannister et al. (2009). This study found that L-type Ca2+ channels in myotubes exhibits a similar pharmacological profile as that shown for ECCE, with inhibition by La3+, Gd3+, SKF-96356, 2-APB and high concentrations of nifedipine. Significant L-type Ca2+ current was also observed under conditions designed to mimic KCl-induced ECCE activation (∼10 s depolarization to −10 mV in 2 mm extracellular Ca2+). Finally, the study also demonstrated Mn2+ permeation through the L-type Ca2+ channel pore and that depolarization-induced Ca2+ entry is not detectable in SkEIIIK-expressing α1S-null myotubes. Together, these results indicate that Ca2+ permeation through the L-type Ca2+ channel pore may indeed represent an important contribution to Ca2+ entry via the ECCE pathway (Bannister et al. 2009).
A major discrepancy in previous studies is the observation that KCl-mediated Mn2+ flux was normal (Cherednichenko et al. 2004) but Ca2+ entry absent (Bannister et al. 2009) following SkEIIIK expression in α1S-null myotubes. Bannister et al. (2009) suggested the difference between the two studies could be explained by KCl depolarization driving the SkEIIIK channel into a high PO mode that restores divalent ion permeability to levels sufficient to resolve Mn2+ quench, but not Ca2+ influx. Indeed, strong depolarization in the presence of Bay K 8644 produced a transient inward tail current during repolarization (Bannister et al. 2009). However, neither the identity of the charge carrier (e.g. Ca2+, Cl−, TEA+, H+) during the inward tail current nor the degree to which SkEIIIK divalent permeation is promoted by KCl application protocols typically used to activate ECCE were determined. Thus, the role of KCl-induced potentiation of Mn2+ flux through SkEIIIK channels for the normal rate of fura-2 quench reported by Cherednichenko et al. (2004) remains unclear.
Additional discrepancies with prior published results are also inconsistent with the notion that ECCE-mediated Ca2+ influx arises solely from Ca2+ permeation through the DHPR pore. First, ECCE is abolished following knockdown of the DHPR α2-δ subunit, in spite of the fact that the L-type Ca2+ current conductance is unaffected and the kinetics of channel activation/inactivation only marginally altered (Gach et al. 2008). Second, ECCE activity is inhibited ∼70% by dantrolene (10 μm) and augmented >6-fold by ryanodine (250 μm) (Cherednichenko et al. 2004, 2008), in spite of the fact that these agents also do not significantly alter maximal L-type Ca2+ channel conductance and only marginally shift the voltage dependence of channel activation (Balog & Gallant, 1999; Szentesi et al. 2001; Bannister et al. 2009). Third, ECCE is activated during high frequency electrical stimulation (Cherednichenko et al. 2004, 2008; Hurne et al. 2005), a condition seemingly less likely than prolonged KCl depolarization to activate Ca2+ entry through slowly activating skeletal muscle L-type Ca2+ channels. Fourth, mutation of a conserved cysteine residue in RyR1 (C4958S) enhanced ECCE (by slowing deactivation), in spite of the fact that L-type Ca2+ current magnitude was reduced ∼60% without a change in channel inactivation kinetics (Hurne et al. 2005). Finally, ECCE is increased ∼2-fold by mutations in RyR1 that result in MH (Yang et al. 2007; Cherednichenko et al. 2008), while these mutations do not markedly alter the magnitude and voltage dependence of the L-type Ca2+ channel conductance (Dirksen & Avila, 2004). In spite of these apparent discrepancies, quantitative comparisons between effects of interventions on L-type Ca2+ currents and ECCE flux can be misleading as even minor alterations in L-channel activity may result in significant changes in integrated divalent ion influx recorded during prolonged KCl depolarization. Nevertheless, while Ca2+ permeation through the L-type Ca2+ channel pore may contribute to Ca2+ influx through the ECCE pathway, additional factors and/or auxiliary influx pathways may also be involved. Clearly, additional work is required in order to reach a more comprehensive understanding of the permeation pathway that underlies ECCE.
Conclusions and future perspectives
In summary, SOCE in skeletal muscle involves store depletion triggering rapid conformational coupling between STIM1 luminal Ca2+ sensor proteins located in the SR and calcium permeable Orai1 channels present in the transverse (t)-tubule membrane. Unlike T-lymphocytes and other non-excitable cells, STIM1 and Orai1 proteins pre-localize within the triad junction in skeletal muscle under resting conditions, thus permitting extremely fast and efficient trans-sarcolemmal Ca2+ influx during store depletion. Prolonged and repetitive depolarization activates ECCE via conformational coupling between DHPR voltage sensor proteins in the t-tubule membrane and RyR1 Ca2+ release channels in the SR terminal cisternae. The store-operated and excitation-coupled Ca2+ entry pathways reflect two distinct molecular channel complexes within the triad junction that enable trans-sarcolemmal Ca2+ entry across a wide range of transmembrane voltages. SOCE is rapidly activated by ‘inside-out’ STIM1–Orai1 conformational coupling during store depletion that provides a mechanism for trans-sarcolemmal Ca2+ influx at negative voltages. On the other hand, ECCE is activated by ‘outside-in’ DHPR–RyR1 conformational coupling that permits trans-sarcolemmal Ca2+ influx at depolarized potentials.
