Abstract
Sarcosine is an amino acid involved in one-carbon metabolism and a promising therapy for schizophrenia because it enhances NMDA receptor (NMDAR) function by inhibiting glycine uptake. The structural similarity between sarcosine and glycine led us to hypothesize that sarcosine is also an agonist like glycine. We examined this possibility using whole-cell recordings from cultured embryonic mouse hippocampal neurons. We found that sarcosine is an NMDAR co-agonist at the glycine binding site. However, sarcosine differed from glycine because less NMDAR desensitization occurred with sarcosine than with glycine as the co-agonist. This finding led us to examine whether the physiological effects of NMDAR activation with these two co-agonists are the same. The difference in desensitization probably accounts for rises in intracellular Ca2+, as assessed by the fluorescent indicator fura-FF, being larger when NMDAR activation occurred with sarcosine than with glycine. In addition, Ca2+-activated K+ currents following NMDAR activation were larger with sarcosine than with glycine. Compared to glycine, NMDAR-mediated autaptic currents decayed faster with sarcosine suggesting that NMDAR deactivation also differs with these two co-agonists. Despite these differences, NMDAR-dependent neuronal death as assessed by propidium iodide was similar with both co-agonists. The same was true for neuronal bursting. Thus, sarcosine may enhance NMDAR function by more than one mechanism and may have different effects from other NMDAR co-agonists.
Sarcosine (N-methylglycine, Fig. 1A) is an endogenous amino acid having several important biological functions. It participates in one-carbon metabolism, which refers to folate-dependent pathways involved in activating single carbons for protein synthesis, nucleotide synthesis and DNA methylation (Ueland et al. 2007). In particular, it is an important intermediate in the metabolism of choline, a major donor of one-carbon groups along with folate. Second, sarcosine is a competitive inhibitor of the type I glycine transporter (GlyT1) (Lopez-Corcuera et al. 1998; Herdon et al. 2001). This property has led sarcosine to receive consideration as a potential antipsychotic agent.
Figure 1. Sarcosine acts as an NMDAR co-agonist at the glycine binding site.
A, structures for sarcosine and glycine. B, currents evoked from one neuron by applying 100 μm NMDA with the indicated sarcosine (Sar) concentrations. The spikes in the current traces are an artifact of the drug perfusion system. C, mean peak current evoked by 100 μm NMDA with varying sarcosine concentrations in no DCKA (N= 12) or in 1 μm DCKA (N= 7). Peak currents were normalized to that evoked by 100 μm NMDA + 300 μm sarcosine in no DCKA in the same cell. For each cell studied in the absence of DCKA, the peak current obtained in the absence of sarcosine was subtracted from the peak current obtained with sarcosine. Lines show fits to a logistic equation with an EC50 of 26 μm and an N of 1.8 in no DCKA and an EC50 of 53 μm and an N of 2.1 in 1 μm DCKA. D, currents evoked from one neuron by applying 100 μm NMDA with the indicated glycine (Gly) concentrations. E, mean peak current evoked by 100 μm NMDA in varying glycine concentrations (N= 6). Peak currents were normalized to that evoked with 10 μm glycine in the same cell. Line shows a fit to a logistic equation with an EC50 of 61 nm and an N of 1.7. F, currents evoked from one neuron by applying 100 μm NMDA with the indicated sarcosine and DCKA concentrations. G, mean 100 μm NMDA peak currents in 10 μm DCKA, 30 μm sarcosine, and in 30 μm sarcosine + 10 μm DCKA. Peak currents were normalized to that induced by 100 μm NMDA in the absence of sarcosine and DCKA (N= 5) in the same cell. *P < 0.0001 versus other bars by one-way ANOVA. H, currents evoked from one neuron by applying 100 μm NMDA ± 30 μm sarcosine (top panel), 1 μm NFPS (middle panel), and 100 μm NMDA ± 30 μm sarcosine in 1 μm NFPS (bottom panel). The traces in the bottom panel were obtained after applying 1 μm NFPS for 10 min. I, mean 100 μm NMDA + 30 μm sarcosine peak currents before and after a 10 min treatment with 1 μm NFPS. Peak currents were normalized to that induced by 100 μm NMDA in the absence of sarcosine before NFPS treatment in the same cell (N= 5). Mean peak currents before and after NFPS do not differ (P= 0.08 by paired t test). J, mean 100 μm NMDA + 30 μm sarcosine peak currents in neurons from control cultures (N= 6), cultures treated with 1 μm NFPS for 1 h (N= 9), and astrocyte-free cultures (N= 4). Peak currents were normalized to that evoked by 100 μm NMDA alone. P= 0.50 by one-way ANOVA. K, currents evoked from one neuron by applying 100 μm NMDA in the absence and presence of 30 μm sarcosine. Traces are from a neuron that was not plated on an astrocytic feeder layer.
Schizophrenia is a debilitating illness characterized by positive symptoms including hallucinations, negative symptoms such as a lack of motivation, and cognitive impairment (Schultz et al. 2007). According to one hypothesis, hypofunction of the NMDA receptor (NMDAR) class of glutamate receptors contributes to schizophrenia (Coyle, 2006). Consequently, potentiators of NMDAR function have emerged as a therapeutic strategy for schizophrenia. They appear to be particularly effective against the negative symptoms and cognitive impairment, which currently available medications targeting dopamine receptors are not (Gray & Roth, 2007).
Opening of the NMDAR channel complex requires occupation of its glutamate binding site by glutamate or another agonist and its glycine binding site by glycine or d-serine (Wolosker et al. 2008). The glycine binding site is a more attractive target for enhancing NMDAR function than the glutamate binding site because the risk of neurotoxicity and seizures may be lower (Gray & Roth, 2007). Sarcosine and other GlyT1 inhibitors provide a potential means for enhancing NMDAR function because they may increase the glycine concentration available to activate these receptors (Eulenburg et al. 2005). GlyT1 is primarily found on glia and helps modulate the amplitude and kinetics of NMDAR-mediated postsynaptic currents (Chen et al. 2003; Tsai et al. 2004b) and NMDAR-dependent long-term potentiation (Kinney et al. 2003; Martina et al. 2004). Evidence for targeting GlyT1 in the treatment of schizophrenia comes from GlyT1 knockout mice, which have enhanced cognition and are resistant to psychotic drugs (Tsai et al. 2004b; Yee et al. 2006). The antipsychotic effects of GlyT1 inhibitors in mice give further support for using these agents to treat schizophrenia (Kinney et al. 2003). These findings appear to extend to humans because preliminary clinical studies suggest that sarcosine improves the positive and negative symptoms of schizophrenia (Tsai et al. 2004a; Lane et al. 2008).
The clinical benefits of sarcosine may arise from its inhibition of GlyT1, but other possible mechanisms are important to consider if GlyT1 antagonists are to become a mainstay in schizophrenia treatment. In particular, the structural similarity between sarcosine and glycine raises the possibility that sarcosine itself is an NMDAR co-agonist, though prior studies found no evidence of such an effect (McBain et al. 1989; Rabe & Tabakoff, 1990). Here we show that sarcosine is an NMDAR co-agonist using whole-cell voltage clamp recordings from cultured embryonic mouse hippocampal neurons. We also show that some physiological effects of hippocampal NMDAR activation with sarcosine and glycine differ.
Methods
Ethical approval
The Washington University Animal Studies Committee approved all experimental protocols.
