Abstract
Chronic exercise has been reported to improve cognitive function. However, whether and how different types of exercise affect various learning and memory tasks remain uncertain. To address this issue, male BALB/c mice were trained for 4 weeks under two different exercise protocols: moderate treadmill running or voluntary wheel running. After exercise training, their spatial memory and aversive memory were evaluated by a Morris water maze and by one-trial passive avoidance (PA), respectively. Levels of neural plasticity-related proteins, i.e. brain-derived neurotrophic factor (BDNF), tropomyosin-related kinase B (TrkB) and synaptotagmin I (Syt I), in hippocampus and amygdala were determined by ELISA or immunoblotting. Finally, the functional roles of these proteins in the basolateral amygdala were verified by locally blocking them with K252a (a TrkB kinase inhibitor), or lentivirus expressing Syt I shRNA. We found that (1) although both moderate treadmill running and wheel running improved the Morris water maze performance, only the former improved PA performance; (2) likewise, both exercise protocols upregulated the BDNF–TrkB pathway and Syt I in the hippocampus, whereas only treadmill exercise upregulated their expression levels in the amygdala; (3) local injection of K252a abolished the treadmill exercise-facilitated PA performance and upregulation of amygdalar TrkB and Syt I; and (4) local administration of Syt I shRNA abolished the treadmill exercise-facilitated PA performance and upregulation of amygdalar Syt I. Therefore, our results support the notion that different forms of exercise induce neuroplasticity changes in different brain regions, and thus exert diverse effects on various forms of learning and memory.
Physical activity has beneficial effects on cognitive function (for reviews see Vaynman & Gomez-Pinilla, 2006; Cotman et al. 2007). Although forced running and voluntary running are the two most commonly used chronic exercise protocols in rodent models, whether and how they exert differential effects on the brain function are often controversial. Studies using both exercise protocols show that they have different effects on anxiety-like behaviours (Dishman, 1997; Burghardt et al. 2004; Leasure & Jones, 2008), neurogenesis (Leasure & Jones, 2008), hippocampal brain-derived neurotrophic factor (BDNF) and synapsin-1 expression (Ploughman et al. 2005), monoamine neurotransmitters (Dishman, 1997), and neuroprotection in stroke (Hayes et al. 2008). In contrast, either treadmill exercise or wheel running improves hippocampus-dependent spatial learning and memory (van Praag et al. 1999; Ang et al. 2006; Huang et al. 2006). Recent reports indicate that treadmill exercise improves aversive memory (Alaei et al. 2006; Chen et al. 2008; Liu et al. 2008). Whether voluntary wheel running affects aversive memory remains to be clarified, as it only improves contextual fear conditioning, but not auditory-cued fear conditioning (Baruch et al. 2004).
Microarray studies have demonstrated that voluntary wheel running increases the hippocampal expression of BDNF and other genes involved with synaptic trafficking, such as synaptotagmin (Syt) (Tong et al. 2001; Molteni et al. 2002). We and others have reported recently that treadmill running increased the hippocampal protein levels of BDNF, tropomyosin-related kinase B (TrkB) and Syt I (Soya et al. 2007b; Liu et al. 2008). Previous studies have shown that over-expression of TrkB, the receptor of BDNF, facilitates learning and memory (Koponen et al. 2004), whereas inhibition or downregulation of BDNF or TrkB impairs memory formation (Ma et al. 1998; Mu et al. 1999; Minichiello et al. 1999; Rattiner et al. 2004). In addition, knockout of mouse Syt, a Ca2+-dependent synaptic vesicle fusion protein, results in impairments in contextual fear conditioning and associative passive avoidance memory (Ferguson et al. 2000, 2004). Since these molecules are very important for cognitive function, they are good candidates for mediating the exercise effects on different types of learning and memory.
Previously, we have demonstrated that hippocampal protein levels of Syt and TrkB were positively correlated with passive avoidance (PA) testing latencies (Liu et al. 2008). As the amygdala represents the primary brain region associated with the emotion of fear, and the basolateral amygdala (BLA), in particular, modulates fear memory consolidation (Davis, 1992; McGaugh et al. 2002), it is essential to unravel whether different exercise regimens differentially alter the expression of these proteins in the amygdala, and to verify their roles in the differential effects of various exercise protocols on aversive memory. This study was aimed to answer these questions, and our results showed that moderate treadmill exercise, but not voluntary wheel running, facilitated aversive memory, at least in part, by upregulating the BDNF–TrkB pathway and Syt I expression in the amygdala.
Methods
Animals
The present study was conducted in accordance with the policies and procedures described in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and the procedures were approved by the Institutional Animal Care and Use Committee of National Cheng Kung University, Tainan, Taiwan. Male BALB/c mice (3 months old), purchased from National Cheng Kung University Animal Center (Tainan, Taiwan), were divided into exercise groups (treadmill or wheel running) and their corresponding control groups. Mouse chow and water were available ad libitum. All animals were housed in an environmentally controlled room (temperature 25 ± 1°C; 12 h light/12 h dark cycle) in groups of four (for treadmill study) or two (for wheel running study) per cage. All efforts were made to minimize the number of animals used and their suffering.