A number of important unresolved issues surrounding SOCE and ECCE in skeletal muscle remain to be addressed. With regard to SOCE, future work will need to determine the mechanism responsible for targeting STIM1 to the SR terminal cisternae in resting muscle, as well as the molecular mechanism for rapid and graded activation. In addition, it will also be important to determine if other potential store-operated Ca2+ influx mechanisms (e.g. STIM1 coupling to TRPC or Orai1/TRPC channels) are also operable in skeletal muscle. Finally, functional roles of STIM2 and Orai2/3, as well as other potential functions for STIM1 (e.g. in ER/SR stress, activation of unfolded protein response) will also require further investigation. With regard to ECCE, the relative contribution of Ca2+ permeation through the α1S pore and/or via another associated channel certainly merits additional attention. However, the most important long-term goal of future work will be to determine the respective physiological roles of SOCE and ECCE in muscle development (Stiber et al. 2008), fatigue (Pan et al. 2002; Zhao et al. 2005), aging (Zhao et al. 2008), and as modifiers of muscle disease (Vandebrouck et al. 2002; Zhao et al. 2006; Yang et al. 2007; Cherednichenko et al. 2008).
Acknowledgments
I would like to thank Drs. Alla Lyfenko, Trevor Shuttleworth and Olivier Mignen for numerous‘STIM-ulating’ and ‘Orai-ginal’ discussions. I also thank Drs Kurt Beam and Isaac Pessah for many similar insightful discussions regarding ECCE. This work was supported by National Institute of Health Grants AR044657 and 5P01AR052354.
Author Contributions
R.T.D prepared/revised the manuscript and secured the funding.
References
- Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N¢-tetracetic acid. Biochim Biophys Acta. 1972;267:605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- Balog EM, Gallant EM. Modulation of the sarcolemmal L-type current by alteration in SR Ca2+ release. Am J Physiol Cell Physiol. 1999;276:C128–C135. doi: 10.1152/ajpcell.1999.276.1.C128. [DOI] [PubMed] [Google Scholar]
- Bannister RA, Pessah IN, Beam KG. The skeletal L-type Ca2+ current is a major contributor to excitation-coupled Ca2+ entry. J Gen Physiol. 2009;133:79–91. doi: 10.1085/jgp.200810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Bootman MD, Berridge MJ, Cheek TR. Ca2+ entry into PC12 cells initiated by ryanodine receptors or inositol 1,4,5-trisphosphate receptors. Biochem J. 1998;329:349–357. doi: 10.1042/bj3290349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotina VM. Orai, STIM1 and iPLA2β: a view from a different perspective. J Physiol. 2008;586:3035–3042. doi: 10.1113/jphysiol.2008.154997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko G, Hurne AM, Fessenden JD, Lee EH, Allen PD, Beam KG, Pessah IN. Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc Natl Acad Sci U S A. 2004;101:15793–15798. doi: 10.1073/pnas.0403485101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko G, Ward CW, Feng W, Cabrales E, Michaelson L, Samso M, Lopez JR, Allen PD, Pessah IN. Enhanced excitation-coupled calcium entry in myotubes expressing malignant hyperthermia mutation R163C is attenuated by dantrolene. Mol Pharmacol. 2008;73:1203–1212. doi: 10.1124/mol.107.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbellay B, Arnaudeau S, Konig S, Jousset H, Bader C, Demaurex N, Bernheim L. STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J Biol Chem. 2009;284:5370–5380. doi: 10.1074/jbc.M806726200. [DOI] [PubMed] [Google Scholar]
- Dirksen RT, Avila G. Distinct effects on Ca2+ handling caused by malignant hyperthermia and central core disease mutations in RyR1. Biophys J. 2004;87:3193–3204. doi: 10.1529/biophysj.104.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen RT, Beam KG. Role of calcium permeation in dihydropyridine receptor function. Insights into channel gating and excitation-contraction coupling. J Gen Physiol. 1999;114:393–403. doi: 10.1085/jgp.114.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada M, Espinosa A, Gibson CJ, Uhlen P, Jaimovich E. Capacitative calcium entry in testosterone-induced intracellular calcium oscillations in myotubes. J Endocrinol. 2005;184:371–379. doi: 10.1677/joe.1.05921. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Gach MP, Cherednichenko G, Haarmann C, Lopez JR, Beam KG, Pessah IN, Franzini-Armstrong C, Allen PD. Alpha2delta1 dihydropyridine receptor subunit is a critical element for excitation-coupled calcium entry but not for formation of tetrads in skeletal myotubes. Biophys J. 2008;94:3023–3034. doi: 10.1529/biophysj.107.118893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Serratos H, Valle-Aguilera R, Lathrop DA, Garcia MC. Slow inward calcium currents have no obvious role in muscle excitation-contraction coupling. Nature. 1982;298:292–294. doi: 10.1038/298292a0. [DOI] [PubMed] [Google Scholar]