Embryonic mouse hippocampal cultures
Hippocampal neurons were cultured from Swiss Webster mouse embryos at day 16 of gestation as described previously (Thio & Zhang, 2006). Briefly, timed pregnant mice were deeply anaesthetized with isoflurane and killed by cervical dislocation. Embryos were obtained by Caesarean section and decapitated. Two hundred embryos from 35 pregnant mice were used in this study. Brains were removed and hippocampi were dissected and sliced. A single-cell suspension was obtained by enzymatically digesting hippocampal slices with papain followed by gentle trituration. Mass cultures were used for the majority of experiments and were derived by plating the cell suspension on coverslips covered with a monolayer of cortical astrocytes. Microisland cultures were used for recording autaptic currents. These cultures were obtained by plating the cell suspension on islands of astrocytes made by plating astrocytes on coverslips that had been sprayed with collagen droplets using a microatomizer (Thomas Scientific, Swedesboro, NJ, USA) (Thio & Yamada, 2004). In a few experiments, the cells were plated directly on poly-l-lysine-coated coverslips lacking a glial monolayer. These coverslips were maintained in a culture dish containing an astrocytic monolayer. Experiments were performed using neurons incubated for 5–16 days with most using neurons cultured for 7–9 days.
Electrophysiology
Whole-cell patch clamp electrophysiology
NMDAR-mediated currents were recorded from voltage-clamped neurons using an Axopatch 200A amplifier (Molecular Devices, Sunnyvale, CA, USA) in the whole-cell patch clamp mode as described previously (Thio & Zhang, 2006). Recordings were performed at a holding potential of −65 mV unless otherwise indicated. Excitatory autaptic currents were obtained by voltage clamping single neurons on glial microislands at −75 mV and subjecting them to a 1 ms depolarizing voltage step to 0 mV as described before (Thio & Yamada, 2004). Holding potentials were corrected for empirically measured junction potentials. Series resistance compensation was set at 60–90%, and the 4-pole low-pass filter on the amplifier was set at 2 kHz. Whole-cell currents were digitized at 10 kHz using pCLAMP 9 (Molecular Devices). All experiments were performed at room temperature.
Solutions
Neurons were bathed in an extracellular solution containing (in mm): 140 NaCl, 5 KCl, 1.5 CaCl2, 10 d-glucose, 2.5 × 10−4 TTX and 10 Hepes (pH 7.35–7.39). Mg2+ was omitted for all experiments. TTX was omitted for experiments examining autaptic currents and neuronal bursting. The extracellular Ca2+ concentration was raised to 3 mm for recording Ca2+-activated K+ currents. Patch pipettes had resistances of 2–6 MΩ when filled with a solution containing (in mm): 140 CsCH3SO3, 4 NaCl, 0.5 CaCl2, 5 EGTA, 0.5 Na3GTP, 2 MgATP and 10 Hepes (pH 7.20–7.30). Equimolar potassium gluconate replaced the CsCH3SO3 for recording Ca2+-activated K+ currents and autaptic currents. For recording Ca2+-activated K+ currents, the pipette solution contained 0.5 mm rather than 5 mm EGTA (Zorumski et al. 1989). The pipette solution included 5 mm QX 314 for the neuronal bursting experiments.
Drug application
For the electrophysiological experiments, agonists and antagonists were applied to neurons at 2 ml min−1 using a multibarrel, gravity-driven, flow tube system (Thio & Zhang, 2006). All antagonists were pre-applied for at least 60 s. Similarly, glycine and sarcosine were pre-applied for at least 60 s when NMDAR-mediated currents were examined. When drugs were not applied, the neuron being studied was continuously perfused with extracellular solution alone. In addition, the recording chamber was continuously perfused with extracellular solution at 0.5 ml min−1.
Data analysis
Current traces were analysed using pCLAMP 9. In general, control and experimental applications were interleaved, and the data were not used if the bracketing control peak currents were not within 10–15% of each other. Using this criterion, two to three trials from each neuron were analysed typically. The desensitizing phase of currents evoked by exogenous NMDA applications was fitted to an exponential function using the Levenberg–Marquardt algorithm. The number of exponentials needed was determined using an F test with P < 0.05. For these experiments, traces from single agonist/antagonist applications, as shown in the figures, were analysed. In contrast, three to five consecutive autaptic traces evoked in a given condition were averaged before analysis. Averaged traces as shown in the figures were used to determine the peak amplitude and the 20–80% rise time, which was the time needed for the current to rise from 20% of its peak amplitude to 80% of its peak amplitude. The decay of averaged autaptic currents was fitted to an exponential function in the same manner as NMDA current desensitization.
Agonist dose–response curves for glycine and sarcosine were fitted to the logistic equation
| (1) |
where R(A) is the response to a given agonist concentration [A], Rmax is the response to a saturating agonist concentration, EC50 is the agonist concentration producing a half-maximal response, and n is the Hill coefficient. The initial points of the dose–response curves for glycine were fitted to a line by linear regression to estimate the background glycine concentration in the extracellular solution (Lerma et al. 1990). Inhibitory dose–response curves were fitted to the logistic equation
| (2) |
where R(Agonist) is the response to the agonist in the presence of a given inhibitor concentration [Inhibitor], Ragonist is the response to agonist in the absence of inhibitor, IC50 is the inhibitor concentration producing half-maximal inhibition, and n is the Hill coefficient. Fits were obtained using the Levenberg–Marquardt algorithm.
Neuronal bursts were detected using the threshold mode for event detection in pCLAMP 9 with a threshold of three times the s.d. of the baseline noise and a duration 200 ms or longer. Thus, bursts were a series of EPSCs superimposed on a prolonged inward current.
Ca2+ imaging
Changes in intracellular Ca2+ in neuronal soma were measured using ratiometric fluorescence imaging with fura-FF. Neurons were loaded with fura-FF by incubating them for 60 min in 5 μm acetoxymethyl (AM) ester of fura-FF (Invitrogen, Carlsbad, CA, USA) and 0.1% Pluronic F-127 (Invitrogen) at room temperature in a solution containing (in mm): 140 NaCl, 5.4 KCl, 1 NaH2PO4, 1.8 CaCl2, 1 MgSO4, 12 Hepes and 5.5 d-glucose (pH 7.20). Then, the neurons were washed and incubated in the same solution (pH 7.40) for another 60 min to allow for ester hydrolysis. After loading, the neurons were bathed in the same solution used for recording NMDAR-mediated currents except that 10 μm nimodipine was added to block L-type voltage-gated Ca2+ channels. The neurons were imaged on an inverted microscope (Eclipse TE300, Nikon, Melville, NY, USA) using a 40×, 1.3 NA fluorite oil immersion objective (Nikon), a cooled CCD camera (Cooke Corp., Auburn Hill, MI, USA), and a 75 W xenon arc lamp. The fluorescence excitation was provided at alternate wavelengths with a band-specific filter (340/26 and 387/11 nm; Semrock, Rochester, NY, USA) in combination with DM400 dichroic beam splitter (Nikon). The images were obtained every second using alternate excitation wavelengths of 340 nm and 387 nm and collecting emission wavelengths above 510 nm. After subtracting the matching background, the images were divided by one another to yield ratios for individual cells. Image acquisition and processing were controlled by an imaging software package, MetaFluor (Molecular Devices). Agonists were applied by exchanging the control external solution with one containing the agonists.