Exercise protocols
Two different types of exercise protocols were used: mandatory treadmill running and voluntary wheel running. In the former, a moderate and relatively stress-free treadmill exercise running protocol was performed (Liu et al. 2008). In brief, after 1 week treadmill familiarization to eliminate novel and stress effects, animals in treadmill exercise groups ran on a levelled motor-driven treadmill (Model T510E, Diagnostic and Research Instruments Co., Taoyuan, Taiwan) at the speed of 10 m min−1 for 20–60 min per day, 5 days per week for the first week, followed by 60 min per day at the same speed, 5 days per week for the other 3 weeks. In order to minimize the possible stress associated with treadmill exercise, the front end of the treadmill was covered with a dark cloth to attract the mice to run forward. A gentle tail touch was sufficient to keep mice running and the entire training process was carried out without using any tail shock. The control groups were placed on the treadmill for 10 min each day without receiving any exercise training. For the study of voluntary wheel running, age-matched animals in the exercise group were placed in cages equipped with running wheels for mice (TSE, Bad Homburg, Germany), whereas animals in the control group were housed in cages with an immobile control wheel for 28 days. Each cage accommodated two mice. After exercise training, the animals were used for different types of experiments. The first set of mice was subjected to behavioural tests. In a second set, mice were killed by CO2 inhalation, followed by decapitation, at different time points (i.e. 0 and 24 h after the last treadmill running; or immediately and 24 h after wheel removal) to collect brain tissues for measuring protein expression in the hippocampus and amygdala. The third set of mice was injected with inhibitors into their BLA and subsequently used for either behavioural tests or brain tissue collections.
Behavioural tests
One-trial PA test
One day after the completion of exercise running, a set of animals was subjected to PA tests to assess their performance in aversive learning and memory as described in our recent study (Liu et al. 2008). In the training trial, the mouse was placed at the far end of the illuminated compartment and received a footshock (0.5 mA, 1 s) immediately after entering the dark compartment. The length of time that they stayed in the light compartment was recorded as the pre-shock training retention latency. Twenty-four hours later, the animal was placed into the illuminated compartment again, and the time that they stayed in the light compartment before stepping into the dark compartment was recorded as the testing retention latency. The ceiling score was assigned as 600 s.
Morris water maze test
The spatial learning and memory capability was evaluated by Morris water maze in a separate set of mice. A circular plastic pool, 1.2 m in diameter, and 0.6 m in height, was filled with water (20 ± 2°C) 0.5 cm above a hidden plexiglass platform (10 cm in diameter), which was placed at a specific location away from the edge of the pool. Mice were subjected to four trials a session with a 10 min intertrial interval, two sessions a day, one in the morning and the other 6 h later in the afternoon. A total of four sessions were completed in 2 days. In each trial, animals were allowed to find the platform within 120 s. Those who could not find the platform within this period of time would be guided to the platform. Animals were allowed to stay on the platform for 30 s before the next trial. The time each animal took to reach the platform was recorded as the escape latency. A probe test was performed 2 days after the acquisition phase was finished. All data were recorded and analysed by using a computerized video-tracking system (EthoVision, Noldus, Wageningen, The Netherlands). To rule out the effects of differential motor activities/motivation between control and exercise animals, the visible platform experiments were performed following the water maze acquisition.
Immunoblotting of TrkB and Syt I
The hippocampal or amygdalar homogenate was centrifuged at 13 000 g for 15 min at 4°C and the supernatant was collected. Protein concentration was determined by Bio-Rad Protein Assay (BioRad, Hercules, CA, USA). Sample proteins were separated on a 10% SDS-polyacrylamide gel, transferred to a polyvinylidine difluoride membrane, blocked by 5% non-fat milk and incubated with primary mouse monoclonal antibody against Syt I (SYA-130, 1:10 000, Stressgen, Victoria, BC, Canada), or rabbit polyclonal antibody against TrkB (anti-TrkB: sc-8316, 1:5000; Santa Cruz, CA, USA). After being washed, the membrane was incubated with secondary antibodies (anti-mouse IgG for synaptotagmin, 1:10 000; anti-rabbit IgG for TrkB, 1:5000; Amersham, Buckinghamshire, UK), and the bound antibody was detected using an enhanced chemiluminescence detection kit (Amersham, Buckinghamshire, UK). For the gel loading control, membranes were re-probed with a monoclonal anti-β-actin antibody (Sigma, A5441, 1:10 000; St Louis, MO, USA). Protein levels in the exercise groups were expressed as a percentage of respective control values.
Measurement of BDNF and serum corticosterone levels
The BDNF protein concentrations in the hippocampal and amygdalar homogenates or serum corticosterone levels were determined in duplicates using enzyme-linked immunosorbent assay (ELISA) kits (BDNF: Promega, Madison, WI, USA; corticosterone: Cayman, Ann Arbor, MI, USA) following the manufacturer's instructions.
Measurement of skeletal muscle aerobic capacity
An increase in citrate synthase activity (reflecting an improved aerobic metabolism in mitochondria) in the skeletal muscle is commonly used to confirm the exercise training effect. Therefore, the citrate synthase activity in soleus muscles was measured using a method described previously (Jen et al. 2002). Briefly, the homogenized muscle specimens were centrifuged at 17 000 g at 4°C for 15 min and the supernatant was collected. After adding dithionitrobenzoate (DTNB), acetyl CoA and oxaloacetate to the sample, the mitochondrial citrate synthase activity was determined spectrophotometrically by measuring the time-dependent absorbance changes at 412 nm.