- Hurne AM, O’Brien JJ, Wingrove D, Cherednichenko G, Allen PD, Beam KG, Pessah IN. Ryanodine receptor type 1 (RyR1) mutations C4958S and C4961S reveal excitation-coupled calcium entry (ECCE) is independent of sarcoplasmic reticulum store depletion. J Biol Chem. 2005;280:36994–37004. doi: 10.1074/jbc.M506441200. [DOI] [PubMed] [Google Scholar]
- Ji W, Xu P, Li Z, Lu J, Liu L, Zhan Y, Chen Y, Hille B, Xu T, Chen L. Functional stoichiometry of the unitary calcium-release-activated calcium channel. Proc Natl Acad Sci U S A. 2008;105:13668–13673. doi: 10.1073/pnas.0806499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov KI, Shin DM, Wang Y, Pessah IN, Allen PD, Muallem S. Gating of store-operated channels by conformational coupling to ryanodine receptors. Mol Cell. 2000;6:421–431. doi: 10.1016/s1097-2765(00)00041-1. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol. 2001;533:185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Barnes M, Stephenson DG. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc Natl Acad Sci U S A. 2003;100:2941–2944. doi: 10.1073/pnas.0536227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Rios E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J Physiol. 2007;583:81–97. doi: 10.1113/jphysiol.2007.135046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EH, Cherednichenko G, Pessah IN, Allen PD. Functional coupling between TRPC3 and RyR1 regulates the expressions of key triadic proteins. J Biol Chem. 2006;281:10042–10048. doi: 10.1074/jbc.M600981200. [DOI] [PubMed] [Google Scholar]
- Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J Physiol. 2008;586:4815–4824. doi: 10.1113/jphysiol.2008.160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Luttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochimica et Biophysica Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J Physiol. 2008;586:419–425. doi: 10.1113/jphysiol.2007.147249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C. A cytosolic homomerization and a modulatory domain within STIM1 C-terminus determine coupling to ORAI1 channels. J Biol Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J, Dirksen RT, Nguyen HT, Pessah IN, Beam KG, Allen PD. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380:72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, Takeshima H, Cheng H, Ma J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol. 2002;4:379–383. doi: 10.1038/ncb788. [DOI] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna A, Demuro A, Yeromin AV, Zhang SL, Safrina O, Parker I, Cahalan MD. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature. 2008;456:116–120. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Putney JWJ. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg P, Hawkins A, Stiber J, Shelton JM, Hutcheson K, Bassel-Duby R, Shin DM, Yan Z, Williams RS. TRPC3 channels confer cellular memory of recent neuromuscular activity. Proc Natl Acad Sci U S A. 2004;101:9387–9392. doi: 10.1073/pnas.0308179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentesi P, Collet C, Sarkozi S, Szegedi C, Jona I, Jacquemond V, Kovacs L, Csernoch L. Effects of dantrolene on steps of excitation-contraction coupling in mammalian skeletal muscle fibers. J Gen Physiol. 2001;118:355–375. doi: 10.1085/jgp.118.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, McCarthy TV, MacLennan DH. Measurement of resting cytosolic Ca2+ concentrations and Ca2+ store size in HEK-293 cells transfected with malignant hyperthermia or central core disease mutant Ca2+ release channels. J Biol Chem. 1999;274:693–702. doi: 10.1074/jbc.274.2.693. [DOI] [PubMed] [Google Scholar]
- Vandebrouck C, Martin D, Colson-Van SM, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Weisleder N, Collet C, Zhou J, Chu Y, Hirata Y, Zhao X, Pan Z, Brotto M, Cheng H, Ma J. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat Cell Biol. 2005;7:525–530. doi: 10.1038/ncb1254. [DOI] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Allen PD, Pessah IN, Lopez JR. Enhanced excitation-coupled calcium entry in myotubes is associated with expression of RyR1 malignant hyperthermia mutations. J Biol Chem. 2007;282:37471–37478. doi: 10.1074/jbc.M701379200. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Weisleder N, Han X, Pan Z, Parness J, Brotto M, Ma J. Azumolene inhibits a component of store-operated calcium entry coupled to the skeletal muscle ryanodine receptor. J Biol Chem. 2006;281:33477–33486. doi: 10.1074/jbc.M602306200. [DOI] [PubMed] [Google Scholar]
- Zhao X, Weisleder N, Thornton A, Oppong Y, Campbell R, Ma J, Brotto M. Compromised store-operated Ca2+ entry in aged skeletal muscle. Aging Cell. 2008;7:561–568. doi: 10.1111/j.1474-9726.2008.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yoshida M, Brotto L, Takeshima H, Weisleder N, Hirata Y, Nosek TM, Ma J, Brotto M. Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol Genomics. 2005;23:72–78. doi: 10.1152/physiolgenomics.00020.2005. [DOI] [PubMed] [Google Scholar]