Propidium iodide staining
Sister neuronal cultures were washed with the same external solution used for recording NMDAR-mediated currents except that TTX was omitted. Then, the agonists were applied by adding them to the external solution and incubating for 3 h at 37°C. After replacing the external solution with culture media, the neurons were returned to the incubator for 24 h before being stained with 5 μg ml−1 propidium iodide in phosphate-buffered saline at 37°C for 10 min. For each condition, images from six randomly selected fields on a coverslip were captured with a Zeiss LSM 5 PASCAL confocal microscope (Zeiss, Thornwood, NY, USA). Each field had an area of 0.4 mm2 and contained 100–200 neurons. Images were acquired using an excitation wavelength of 543 nm and an emission wavelength of 560 nm and above. A blinded observer determined the percentage of cell death by counting the number of propidium iodide-labelled cells and expressing it as a percentage of the total number of cells. These experiments were repeated with four different platings.
Glycine contamination of sarcosine
We measured the sarcosine and glycine concentration in two 1 m stock solutions of sarcosine by high-performance liquid chromatography–tandem mass spectrometry. The assay confirmed that both stocks contained 1 m sarcosine with one stock containing 8.1 ± 1.7 μm glycine and the other containing 9.9 ± 4.1 μm glycine (measurements in triplicate, mean ±s.e.m.).
Statistics
Statistical analysis was performed with Origin 7 (OriginLab, Northampton, MA, USA), GraphPad (GraphPad Software, La Jolla, CA, USA) and Microsoft Excel 2000 (Microsoft, Redmond, WA, USA). Data are presented as the mean ±s.e.m. with N being the number of neurons studied or experiments performed. Error bars smaller than symbols are not shown. Means were compared using a two-tailed t test, a two-tailed paired t test, or a one-way ANOVA followed by Tukey's post hoc comparison of means. Statistical significance was defined as P < 0.05.
Materials
All chemicals were obtained from Sigma (St Louis, MO, USA) except for N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS), which was obtained from Tocris Bioscience (Ellisville, MO, USA) and fura-FF, which was obtained from Invitrogen.
Results
Sarcosine increases NMDAR-mediated currents
We first examined whether sarcosine enhances NMDAR-mediated currents in cultured embryonic mouse hippocampal neurons. We found that sarcosine increased 100 μm NMDA peak currents in a dose-dependent manner (Fig. 1B and C). We included 1 μm strychnine in the extracellular solution in these experiments to eliminate a contribution by inhibitory glycine receptors. Sarcosine increased 100 μm NMDA currents with an EC50 of 26 ± 3 μm (N= 12) and an n of 1.8 ± 0.2 (N= 12). Glycine was 430 times more potent with an EC50 of 61 ± 8 nm (N= 6) and an n of 1.7 ± 0.3 (N= 6) (Fig. 1D and E). Saturating sarcosine concentrations produced slightly smaller peak currents than saturating glycine concentrations. Peak currents evoked by 100 μm NMDA with 300 μm sarcosine were 86 ± 2% (N= 4, P= 0.02, two-tailed paired t test) of the peak currents evoked by 100 μm NMDA with 3 μm glycine.
We then used 5,7-dichlorokynurenic acid (DCKA), an NMDAR glycine binding site antagonist (Baron et al. 1990), to determine whether sarcosine acts at this site on the NMDAR. DCKA (10 μm) prevented 20 μm sarcosine from increasing peak 100 μm NMDA currents as expected if sarcosine acts at the NMDAR glycine binding site (Fig. 1F and G). In accord with this hypothesis, increasing the sarcosine concentration overcame the inhibition with 1 μm DCKA (Fig. 1C). Sarcosine had an EC50 of 53 ± 2 μm (N= 6, P= 0.0005 versus sarcosine alone, two-tailed t test) and an n of 2.1 ± 0.1 (N= 6, P= 0.65 versus sarcosine alone, two-tailed t test) in 1 μm DCKA. DCKA also completely inhibited 100 μm NMDA currents in the absence of added sarcosine indicating that background concentrations of glycine or another NMDAR co-agonist were sufficient to activate the NMDAR. We estimated that the equivalent of 19 ± 6 nm (N= 6) glycine contaminated our extracellular solutions by extrapolating the linear region of the glycine dose–response curve (no added – 10 nm added glycine) to zero current (Lerma et al. 1990). Others have obtained similar results (Vyklicky et al. 1990; Lerma et al. 1990), and we corrected all glycine concentrations, including those in Fig. 1, for a background glycine concentration of 20 nm. Together, these results suggest that sarcosine increases NMDA currents via the NMDAR glycine binding site.
Sarcosine may increase NMDAR-mediated currents because it binds directly to the NMDAR, causes glycine to accumulate in the extracellular solution, or contains glycine as a contaminant. The increase did not result from contaminating glycine because 30 μm sarcosine (EC60) only contains 300 pm glycine (see Methods), a concentration that would not increase NMDA currents under our conditions. Furthermore, 3 mm sarcosine, which contains 30 nm glycine, increased peak 100 μm NMDA currents by 300 ± 42% (N= 6). This increase was greater than the 180 ± 27% (N= 6, P= 0.01, two-tailed paired t test) increase produced by 30 nm glycine in the same neurons. Sarcosine may cause glycine to accumulate by blocking glycine uptake by GlyT1 or by heteroexchange of sarcosine for glycine via GlyT1 (Herdon et al. 2001). We excluded these possibilities by using the GlyT1 inhibitor NFPS. NFPS is an essentially irreversible GlyT1 inhibitor with 1 μm NFPS producing complete inhibition after 6 min (Aubrey & Vandenberg, 2001; Atkinson et al. 2001). Thirty micromolar sarcosine increased 100 μm NMDA peak currents by a similar amount before and after applying 1 μm NFPS for 10 min (Fig. 1H and I). Even in cultures treated with 1 μm NFPS for 60 min, 30 μm sarcosine increased 100 μm NMDA peak currents to the same extent as in untreated cultures (Fig. 1J). Finally, we eliminated the effect of astrocytic GlyT1 by examining whether sarcosine potentiated NMDA currents in neurons plated in the absence of an astrocytic feeder layer. In such neurons, 30 μm sarcosine potentiated 100 μm NMDA peak currents to the same extent as in neurons plated on an astrocytic feeder layer (Fig. 1J and K). The above data indicate that sarcosine is a NMDAR co-agonist at the glycine binding site.
NMDAR desensitization is weaker with sarcosine than with glycine as the co-agonist
While performing the preceding experiments, we noted that 100 μm NMDA currents showed less desensitization with sarcosine than with glycine as the co-agonist (Fig. 2A). This difference was evident at an EC20 and EC50 but not a saturating concentration (Fig. 2B). However, these results did not indicate whether sarcosine altered ‘glycine’-sensitive desensitization, ‘glycine’-insensitive desensitization or Ca2+-dependent inactivation of NMDARs (Vyklicky et al. 1990; Clark et al. 1990; Sather et al. 1990; Krupp et al. 1996). ‘Glycine’-insensitive desensitization, which includes a zinc-independent and a zinc-induced component (Erreger & Traynelis, 2005), remains at saturating glycine concentrations (Krupp et al. 1996). We expected ‘glycine’-insensitive desensitization to occur because cultured embryonic hippocampal neurons express NR2A subunits (Brewer et al. 2007), and because this form of desensitization is especially prominent in NR1/NR2A NMDARs (Krupp et al. 1996; Erreger & Traynelis, 2005). We reported previously that our extracellular solution contains ∼800 nm zinc (Thio & Zhang, 2006), a sufficient concentration for zinc-induced desensitization (Erreger & Traynelis, 2005).