Preparation of Syt I shRNA-expressing lentivirus
The plasmids (pLKO.1-puro) containing Syt I shRNA (5′-CAGTCTTCAATGAACAGTTTA-3′) or luciferase shRNA (5′-CTTCGAAATGTCCGTTCGGTT-3′), which served as a negative control (NC shRNA), were obtained from the National RNAi Core Facility in Academia Sinica (Taipei, Taiwan). The effectiveness of Syt I shRNA was validated by transfecting the Syt I shRNA- or NC shRNA-expressing plasmid into cultured mouse neuroblastoma (N2a) cells followed by Western blotting of Syt I. There was about 85% inhibition of Syt I protein expression in the Syt I shRNA-transfected cells. Lentivirus packaging was done by transient cotransfection of the shRNA expression plasmid, psPAX2 packaging plasmid and pMD2G envelope plasmid into cells of the human embryonic kidney cell line HEK293T. The medium was collected 48 h after transfection, and the debris was cleared by low-speed centrifugation for 5 min at 1000 r.p.m. High-titre stocks were prepared by ultracentrifugation for 100 min at 26 000 r.p.m. The viral pellet was re-suspended in serum-free medium and stored at −80°C. Viral titres were determined by the infection of HEK293T cells. The final viral titres were ∼107 transducing units per millilitre.
Inhibition of BDNF pathway or Syt I expression by microinjection of K252a or lentivirus into BLA
For BDNF pathway-blocking experiments, a set of mice received a microinjection of K252a after the last bout of treadmill exercise. Both control and exercised mice were anaesthetized with an intraperitoneal injection of a mixed anaesthetic (2 ml kg−1; final concentrations: ketamine 40 mg ml−1, atropine 0.01 mg ml−1 and rompun 6 mg ml−1). The skull was exposed and the microinjection was performed bilaterally at BLA (anteroposterior −1.4 mm, dorsoventral −3.9 mm, mediolateral ± 2.9 mm from bregma). One microlitre of either 15 μm K252a (Calbiochem, La Jolla, CA, USA) or artificial cerebrospinal fluid (ACSF) in 50% DMSO was injected into each side at an infusion rate of 0.2 μl min−1 from a 29-gauge needle attached to a 10 μl syringe. Three days after surgery, the mice were subjected to PA tests, and the amygdalar tissues were obtained afterward for immunoblot analyses.
For Syt I-blocking experiments, mice received a microinjection of lentivirus in a similar way after the first week of treadmill running. Briefly, each side of BLA received 3 μl of lentiviruses, which expressed Syt I shRNA or NC shRNA. Animals in the exercise groups continued the remaining exercise programme 3 days after the injection. At the end of the exercise training period, mice were subjected to PA tests, and subsequently killed to collect the amygdala for further analysis.
Statistical analysis
Data were expressed as mean ±s.e.m., except that the PA retention latencies were expressed as median ± quartiles. Because the PA retention latency values were truncated at 600 s, the comparison of this parameter was performed by using the non-parametric Mann–Whitney U test or the Kruskal–Wallis test followed by Dunn's multiple comparison test whenever appropriate. ANOVA with repeated measures was used to analyse the learning curve of the Morris water maze. The probe test of the Morris water maze and protein levels were analysed by unpaired Student's t tests or one-way ANOVA followed by Tukey's multiple comparison whenever appropriate. Significance was established at P < 0.05. Sample sizes were indicated by n.
Results
Effects of different types of exercise on skeletal muscle aerobic capacity
Our standard treadmill exercise protocol significantly elevated the citrate synthase activity in soleus muscles (0.31 ± 0.01 vs. 0.26 ± 0.01 μmol min−1 (mg protein)−1 for treadmill and control, respectively, P < 0.05, n= 7), indicating that the moderate-intensity treadmill exercise training was effective to elevate aerobic capacity. In contrast, voluntary wheel running did not significantly alter this parameter (0.27 ± 0.01 vs. 0.26 ± 0.01 μmol min−1 (mg protein)−1 for wheel running and control, respectively, P > 0.05, n= 9). The increase in skeletal muscle aerobic capacity was dependent on exercise intensity, as mice trained at mild intensity (7 m min−1 instead of 10 m min−1 running speed) on the treadmill showed unaltered citrate synthase activity (0.27 ± 0.01 vs. 0.26 ± 0.01 μmol min−1 (mg protein)−1 for exercise and control groups, respectively, P > 0.05, n= 8).