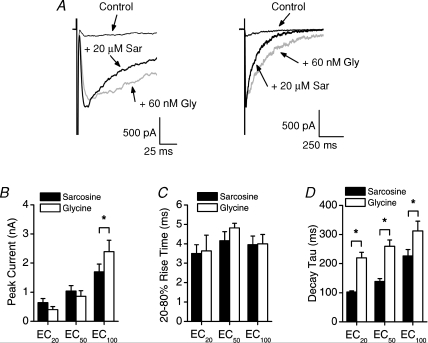
Figure 2. NMDAR desensitization is weaker with sarcosine than glycine as a co-agonist.
A, currents evoked from one neuron by applying 100 μm NMDA with an EC50 sarcosine (20 μm) or glycine (60 nm) concentration. B, mean percentage desensitization for currents evoked by 100 μm NMDA with an EC20, EC50 or EC100 concentration of sarcosine (N= 6–7) or glycine (N= 6–7). Percentage desensitization was calculated by 100 × (peak current amplitude – steady-state current amplitude)/peak current amplitude. *P < 0.0001 by two-tailed paired t test. C, currents evoked from one neuron by applying 100 μm NMDA with an EC20 (6 μm) or an EC50 (20 μm) sarcosine concentration. Lines show a fit to a single exponential with the indicated time constants. D, mean time constant of desensitization for currents evoked by 100 μm NMDA with an EC20, EC50 or EC100 concentration of sarcosine (N= 6) or glycine (N= 6). Sarcosine concentrations used in B and D were 6 μm, 20 μm and 300 μm; corresponding glycine concentrations were 30 nm, 60 nm and 3 μm. P < 0.05 for * bars versus all *** bars, and P < 0.05 for ** bar versus* bars, except the EC20 glycine concentration, by one-way ANOVA. E, currents evoked from one neuron by applying 100 μm NMDA with an EC50 sarcosine (20 μm) or glycine (60 nm) concentration. The extracellular solution contained 1.5 mm Ba2+ instead of 1.5 mm Ca2+. F, mean percentage desensitization for currents evoked by 100 μm NMDA with an EC50 sarcosine (20 μm) or glycine (60 nm) concentration in an extracellular solution containing 1.5 mm Ca2+ (N= 6 for both sarcosine and glycine, data from B) or 1.5 mm Ba2+ (N= 9 for both sarcosine and glycine). *P < 0.001 by one-way ANOVA.
We next examined whether NMDAR desensitization kinetics differed depending on whether sarcosine or glycine was the co-agonist. We found that NMDAR desensitization followed a single exponential at an EC20, EC50 and EC100 concentration of both agonists (Fig. 2C). This finding suggests that one form of NMDAR desensitization predominated at each of these co-agonist concentrations, though the predominant form probably was different at subsaturating and saturating concentrations as outlined below. The desensitization time constants for sarcosine and glycine were similar at an EC20 and an EC100 concentration, but NMDAR desensitization was slower with sarcosine than glycine at an EC50 concentration (Fig. 2D). Thus, increasing sarcosine from an EC20 to an EC50 concentration markedly slowed NMDAR desensitization whereas increasing glycine over the same range produced a slight but not significant increase in the desensitization rate. The similar degree and rate of desensitization for sarcosine and glycine at an EC100 concentration suggests that ‘glycine’-insensitive desensitization is similar for both co-agonists (Fig. 2B and D).
Less ‘glycine’-sensitive NMDAR desensitization occurs with higher glycine concentrations, and less Ca2+-dependent inactivation occurs with lower extracellular Ca2+ concentrations (Benveniste et al. 1990; Vyklicky et al. 1990; Clark et al. 1990). To determine whether a change in Ca2+-dependent inactivation contributed to our observations, we replaced the extracellular Ca2+ with Ba2+ to eliminate Ca2+-dependent inactivation (Clark et al. 1990; Krupp et al. 1996). Substituting Ba2+ for Ca2+ did not change the degree of NMDAR desensitization with an EC50 sarcosine or glycine concentration (Fig. 2E and F). This result suggests that significant Ca2+-dependent inactivation did not occur under these experimental conditions. More importantly for the question being considered here, NMDAR desensitization still was less with an EC50 concentration of sarcosine than glycine when Ba2+ replaced the extracellular Ca2+ (Fig. 2E and F). These results suggest that sarcosine did not alter Ca2+-dependent inactivation under our experimental conditions. However, Ca2+-dependent inactivation may differ with sarcosine and glycine at higher NMDA concentrations and with different intracellular Ca2+ buffers because Ca2+-dependent inactivation depends on these factors (Clark et al. 1990; Krupp et al. 1996). All of these results together suggest that less ‘glycine’-sensitive NMDAR desensitization occurs with sarcosine than glycine as the co-agonist.
NMDAR activation produces a larger Ca2+ influx and Ca2+-activated K+ current with sarcosine than with glycine
A well-known effect of NMDAR activation is a rise in intracellular Ca2+. We predicted that NMDAR activation would produce a greater Ca2+ influx with sarcosine than with glycine as the co-agonist. We expected this difference with subsaturating concentrations at which less NMDAR desensitization occurred with sarcosine than with glycine as the co-agonist. Accordingly, Ca2+ imaging using fura-FF showed that 100 μm NMDA plus an EC50 sarcosine concentration produced higher free intracellular Ca2+ concentrations than 100 μm NMDA plus an EC50 glycine concentration. We obtained this result using an extracellular Ca2+ of 1.5 mm (data not shown) and 3 mm (Fig. 3A).
Figure 3. Ca2+ influx and Ca2+-activated K+ currents following NMDAR activation are larger with sarcosine than with glycine as the co-agonist.
A, time course of change in fura-FF ratio induced by 100 μm NMDA + an EC50 sarcosine concentration (20 μm) (54 neurons examined in 4 separate experiments), 100 μm NMDA + an EC50 glycine concentration (60 nm) (61 neurons examined in 4 separate experiments), and 100 μm NMDA alone (61 neurons examined in 4 separate experiments). Symbols show the mean fura-FF ratio normalized to the baseline ratio. Only every third point is shown for clarity. B, 100 μm NMDA with an EC50 sarcosine (20 μm) or glycine (60 nm) concentration evoked an inward current followed by an outward current. Traces are from one neuron. C, currents evoked by 100 μm NMDA + 20 μm sarcosine without and with 1 mm Ba2+ added to the extracellular solution. Traces are from one neuron. In B and for the trace in C obtained without Ba2+, the decline of the NMDA current during NMDA application reflects both NMDAR desensitization and the opening of Ca2+-activated K+ channels. Accordingly, the NMDA current in C obtained in Ba2+ shows less decay during the NMDA application than the current in no Ba2+. D, mean charge transferred by Ca2+-activated K+ currents evoked by 100 μm NMDA with an EC20 or an EC50 sarcosine (N= 11) or glycine (N= 9) concentration. Charge transferred was normalized to cell capacitance. Sarcosine concentrations used were 6 μm and 20 μm; corresponding glycine concentrations were 30 nm and 60 nm. *P= 0.02 by paired t test. Data in B–D were obtained at −20 mV.
The Ca2+ influx associated with NMDAR activation results in the opening of Ca2+-activated K+ channels (Zorumski et al. 1989; Isaacson & Murphy, 2001; Shah & Haylett, 2002). Using an extracellular Ca2+ concentration of 3 mm and a pipette solution with 0.5 mm EGTA (see Methods), 100 μm NMDA plus glycine or sarcosine elicited an inward current followed by a slow outward current that outlasted the agonist application (Fig. 3B). Reducing extracellular Ca2+ to 0.5 mm or adding 1 mm Ba2+ to the extracellular solution decreased the amplitude of the outward current to 15 ± 6% (N= 6) and 14 ± 3% (N= 6) of control (Fig. 3C). The outward current had a reversal potential of −86 ± 5 mV (N= 5) as expected of a K+ current. These findings are consistent with the Ca2+-activated K+ currents described previously in cultured hippocampal neurons (Zorumski et al. 1989; Shah & Haylett, 2002).
Based on the Ca2+ imaging results, we predicted that NMDAR activation with a subsaturating sarcosine concentration would result in larger Ca2+-activated K+ currents than with a comparable glycine concentration. Ca2+-activated K+ currents evoked by 100 μm NMDA plus a subsaturating sarcosine concentration were larger than those evoked by 100 μm NMDA plus a comparable glycine concentration (Fig. 3B and D). The results were similar whether assessed using total charge transferred or peak amplitudes (data not shown). We note that less NMDAR desensitization still occurred with sarcosine than glycine at subsaturating concentrations under the conditions used to record Ca2+-activated K+ currents. One hundred micromolar NMDA currents desensitized by 48 ± 6% (N= 4) with an EC50 sarcosine concentration (20 μm) compared to 85 ± 3% (N= 4) with an EC50 glycine concentration (60 nm) under these conditions. Thus, these findings suggest that sustained NMDAR activation with sarcosine causes a larger Ca2+ influx and Ca2+-activated K+ current than NMDAR activation with glycine at subsaturating concentrations.
NMDA-induced neuronal death is similar whether sarcosine or glycine is the co-agonist
The smaller amount of NMDAR desensitization with sarcosine than with glycine at comparable subsaturating concentrations results in greater ion flux with sarcosine. We hypothesized that this difference in desensitization may translate into a difference in NMDAR-mediated neuronal death with these two co-agonists. The magnitude and direction of the difference may depend on the NMDAR subunit composition and whether extrasynaptic or synaptic NMDAR effects predominate (Vanhoutte & Bading, 2003; Waxman & Lynch, 2005). For example, synaptic NMDARs tend to promote survival whereas extrasynaptic NMDARs may promote neuronal death. We examined NMDAR-mediated neuronal death using a protocol similar to that used in prior studies focusing on the NMDAR glycine binding site in neurotoxicity (Shleper et al. 2005). We exposed cultured hippocampal neurons to 100 μm NMDA and an EC50 sarcosine (20 μm) or an EC50 glycine (60 nm) concentration for 3 h and assessed neuronal death by propidium iodide 24 h later. In parallel, we exposed neurons to 20 μm sarcosine, 60 nm glycine, 100 μm NMDA or vehicle using the same protocol. Only 100 μm NMDA + 20 μm sarcosine and 100 μm NMDA + 60 nm glycine increased death above baseline, though the amount of death in these two conditions was similar (Fig. 4). In these two conditions, we estimated that the extracellular solution contained an additional 100–200 nm glycine at the end of the 3 h incubation assuming a cytoplasmic glycine concentration of 1–2 mm (Supplisson & Roux, 2002). Our results suggest that sarcosine does not enhance NMDAR-mediated neuronal death compared to glycine despite producing less desensitization.
Figure 4. NMDAR-mediated neuronal death is similar with sarcosine or glycine as the co-agonist at subsaturating concentrations.
A, representative fields of propidium iodide-stained hippocampal neurons 24 h after being exposed to the indicated combinations of 100 μm NMDA, 20 μm sarcosine and 60 nm glycine for 3 h. Merged brightfield and fluorescence images are shown. B, mean percentage of propidium iodide-stained neurons 24 h after a 3 h exposure to vehicle alone, 20 μm sarcosine, 60 nm glycine or 100 μm NMDA, or the indicated combinations (N= 4 experiments). *P < 0.001 versus all other conditions except 100 μm NMDA + 60 nm glycine by one-way ANOVA. #P < 0.05 versus all other conditions except 100 μm NMDA + 20 μm sarcosine by one-way ANOVA.
Sarcosine and glycine affect synaptically activated NMDARs differently
Deactivation determines the decay rate of NMDAR-mediated postsynaptic currents (Cais et al. 2008). Prior studies indicate that ‘glycine’-insensitive but not ‘glycine’-sensitive desensitization influences deactivation and thereby helps to shape the decay of NMDAR-mediated postsynaptic currents (Lester & Jahr, 1992; Lester et al. 1993; Cais et al. 2008). Thus, if sarcosine and glycine only differ with respect to ‘glycine’-sensitive desensitization, then the kinetics of NMDAR-mediated postsynaptic currents with these co-agonists should be similar. To explore whether other NMDAR properties differ when sarcosine is the co-agonist rather than glycine, we examined the effect of sarcosine on NMDAR-mediated excitatory autaptic currents (EACs) (Fig. 5A). We isolated NMDAR-mediated EACs by using an extracellular solution with 10 μm 2,3-dioxo-6-nitro-1,2,3,4-tetrhydrobenzo[f]quinoxaline-7-sulfonamide (NBQX) to block AMPA receptors (AMPARs) and 10 μm bicuculline to block GABAA receptors (GABAAR). NMDAR-mediated EACs decayed according to a single exponential as described previously in this preparation (Wilcox et al. 1996). Peak amplitudes of NMDAR-mediated EACs were similar for sarcosine and glycine at an EC20 and an EC50 concentration, but they were smaller with sarcosine than glycine at an EC100 concentration (Fig. 5B). NMDAR-mediated EACs decayed faster with sarcosine than glycine, though 20–80% rise times were similar at all concentrations tested (Fig. 5C and D). The difference in decay rates suggests that sarcosine and glycine affect NMDAR deactivation differently.
Figure 5. NMDAR-mediated autaptic currents decay faster with sarcosine than glycine as the co-agonist.
A, NMDAR-mediated EACs evoked from a single neuron in the absence of added glycine or sarcosine (Control), in 20 μm sarcosine, and in 60 nm glycine at a faster (left panel) and slower (right panel) time scale. The extracellular solution contained 10 μm NBQX to block AMPARs, 10 μm bicuculline to block GABAARs, and no added Mg2+. All traces are the average of 5 traces in each condition. B, mean peak amplitudes of NMDAR-mediated EACs with an EC20 (N= 8), EC50 (N= 8) or EC100 (N= 8) concentration of sarcosine or glycine. *P= 0.001 by two-tailed paired t test. C, mean 20–80% rise times for NMDAR-mediated EACs with an EC20 (N= 3), EC50 (N= 5) or EC100 (N= 7) concentration of sarcosine or glycine. D, mean time constant of decay for NMDAR-mediated EACs with an EC20 (N= 8), EC50 (N= 8) or EC100 (N= 8) concentration of sarcosine or glycine. *P < 0.001 by two-tailed paired t test. Sarcosine concentrations used were 6 μm, 20 μm and 300 μm; corresponding glycine concentrations were 30 nm, 60 nm and 3 μm. All data in this figure were obtained at −75 mV.