Effects of different types of exercise on spatial and aversive learning and memory
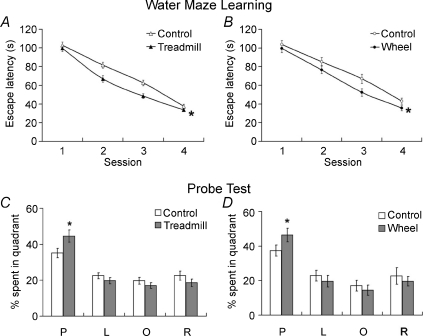
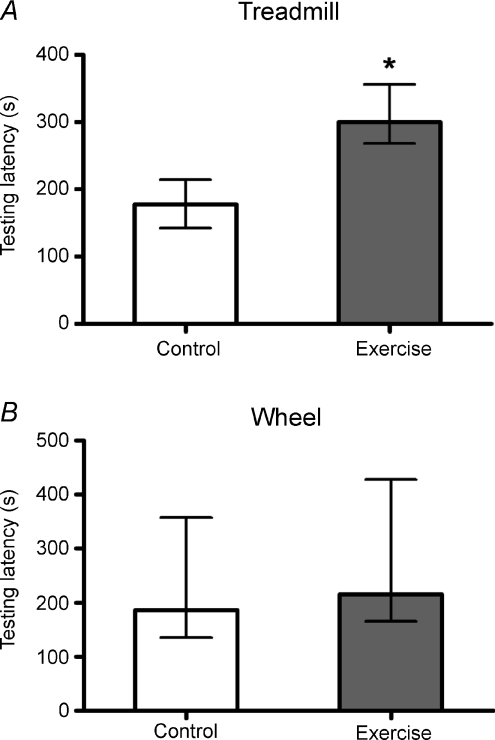
In this study, the spatial learning and memory was assessed by the Morris water maze. We confirmed that either treadmill running or voluntary wheel running for 4 weeks facilitated the spatial learning curve (Fig. 1A and B). Moreover, the probe test results indicated that mice trained either way spent more time in the zone with the platform than their control groups (Fig. 1C and D). However, their effects on aversive learning and memory, as assessed by the PA performance, were different. Our results showed that treadmill running, but not wheel running, significantly increased the 24 h retention latency (Fig. 2). Taken together, these two types of exercise training exert similar effects on spatial learning and memory; but they differentially affected aversive learning and memory. In order to investigate the possible role of exercise intensity in this regard, we also evaluated the PA performance in mice trained with a mild-intensity of treadmill running. Their PA performance remained the same as the control (see Fig. S1 in the Supplemental material, available online only).
Figure 1. Effects of moderate treadmill running or voluntary wheel running on learning curves of Morris water maze tests (A and B) and 48 h post-training probe tests (C and D), respectively.
Both exercise protocols improved learning curves (*P < 0.05, ANOVA with repeated measures design) and memory retention times (*P < 0.05, unpaired Student's t test). n= 10–18 for each group. Abbreviations: P, platform; O, opposite to the platform; R, right to the platform; L, left to the platform.
Figure 2. Effects of moderate treadmill running (A) or voluntary wheel running (B) on 24 h testing latencies of PA performance.
Data are presented as median ± quartile. It was noticed that treadmill running, but not voluntary wheel running, increased PA testing retention latency (*P < 0.05, Mann–Whitney U test; n= 9–13 for each group).
Effects of different types of exercise on the BDNF pathway and Syt I expression in the hippocampus and amygdala
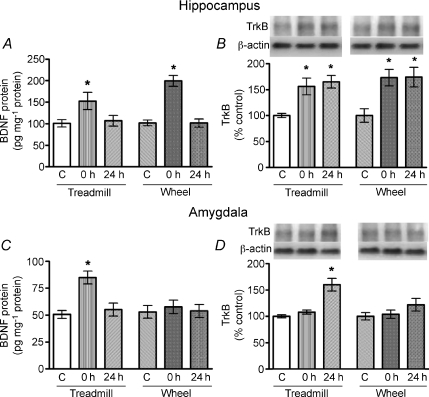
To examine the exercise-induced molecular changes in the limbic system, i.e. the hippocampus and amygdala, protein levels of BDNF and TrkB in these brain regions were measured at 0 h and 24 h after the last episode of exercise. Our results revealed that both treadmill and wheel running acutely elevated the BDNF level (Fig. 3A) and persistently upregulated the protein expression of TrkB (Fig. 3B) in the hippocampus. However, only treadmill exercise, but not wheel running, increased BDNF and TrkB protein expression in the amygdala (Fig. 3C and D).
Figure 3. Effects of moderate treadmill running or voluntary wheel running on BDNF protein concentrations and TrkB expression in the hippocampus and the amygdala.
Results showed that both types of exercise transiently elevated hippocampal BDNF levels (A) and persistently increased TrkB protein expression (B). However, only treadmill running significantly increased BDNF (C) and TrkB (D) in the amygdala. *P < 0.05 (compared to the control; ANOVA followed by Tukey's post hoc test, n= 6–9). Abbreviations: C, control; 0 h, immediately after the last run of exercise; 24 h, 24 h after the last run.
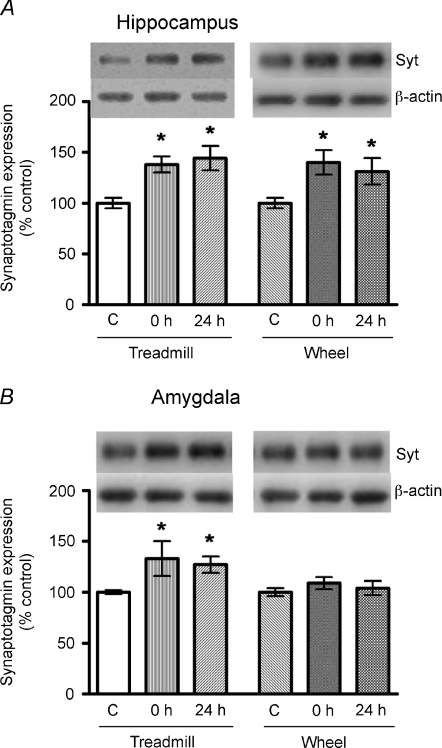
Similarly, both treadmill exercise and wheel running significantly increased the hippocampal protein expression of Syt I at 0 or 24 h after the last episode of exercise (Fig. 4A). In contrast, moderate treadmill running, but not voluntary wheel running, significantly elevated Syt I protein levels in the amygdala (Fig. 4B). Therefore, both exercise protocols exerted similar effects on the hippocampus, while only treadmill running was effective in modulating the BDNF pathway and Syt I expression in the amygdala.
Figure 4. Effects of moderate treadmill running or voluntary wheel running on the protein levels of Syt I in the hippocampus or in the amygdala.
Both exercise protocols significantly enhanced hippocampal Syt I protein levels (A), but only treadmill exercise increased amygdalar Syt I protein expression (B). *P < 0.05 (compared to the control; ANOVA followed by Tukey's post hoc test, n= 8–9).
Local blockade of TrkB activity or Syt I expression abolished the treadmill exercise-facilitated PA performance
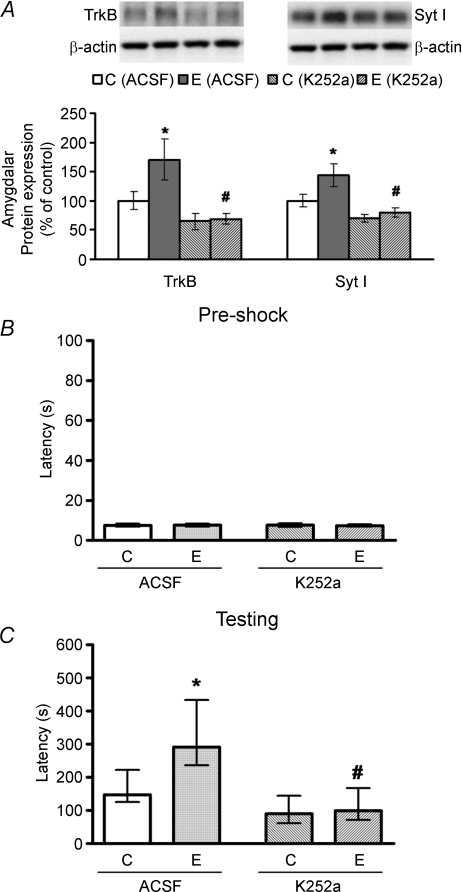
We then aimed to investigate the functional role of amygdalar adaptation in the treadmill exercise-facilitated PA performance. K252a, a TrkB kinase inhibitor, was stereotaxically injected into BLA after the last run. This treatment effectively eliminated the exercise-upregulated amygdalar protein expression in TrkB and Syt I (Fig. 5A). In addition, it also abolished the treadmill exercise-facilitated PA testing latency without affecting the pre-shock values (Fig. 5B and C).
Figure 5. Effects of TrkB kinase blockage on the protein expression of amygdalar TrkB and Syt I (A), and on the pre-shock latencies (B) and 24 h testing latencies (C) of PA performance.
Data of TrkB and Syt I were expressed as mean ±s.e.m., and were analysed by ANOVA followed by Tukey's post hoc test. In contrast, data of PA tests were presented as median ± quartile, and were analysed by Kruskal–Wallis test followed by Dunn's multiple comparison test. Results indicated that TrkB kinase blockage by K252a abolished treadmill exercise-enhanced PA testing latencies and amygdalar TrkB/Syt I expression. *P < 0.05 (E vs. C), #P < 0.05 (E-K252a vs. E-ACSF). n= 8. Abbreviations: ACSF, artificial CSF; C, control; E, exercise.
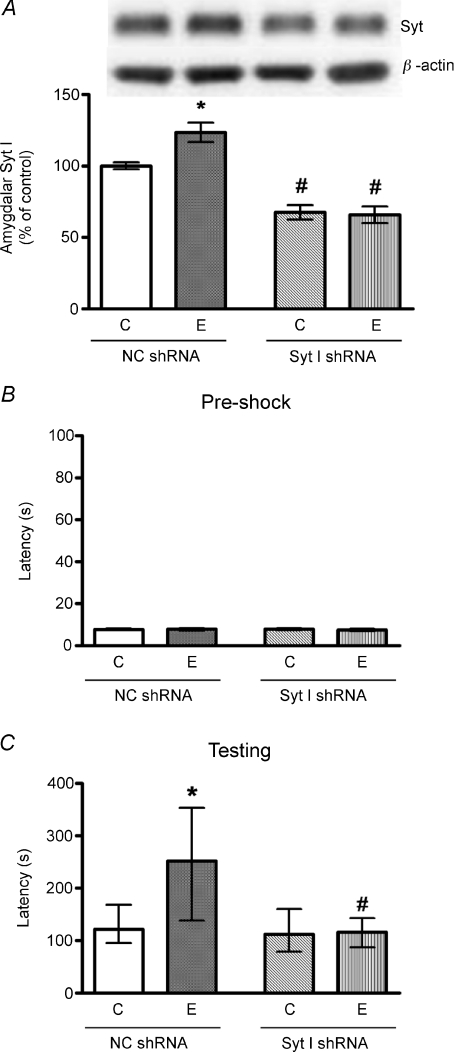
In another experiment, the administration of Syt I shRNA lentivirus into BLA effectively abolished the exercise-increased amygdalar Syt I protein expression (Fig. 6A). The treatment of Syt I shRNA lentivirus, but not NC shRNA lentivirus (the negative control), abolished treadmill exercise-enhanced PA testing latency without affecting the pre-shock values (Fig. 6B and C). To rule out the possible non-specific effects, we measured the amygdalar TrkB and hippocampal Syt I; neither was affected by amygdalar injection of lentiviral-Syt I shRNA (Supplemental Fig. S1).