NMDAR-dependent neuronal bursting is similar whether sarcosine or glycine is the co-agonist
The smaller amount of desensitization exhibited by NMDA currents with sarcosine as the co-agonist raises the possibility that NMDAR activation with sarcosine is more likely to promote hyperexcitability than NMDAR activation with glycine. We examined this possibility by comparing the effect of subsaturating sarcosine and glycine concentrations on neuronal bursting induced by omitting Mg2+ from the extracellular solution (Mangan & Kapur, 2004). We used this model because neuronal bursting in a nominally Mg2+ solution depends on NMDAR activation and on a neuronal network (Mangan & Kapur, 2004). Neurons exhibited spontaneous postsynaptic currents in an extracellular solution containing no added Mg2+. Prolonged spontaneous inward currents with superimposed postsynaptic currents occurred with the addition of an EC50 sarcosine (20 μm) or glycine (60 nm) concentration to the extracellular solution (Fig. 6A). d-2-amino-5-phosphonovaleric acid (d-APV, 100 μm) completely inhibited this bursting (N= 3). The prolonged inward currents probably correspond to the prolonged depolarizations observed with current-clamp recordings (Mangan & Kapur, 2004). However, the duration of these prolonged inward currents and their frequency were the same with 20 μm sarcosine and 60 nm glycine (Fig. 6B). This result suggests that sarcosine does not promote more excitability than glycine despite promoting less desensitization at subsaturating concentrations.
Figure 6. NMDAR-mediated bursting is similar with sarcosine or glycine as the co-agonist at a subsaturating concentration.
A, current traces from a neuron in an extracellular solution with no added Mg2+ (left panel) to which 20 μm sarcosine (middle panel) or 60 nm glycine (right panel) was added. Panels in bottom row show an expanded view of the trace above at the point marked with an asterisk. Scale bar is the same for all traces in top row. B, mean burst duration (left panel, N= 5, P= 0.51 by two-tailed paired t test) and interval between bursts (right panel, N= 5, P= 0.82 by two-tailed paired t test) with 20 μm sarcosine or 60 nm glycine.
Discussion
The principal finding of this study is that sarcosine is an NMDAR co-agonist, but some physiological effects of NMDAR activation with sarcosine and glycine differ. Sarcosine probably functions as an NMDAR co-agonist at the same binding site as glycine and d-serine because it increased NMDA currents in a DCKA-sensitive, dose-dependent manner. The results with NFPS and with the neurons grown in the absence of an astrocytic feeder layer indicate that glycine is not responsible for the effects of sarcosine on NMDARs. Specifically, sarcosine's actions do not result from an increase in extracellular glycine via GlyT1 inhibition or GlyT1-mediated heteroexchange. The differing effects of sarcosine and glycine on NMDAR desensitization and on the time course of NMDAR-mediated EACs also indicate that glycine does not mediate the effects of sarcosine. These results argue against glycine being produced by sarcosine dehydrogenase demethylating sarcosine to glycine (Deutsch et al. 2006). More importantly, our findings indicate that the identity of the NMDA co-agonist may help determine the physiological consequences of NMDAR activation. The clinical implication is that structural differences in NMDAR co-agonists may influence the efficacy and tolerability of these agents in schizophrenics.
Functional effects of NMDAR activation by sarcosine
The finding that sarcosine probably is an agonist at a known glycine binding site is not surprising given its structural similarity to glycine. Sarcosine joins guanidinoethyl sulphonate (Mellor et al. 2000; Sergeeva et al. 2002) and dihydrokainate (Thio et al. 1991) as transport inhibitors capable of interacting with their respective receptors. However, sarcosine was less potent than glycine at NMDARs, and it did not quite appear to be a full agonist. Thus, sarcosine is slightly less effective than glycine at gating NMDARs. Perhaps the addition of a methyl group creates steric hindrance with the glycine binding site.
NMDAR-mediated effects with sarcosine as the co-agonist differed from those with glycine. A notable difference was that less NMDAR desensitization occurred with sarcosine than glycine at comparable subsaturating concentrations on their respective dose–response curves. The smaller amount of desensitization with sarcosine as the co-agonist resulted in greater Ca2+ influx with sustained NMDA applications and probably explains the larger Ca2+-activated K+ currents with these applications. Physiologically, repetitive stimulation of synaptic NMDARs results in activation of Ca2+-activated K+ currents (Isaacson & Murphy, 2001). Thus, repetitive stimulation of NMDARs in the presence of sarcosine and glycine may have different effects on neuronal excitability. Another consequence of sustained NMDA applications is neuronal death. Although sustained NMDA applications produced greater Ca2+ influx with sarcosine than with glycine as the co-agonist, NMDAR activation with sarcosine did not cause more death than with glycine. The reason for sarcosine not producing greater toxicity than glycine is unclear, but it could be a consequence of other effects of weaker NMDAR desensitization or a differential effect on synaptic versus extrasynaptic NMDARs.
Compared to glycine, sarcosine decreased the extent and the rate of NMDAR desensitization but increased the decay rate of NMDAR-mediated EACs. In addition, sarcosine and glycine affected NMDAR desensitization differently only at subsaturating concentrations, but they affected NMDAR-mediated EAC decay rates differently at all concentrations tested. This difference in concentration dependence supports the notion that ‘glycine’-sensitive desensitization does not contribute significantly to the NMDAR-mediated EAC decay as reported earlier (Lester et al. 1993). A more important process governing the decay of NMDAR-mediated EACs is deactivation (Lester & Jahr, 1992; Cais et al. 2008). Although these prior studies show that desensitization influences NMDAR deactivation, only ‘glycine’-insensitive desensitization occurred in these studies because they used saturating glycine concentrations and very low extracellular Ca2+ concentrations (Lester & Jahr, 1992; Cais et al. 2008). Given that sarcosine and glycine were similar with respect to ‘glycine’-insensitive desensitization, the faster NMDAR-mediated EAC decay rate with sarcosine suggests that NMDAR deactivation is faster with sarcosine than glycine. Taken together, our results suggest that both NMDAR desensitization and deactivation differ depending on whether sarcosine or glycine is the co-agonist. The opposing effects of sarcosine on NMDAR desensitization and deactivation may explain why bursting did not differ with the two co-agonists.
The extent of NMDAR desensitization decreased and the rate slowed as the sarcosine concentration increased from an EC20 to an EC50 concentration. This result suggests that when sarcosine is the co-agonist, NMDAR desensitization does not reflect sarcosine dissociating from the receptor as proposed by one model for ‘glycine’-sensitive desensitization (Benveniste et al. 1990). Rather, the effect of sarcosine on NMDAR desensitization resembles the inhibition of AMPAR desensitization by cyclothiazide, which produces less and slower desensitization with increasing concentrations (Rammes et al. 1996). Perhaps sarcosine slows NMDAR entry into a desensitized state, which is one of cyclothiazide's many effects on AMPARs (Fucile et al. 2006).
Clinical implications of sarcosine being an NMDAR co-agonist and GlyR agonist
The agonist effects of sarcosine at NMDARs may contribute to the beneficial effects schizophrenics treated with sarcosine have experienced in clinical trials (Tsai et al. 2004a; Lane et al. 2008). The working hypothesis for these trials was that the symptoms would improve by increasing NMDAR function by inhibiting GlyT1 and increasing the ambient glycine concentration. The findings of these trials are consistent with this hypothesis. Although these trials did not measure serum and cerebrospinal fluid sarcosine concentrations, the clinical benefit may also result from sarcosine acting as a NMDAR co-agonist because it is a co-agonist at slightly lower concentrations (EC50 26 μm) than it is a GlyT1 inhibitor (IC50 40–150 μm) (Lopez-Corcuera et al. 1998; Herdon et al. 2001). The extent to which the co-agonist effects of sarcosine are relevant will depend on its concentration relative to that of glycine because glycine is 430 times more potent. Regardless of sarcosine's mechanism of action, the underlying assumption is that the NMDAR glycine binding site is not saturated, though experimental evidence for and against this assumption exists (Chen et al. 2003; Le Meur et al. 2007).