Figure 6. Effects of Syt I lentiviral shRNA treatment on the protein expression of amygdalar Syt I (A), and on the pre-shock latencies (B) and 24 h-testing latencies (C) of PA performance.
Data presentation and analyses were the same as in Fig. 5. Results showed that Syt I shRNA abolished treadmill exercise-increased PA testing latencies and amygdalar Syt I expression. *P < 0.05 (E vs. C), #P < 0.05 (Syt I shRNA vs. NC shRNA). n= 9. Abbreviations: C, control; E, exercise.
Effects of different types of exercise on serum corticosterone levels
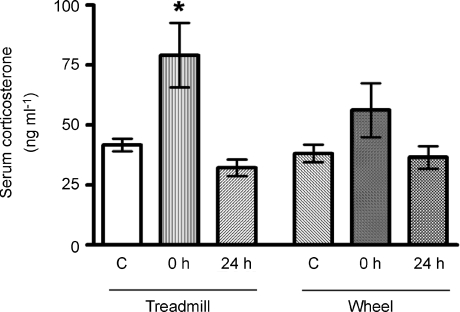
The serum corticosterone levels were measured to investigate possible roles of stress in the differential exercise effects on aversive learning and memory. Our results demonstrated that (i) the basal corticosterone levels were the same in the control groups of either exercise protocol; (ii) treadmill running, but not wheel running, significantly increased serum corticosterone level immediately after the last episode of exercise; and (iii) the treadmill exercise-induced corticosterone change was transient, i.e. it returned to the resting level 24 h after exercise (Fig. 7).
Figure 7. Effects of moderate treadmill running or voluntary wheel running on serum corticosterone levels.
The serum corticosterone level was elevated immediately after treadmill exercise and returned to the resting level thereafter. Voluntary wheel running did not significantly alter this parameter. *P < 0.05 (compared to the control; ANOVA followed by Tukey's post hoc test, n= 6–8).
Discussion
Our results suggest that different types of exercise modulate neural plasticity-related proteins in various associated brain regions, which subsequently have diverse effects on different learning and memory capabilities. The current consensus that exercise improves rodent learning and memory is largely based on two modes of exercise regimens, i.e. forced exercise (such as treadmill running or motorized wheel running) and voluntary exercise (for instance, spontaneous wheel running). In the present study, we found that (1) moderate treadmill exercise and voluntary wheel running differentially affected amygdala-associated aversive learning and memory, although they had similar effects on the hippocampus-dependent spatial learning and memory; (2) these two exercise protocols upregulated the BDNF–TrkB pathway and Syt I in the hippocampus, whereas only treadmill exercise elevated the expression of these molecules in the amygdala; and (3) local blocking of TrkB activation or Syt I expression in BLA abolished the moderate treadmill exercise-facilitated PA performance. Generally speaking, hippocampus plays a dominant role in the performance of the Morris water maze, while both hippocampus and amygdala are important in the PA task. The current study shows that treadmill exercise affects not only the hippocampus but also the amygdala, whereas wheel running only affects the hippocampus. This viewpoint also explains why wheel running improves contextual learning (a hippocampus-associated task) but not cued fear learning (an amygdala-related task) (Baruch et al. 2004).
Exercise has broad beneficial effects on the brain. Many central and peripheral growth factors, such as BDNF, IGF-1 and VEGF, are involved in the underlying mechanisms (for a review, see Ang & Gomez-Pinilla, 2007; Cotman et al. 2007). Among them, BDNF is one of the principal neurotrophic factors. Previous studies have demonstrated that the hippocampal BDNF–TrkB signal pathway is involved in learning and memory (Ma et al. 1998; Mu et al. 1999; Minichiello et al. 1999; Koponen et al. 2004), and that exercise improves spatial learning and memory via upregulating these molecules in the hippocampus (Vaynman et al. 2004; Huang et al. 2006). The current studies also showed that treadmill exercise facilitates PA performance and enhances BDNF–TrkB signalling in the hippocampus (Fig. 3A and B). As the activity-dependent upregulation of the BDNF–TrkB pathway in amygdala plays an important role during the consolidation of fear memory (Ou & Gean, 2007), and the inhibition of TrkB in BLA impairs fear memory in rats (Rattiner et al. 2004), it is likely that the BDNF signalling in the amygdala is also involved in the treadmill exercise-facilitated PA performance. Indeed, treadmill exercise increased not only the hippocampal but also the amygdalar BDNF signalling (Fig. 3C and D). Furthermore, these exercise effects could be abolished by the local administration of K252a, a TrkB inhibitor, in the BLA (Fig. 5). In contrast, wheel running, which did not affect PA performance, did not change the amygdalar BDNF–TrkB pathway either. Therefore, the upregulation of BDNF signalling in the amygdala plays an important role in treadmill exercise-facilitated aversive learning and memory.
It has been reported recently that Syt I is required in synaptic vesicle aggregation during nascent synapse formation (Gardzinski et al. 2007). This would explain why higher Syt I levels in the limbic system can improve learning and memory. The current study suggested that the treadmill exercise upregulated Syt I in the amygdala and thus enhanced aversive learning and memory. Treadmill exercise, but not voluntary wheel running, facilitated PA performance and elevated amygdalar Syt I protein levels (Figs 2 and 4). In addition, these effects were abolished by Syt I shRNA treatment in BLA (Fig. 6). Previous studies have reported that contextual fear conditioning and inhibitory avoidance learning are impaired in Syt IV knockout mice (Ferguson et al. 2000, 2004). Consistent with our findings, both Syt 5 and Syt 11 genes in rat hippocampus are upregulated after voluntary wheel running (Tong et al. 2001; Molteni et al. 2002). These results suggest that the Syt I-associated neural transmission in both hippocampus and amygdala is crucial for the formation of aversive learning and memory.
Both BDNF and Syt are upregulated by exercise, and are involved in exercise-enhanced performance in learning and memory tasks. BDNF has been shown to increase transmitter release probability and the size of a rapidly recycling vesicle pool in hippocampal excitatory synapses (Tyler et al. 2006). Moreover, BDNF can modulate the expression of vesicle fusion proteins; it upregulates several v-SNARE affiliates, such as Syt, synaptophysin and synaptobrevin, without affecting SNAP-25, syntaxin or synapsin-I (Tartaglia et al. 2001). Interestingly, the increase in Syt levels (5-fold) was more pronounced than that in synaptophysin and synaptobrevin (2-fold). Thus, we postulate that exercise enhances the BDNF–TrkB signalling pathway which subsequently activates the protein synthesis of v-SNARE affiliates, particularly the Syt, to modulate synaptic plasticity. This proposition is supported by our findings that blockade of BDNF–TrkB signalling by K252a abolished treadmill exercise-upregulated Syt and PA performance. It remains to be clarified whether other v-SNARE affiliates also participate in the exercise-improved learning and memory.
Treadmill running and voluntary wheel running apparently have distinctive impacts on brain functions. The exercise-associated stress level or pattern could be an underlying modulating factor for their differential effects on brain functions. Although severe stress is known to suppress the hippocampal BDNF expression (Smith et al. 1995), mild stress can enhance the cognitive function (Parker et al. 2005). In our view, repeated mild stresses, such as those encountered in our treadmill exercise protocol, may be necessary to alter the amygdala in a favourable way for aversive learning and memory. The treadmill exercise used in the current study not only improved the muscle aerobic capacity but also transiently increased serum corticosterone level, while wheel running did not alter these physiological parameters. It has been reported that low and high doses of corticosterone exert differential effects on BDNF and TrkB mRNA levels in the hippocampus (Schaaf et al. 1997). Besides, prolonged immobilization stress can enhance the synaptic connectivity in the amygdala (Vyas et al. 2006). The repeated mild stresses associated with treadmill exercise running could potentially increase dendritic branches and spines in amygdala and thus improve PA performance. It is worthy to note that excessive stress, such as prolonged immobilization or forced running at high speeds with tail shock, may exert inhibitory effects. Indeed, our previous study has shown that treadmill exercise at moderate intensity in rats acutely upregulates hippocampal BDNF expression, while high intensity of treadmill running without familiarization has the opposite effect, similar to the stress effects of immobilization or water exposure (Huang et al. 2006). In comparison, chronic voluntary physical activity attenuates neural responses to stress in brain circuits responsible for regulating peripheral sympathetic activity (Dishman et al. 2006). Interestingly, an enriched environment increases neurogenesis and spatial memory (Kempermann et al. 1997; Williams et al. 2001) without affecting the PA performance (Iso et al. 2007). Thus, the effect of voluntary wheel running resembles that of environmental enrichment. This may partially explain the differential effects of two exercise protocols on aversive learning and memory.
It is also likely that exercise facilitates aversive learning and memory in an intensity-dependent manner. A previous study has reported that the hypothalamus responds to running in a threshold-like pattern (Soya et al. 2007a). Different exercise intensities between treadmill exercise and wheel running may activate the hypothalamus–pituitary gland–adrenal gland axis to various extents. Although the total running distance in wheel-running groups (4.4–5.2 km in 12 h, intermittent running) was much greater than that in treadmill groups (600 m in 1 h at a running speed of 10 m min−1), the exercise intensity in the former was lower than that in the latter. This was supported by the results of citrate synthase activities. Moreover, treadmill exercise with mild intensity (420 m in 1 h at a running speed of 7 m min−1) did not affect either citrate synthase activity or PA performance (Supplemental Fig. S2), implying that exercise facilitates aversive learning and memory in a threshold-like pattern.
In conclusion, our results demonstrate that in mice, only moderate treadmill exercise improves aversive memory, although both treadmill exercise and wheel running can improve spatial learning and memory. The differential effects may be due to various impacts on the BDNF–TrkB–Syt pathway in the amygdala, a fear memory-associated brain region.
Acknowledgments
This work was supported by the National Science Council (Grant Nos. NSC95-2320-B-006-041-MY3, NSC95-2320-B-006-045-MY3, and NSC95-2320-B-006-049-MY3) and National Cheng Kung University (Grant No. R026) in Taiwan.