The differences between NMDAR-mediated physiological effects with sarcosine and glycine as the co-agonist may be clinically relevant. For example, the comparatively weaker desensitization with sarcosine as the co-agonist may be important for enhancing tonic NMDAR activation in schizophrenics (Le Meur et al. 2007). The accompanying increase in Ca2+ influx may be important to learning and memory (Franks & Sejnowski, 2002). On the other hand, the faster NMDA EAC decay kinetics and larger Ca2+-activated K+ currents with sarcosine as a co-agonist may allow it to enhance NMDAR function without resulting in hyperexcitability or neuronal death. These characteristics may provide sarcosine with an advantage over other NMDAR co-agonists and GlyT1 inhibitors from a clinical perspective.
Incidentally, our results may provide insight into the pathophysiology of sarcosinaemia, a rare and controversial, autosomal recessive condition diagnosed by elevated sarcosine concentrations in plasma and urine (Deutsch et al. 2006). Sarcosinaemia has variable clinical features ranging from no symptoms to mental retardation, growth retardation, hypertonia and vomiting. Our results support the notion that the symptoms of sarcosinaemia may result from excessive NMDAR activation (Deutsch et al. 2006).
In conclusion, sarcosine is an NMDAR co-agonist in addition to being a GlyT1 inhibitor and an endogenous amino acid involved with one-carbon metabolism. These diverse functions are reminiscent of other endogenous amino acids that are neurotransmitters. If sarcosine is released from neurons or glia, then it would fulfill the criteria for a non-classical neurotransmitter such as nitric oxide or d-serine (Snyder & Ferris, 2000; Wolosker et al. 2008). Its ability to enhance NMDAR function via more than one mechanism may make it an ideal therapeutic agent for schizophrenia. The difference in the physiological effects of NMDAR activation with sarcosine compared to glycine as the co-agonist may be advantageous clinically. Sarcosine may exemplify the concept of the ideal drug for treating schizophrenia being one with multiple targeted mechanisms of action (Roth et al. 2004).
Acknowledgments
We thank Nicholas Rensing for preparing and maintaining the neuronal cultures. We thank Dennis Dietzen, PhD, for performing high performance liquid chromatography/tandem mass spectrometry assays. NIH grant K02 NS043278, the Alafi Neuroimaging Laboratory, the Hope Center for Neurological Disorders and NIH Neuroscience Blueprint Center Core Grant P30 NS057105 to Washington University supported this work.
Glossary
Abbreviations
- DCKA
5,7-dichlorokynurenic acid
- EACs
excitatory autaptic currents
- GlyT1
type I glycine transporter
- NBQX
2,3-dioxo-6-nitro-1,2,3,4-tetrhydrobenzo[f]quinoxaline-7-sulfonamide
- NFPS
N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine
- NMDAR
NMDA receptor
Author contributions
All authors contributed to the conception and design, analysis and interpretation of the data; all drafted and revised the manuscript for important intellectual content; and all approved the final published version. The authors performed the experiments at Washington University.
References
- Atkinson BN, Bell SC, De Vivo M, Kowalski LR, Lechner SM, Ognyanov VI, Tham CS, Tsai C, Jia J, Ashton D, Klitenick MA. ALX 5407: a potent, selective inhibitor of the hGlyT1 glycine transporter. Mol Pharmacol. 2001;60:1414–1420. doi: 10.1124/mol.60.6.1414. [DOI] [PubMed] [Google Scholar]
- Aubrey KR, Vandenberg RJ. N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS) is a selective persistent inhibitor of glycine transport. Br J Pharmacol. 2001;134:1429–1436. doi: 10.1038/sj.bjp.0704381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron BM, Harrison BL, Miller FP, McDonald IA, Salituro FG, Schmidt CJ, Sorensen SM, White HS, Palfreyman MG. Activity of 5,7-dichlorokynurenic acid, a potent antagonist at the N-methyl-D-aspartate receptor-associated glycine binding site. Mol Pharmacol. 1990;38:554–561. [PubMed] [Google Scholar]
- Benveniste M, Clements J, Vyklicky L, Jr, Mayer ML. A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol. 1990;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer LD, Thibault O, Staton J, Thibault V, Rogers JT, Garcia-Ramos G, Kraner S, Landfield PW, Porter NM. Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res. 2007;1151:20–31. doi: 10.1016/j.brainres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Cais O, Sedlacek M, Horak M, Dittert I, Vyklicky L., Jr Temperature dependence of NR1/NR2B NMDA receptor channels. Neuroscience. 2008;151:428–438. doi: 10.1016/j.neuroscience.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Chen L, Muhlhauser M, Yang CR. Glycine tranporter-1 blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. J Neurophysiol. 2003;89:691–703. doi: 10.1152/jn.00680.2002. [DOI] [PubMed] [Google Scholar]
- Clark GD, Clifford DB, Zorumski CF. The effect of agonist concentration, membrane voltage and calcium on N-methyl-D-aspartate receptor desensitization. Neuroscience. 1990;39:787–797. doi: 10.1016/0306-4522(90)90261-2. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Long KD, Gaskins B, Mastropaolo J. Rare neurodevelopmental abnormalities of sarcosinemia may involve glycinergic stimulation of a primed N-methyl-D-aspartate receptor. Clin Neuropharmacol. 2006;29:361–363. doi: 10.1097/01.WNF.0000236767.46526.1F. [DOI] [PubMed] [Google Scholar]
- Erreger K, Traynelis SF. Allosteric interaction between zinc and glutamate binding domains on NR2A causes desensitization of NMDA receptors. J Physiol. 2005;569:381–393. doi: 10.1113/jphysiol.2005.095497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci. 2005;30:325–333. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Franks KM, Sejnowski TJ. Complexity of calcium signalling in synaptic spines. Bioessays. 2002;24:1130–1144. doi: 10.1002/bies.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S, Miledi R, Eusebi F. Effects of cyclothiazide on GluR1/AMPA receptors. Proc Natl Acad Sci U S A. 2006;103:2943–2947. doi: 10.1073/pnas.0511063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Roth BL. The pipeline and future of drug development in schizophrenia. Mol Psychiatry. 2007;12:904–922. doi: 10.1038/sj.mp.4002062. [DOI] [PubMed] [Google Scholar]
- Herdon HJ, Godfrey FM, Brown AM, Coulton S, Evans JR, Cairns WJ. Pharmacological assessment of the role of the glycine transporter GlyT-1 in mediating high-affinity glycine uptake by rat cerebral cortex and cerebellum synaptosomes. Neuropharmacology. 2001;41:88–96. doi: 10.1016/s0028-3908(01)00043-0. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Murphy GJ. Glutamate-mediated extrasynaptic inhibition: direct coupling of NMDA receptors to Ca2+-activated K+ channels. Neuron. 2001;31:1027–1034. doi: 10.1016/s0896-6273(01)00428-7. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Sur C, Burno M, Mallorga PJ, Williams JB, Figueroa DJ, Wittmann M, Lemaire W, Conn PJ. The glycine transporter type 1 inhibitor N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine potentiates NMDA receptor-mediated responses in vivo and produces an antipsychotic profile in rodent behavior. J Neurosci. 2003;23:7586–7591. doi: 10.1523/JNEUROSCI.23-20-07586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Heinemann SF, Westbrook GL. Calcium-dependent inactivation of recombinant N-methyl-D-aspartate receptors is NR2 subunit specific. Mol Pharmacol. 1996;50:1680–1688. [PubMed] [Google Scholar]
- Lane HY, Liu YC, Huang CL, Chang YC, Liau CH, Perng CH, Tsai GE. Sarcosine (N-methylglycine) treatment for acute schizophrenia: a randomized, double-blind study. Biol Psychiatry. 2008;63:9–12. doi: 10.1016/j.biopsych.2007.04.038. [DOI] [PubMed] [Google Scholar]
- Le Meur K, Galante M, Angulo MC, Audinat E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J Physiol. 2007;580:373–383. doi: 10.1113/jphysiol.2006.123570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Zukin RS, Bennett MV. Glycine decreases desensitization of N-methyl-D-aspartate (NMDA) receptors expressed in Xenopus oocytes and is required for NMDA responses. Proc Natl Acad Sci U S A. 1990;87:2354–2358. doi: 10.1073/pnas.87.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RA, Jahr CE. NMDA channel behavior depends on agonist affinity. J Neurosci. 1992;12:635–643. doi: 10.1523/JNEUROSCI.12-02-00635.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RA, Tong G, Jahr CE. Interactions between the glycine and glutamate binding sites of the NMDA receptor. J Neurosci. 1993;13:1088–1096. doi: 10.1523/JNEUROSCI.13-03-01088.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Corcuera B, Martinez-Maza R, Nunez E, Roux M, Supplisson S, Aragon C. Differential properties of two stably expressed brain-specific glycine transporters. J Neurochem. 1998;71:2211–2219. doi: 10.1046/j.1471-4159.1998.71052211.x. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Kleckner NW, Wyrick S, Dingledine R. Structural requirements for activation of the glycine coagonist site of N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Mol Pharmacol. 1989;36:556–565. [PubMed] [Google Scholar]
- Mangan PS, Kapur J. Factors underlying bursting behavior in a network of cultured hippocampal neurons exposed to zero magnesium. J Neurophysiol. 2004;91:946–957. doi: 10.1152/jn.00547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Gorfinkel Y, Halman S, Lowe JA, Periyalwar P, Schmidt CJ, Bergeron R. Glycine transporter type 1 blockade changes NMDA receptor-mediated responses and LTP in hippocampal CA1 pyramidal cells by altering extracellular glycine levels. J Physiol. 2004;557:489–500. doi: 10.1113/jphysiol.2004.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor JR, Gunthorpe MJ, Randall AD. The taurine uptake inhibitor guanidinoethyl sulphonate is an agonist at γ-aminobutyric acidA receptors in cultured murine cerebellar granule cells. Neurosci Lett. 2000;286:25–28. doi: 10.1016/s0304-3940(00)01082-x. [DOI] [PubMed] [Google Scholar]
- Rabe CS, Tabakoff B. Glycine site-directed agonists reverse the actions of ethanol at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1990;38:753–757. [PubMed] [Google Scholar]
- Rammes G, Swandulla D, Collingridge GL, Hartmann S, Parsons CG. Interactions of 2,3-benzodiazepines and cyclothiazide at AMPA receptors: patch clamp recordings in cultured neurones and area CA1 in hippocampal slices. Br J Pharmacol. 1996;117:1209–1221. doi: 10.1111/j.1476-5381.1996.tb16718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Sather W, Johnson JW, Henderson G, Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990;4:725–731. doi: 10.1016/0896-6273(90)90198-o. [DOI] [PubMed] [Google Scholar]
- Schultz SH, North SW, Shields CG. Schizophrenia: a review. Am Fam Physician. 2007;75:1821–1829. [PubMed] [Google Scholar]
- Sergeeva OA, Chepkova AN, Haas HL. Guanidinoethyl sulphonate is a glycine receptor antagonist in striatum. Br J Pharmacol. 2002;137:855–860. doi: 10.1038/sj.bjp.0704940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Haylett DG. K+ currents generated by NMDA receptor activation in rat hippocampal pyramidal neurons. J Neurophysiol. 2002;87:2983–2989. doi: 10.1152/jn.2002.87.6.2983. [DOI] [PubMed] [Google Scholar]
- Shleper M, Kartvelishvily E, Wolosker H. D-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J Neurosci. 2005;25:9413–9417. doi: 10.1523/JNEUROSCI.3190-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH, Ferris CD. Novel neurotransmitters and their neuropsychiatric relevance. Am J Psychiatry. 2000;157:1738–1751. doi: 10.1176/appi.ajp.157.11.1738. [DOI] [PubMed] [Google Scholar]
- Supplisson S, Roux MJ. Why glycine transporters have different stoichiometries. FEBS Lett. 2002;529:93–101. doi: 10.1016/s0014-5793(02)03251-9. [DOI] [PubMed] [Google Scholar]
- Thio LL, Clifford DB, Zorumski CF. Characterization of quisqualate receptor desensitization in cultured postnatal rat hippocampal neurons. J Neurosci. 1991;11:3430–3441. doi: 10.1523/JNEUROSCI.11-11-03430.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thio LL, Yamada KA. Differential presynaptic modulation of excitatory and inhibitory autaptic currents in cultured hippocampal neurons. Brain Res. 2004;1012:22–28. doi: 10.1016/j.brainres.2004.02.077. [DOI] [PubMed] [Google Scholar]
- Thio LL, Zhang HX. Modulation of inhibitory glycine receptors in cultured embryonic mouse hippocampal neurons by zinc, thiol containing redox agents and carnosine. Neuroscience. 2006;139:1315–1327. doi: 10.1016/j.neuroscience.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Tsai G, Lane HY, Yang P, Chong MY, Lange N. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 2004a;55:452–456. doi: 10.1016/j.biopsych.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Tsai G, Ralph-Williams RJ, Martina M, Bergeron R, Berger-Sweeney J, Dunham KS, Jiang Z, Caine SB, Coyle JT. Gene knockout of glycine transporter 1: characterization of the behavioral phenotype. Proc Natl Acad Sci U S A. 2004b;101:8485–8490. doi: 10.1073/pnas.0402662101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland PM, Midttun O, Windelberg A, Svardal A, Skalevik R, Hustad S. Quantitative profiling of folate and one-carbon metabolism in large-scale epidemiological studies by mass spectrometry. Clin Chem Lab Med. 2007;45:1737–1745. doi: 10.1515/CCLM.2007.339. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Bading H. Opposing roles of synaptic and extrasynaptic NMDA receptors in neuronal calcium signalling and BDNF gene regulation. Curr Opin Neurobiol. 2003;13:366–371. doi: 10.1016/s0959-4388(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Vyklicky L, Jr, Benveniste M, Mayer ML. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- Wilcox KS, Fitzsimonds RM, Johnson B, Dichter MA. Glycine regulation of synaptic NMDA receptors in hippocampal neurons. J Neurophysiol. 1996;76:3415–3424. doi: 10.1152/jn.1996.76.5.3415. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Dumin E, Balan L, Foltyn VN. D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J. 2008;275:3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- Yee BK, Balic E, Singer P, Schwerdel C, Grampp T, Gabernet L, Knuesel I, Benke D, Feldon J, Mohler H, Boison D. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J Neurosci. 2006;26:3169–3181. doi: 10.1523/JNEUROSCI.5120-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Thio LL, Clark GD, Clifford DB. Calcium influx through N-methyl-D-aspartate channels activates a potassium current in postnatal rat hippocampal neurons. Neurosci Lett. 1989;99:293–299. doi: 10.1016/0304-3940(89)90462-x. [DOI] [PubMed] [Google Scholar]