Supplemental material
References
- Alaei H, Borjeian L, Azizi M, Orian S, Pourshanazari A, Hanninen O. Treadmill running reverses retention deficit induced by morphine. Eur J Pharmacol. 2006;536:138–141. doi: 10.1016/j.ejphar.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Ang ET, Dawe GS, Wong PT, Moochhala S, Ng YK. Alterations in spatial learning and memory after forced exercise. Brain Res. 2006;1113:186–193. doi: 10.1016/j.brainres.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Ang ET, Gomez-Pinilla F. Potential therapeutic effects of exercise to the brain. Curr Med Chem. 2007;14:2564–2571. doi: 10.2174/092986707782023280. [DOI] [PubMed] [Google Scholar]
- Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behav Neurosci. 2004;118:1123–1127. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behaviour in rats. Brain Res. 2004;1019:84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Chen HI, Lin LC, Yu L, Liu YF, Kuo YM, Huang AM, Chuang JI, Wu FS, Liao PC, Jen CJ. Treadmill exercise enhances passive avoidance learning in rats: the role of down-regulated serotonin system in the limbic system. Neurobiol Learn Mem. 2008;89:489–496. doi: 10.1016/j.nlm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of amygdala in fear and anxiety. Ann Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dishman RK. Brain monoamines, exercise, and behavioural stress: animal models. Med Sci Sports Exerc. 1997;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of exercise. Obesity. 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Ferguson GD, Anagnostaras SG, Silva AJ, Herschman HR. Deficits in memory and motor performance in synaptotagmin IV mutant mice. Proc Natl Acad Sci U S A. 2000;97:5598–5603. doi: 10.1073/pnas.100104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson GD, Wang H, Herschman HR, Storm DR. Altered hippocampal short-term plasticity and associative memory in synaptotagmin IV (−/−) mice. Hippocampus. 2004;14:964–974. doi: 10.1002/hipo.20013. [DOI] [PubMed] [Google Scholar]
- Gardzinski P, Lee DWK, Fei G-H, Hui K, Huang GJ, Sun H-S, Feng Z-P. The role of synaptotagmin I C2A calcium-binding domain in synaptic vesicle clustering during synapse formation. J Physiol. 2007;581:75–90. doi: 10.1113/jphysiol.2006.127472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes K, Sprague S, Guo M, Davis W, Friedman A, Kumar A, Jimenez DF, Ding Y. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol. 2008;115:289–296. doi: 10.1007/s00401-008-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AM, Jen CJ, Chen HF, Yu L, Kuo YM, Chen HI. Compulsive exercise acutely upregulates rat hippocampal brain-derived neurotrophic factor. J Neural Transm. 2006;113:803–811. doi: 10.1007/s00702-005-0359-4. [DOI] [PubMed] [Google Scholar]
- Iso H, Simoda S, Matsuyama T. Environmental change during postnatal development alters behaviour, cognitions and neurogenesis of mice. Behav Brain Res. 2007;179:90–98. doi: 10.1016/j.bbr.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Jen CJ, Chan HP, Chen HI. Chronic exercise improves endothelial calcium signalling and vasodilatation in hypercholesterolemic rabbit femoral artery. Arterioscler Thromb Vasc Biol. 2002;22:1219–1224. doi: 10.1161/01.atv.0000021955.23461.cd. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Koponen E, Voikar V, Rickki R, Saarelainen T, Rauramaa T, Rauvala H, Taira T, Castren E. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCγ pathway, reduced anxiety, and facilitated learning. Mol Cell Neurosci. 2004;26:166–181. doi: 10.1016/j.mcn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behaviour. Neuroscience. 2008;156:456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Liu YF, Chen HI, Yu L, Kuo YM, Wu FS, Chuang JI, Liao PC, Jen CJ. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol Learn Mem. 2008;90:81–89. doi: 10.1016/j.nlm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–967. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiol Learn Mem. 2002;78:539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999;835:259–265. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Transcriptional regulation of brain-derived neurotrophic factor in the amygdala during consolidation of fear memory. Mol Pharmacol. 2007;72:350–358. doi: 10.1124/mol.107.034934. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biol Psychiatry. 2005;57:848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience. 2005;136:991–1001. doi: 10.1016/j.neuroscience.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf MJ, Hoetelmans RW, de Kloet ER, Vreugdenhil E. Corticosterone regulates expression of BDNF and trkB but not NT-3 and trkC mRNA in the rat hippocampus. J Neurosci Res. 1997;48:334–341. [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya H, Mukai A, Deocaris CC, Ohiwa N, Chang H, Nishijima T, Fujikawa T, Togashi K, Saito T. Threshold-like pattern of neuronal activation in the hypothalamus during treadmill running: establishment of a minimum running stress (MRS) rat model. Neurosci Res. 2007a;58:341–348. doi: 10.1016/j.neures.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Soya H, Nakamura T, Deocaris CC, Kimpara A, Iimura M, Fujikawa T, Chang H, McEwen BS, Nishijima T. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007b;358:961–967. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- Tartaglia N, Du J, Tyler WJ, Neale E, Pozzo-Miller L, Lu B. Protein synthesis-dependent and -independent regulation of hippocampal synapses by brain-derived neurotrophic factor. J Biol Chem. 2001;276:37585–37593. doi: 10.1074/jbc.M101683200. [DOI] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8:1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Zhang X-l, Hartman K, Winterer J, Muller W, Stanton PK, Pozzo-Miller L. BDNF increases release probability and the size of a rapidly recycling vesicle pool within rat hippocampal excitatory synapses. J Physiol. 2006;574:787–803. doi: 10.1113/jphysiol.2006.111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. Revenge of the ‘sit’: how lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. J Neurosci Res. 2006;84:699–715. doi: 10.1002/jnr.20979. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioural stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Williams BM, Luo Y, Ward C, Redd K, Gibson R, Kuczaj SA, McCoy JG. Environmental enrichment: effects on spatial memory and hippocampal CREB immunoreactivity. Physiol Behav. 2001;73:649–658. doi: 10.1016/s0031-9384(01)00543-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.