Abstract
Habitual aerobic exercise is associated with enhanced endothelium-dependent dilatation (EDD) in older humans, possibly by increasing nitric oxide bioavailability and reducing oxidative stress. However, the mechanisms involved are incompletely understood. EDD was measured in young (6–8 months) and old (29–32 months) cage control and voluntary wheel running (VR) B6D2F1 mice. Age-related reductions in maximal carotid artery EDD to acetylcholine (74 vs. 96%, P < 0.01) and the nitric oxide (NO) component of EDD (maximum dilatation with ACh and l-NAME minus that with ACh alone was −28%vs.−55%, P < 0.01) were restored in old VR (EDD: 96%, NO: −46%). Nitrotyrosine, a marker of oxidative stress, was increased in aorta with age, but was markedly lower in old VR (P < 0.05). Aortic superoxide dismutase (SOD) activity was greater (P < 0.01), whereas NADPH oxidase protein expression (P < 0.01) and activity (P= 0.05) were lower in old VR vs. old cage control. Increasing SOD (with 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl) and inhibition of NADPH oxidase (with apocynin) improved EDD and its NO component in old cage control, but not old VR mice. VR increased endothelial NO synthase (eNOS) protein expression (P < 0.05) and activation (Ser1177 phosphorylation) (P < 0.05) in old mice. VR did not affect EDD in young mice. Our results show that voluntary aerobic exercise restores the age-associated loss of EDD by suppression of oxidative stress via stimulation of SOD antioxidant activity and inhibition of NADPH oxidase superoxide production. Increased eNOS protein and activation also may contribute to exercise-mediated preservation of NO bioavailability and EDD with ageing.
Older age is a major risk factor for cardiovascular diseases (CVD) (Lakatta & Levy, 2003). This is believed to be attributable in large part to the development of vascular endothelial dysfunction, as indicated by impaired endothelium-dependent dilatation (EDD) (Vita & Keaney, 2002; Bonetti et al. 2003; Widlansky et al. 2003). Impaired EDD with ageing is mediated by reduced nitric oxide (NO) bioavailability associated with the development of oxidative stress (Cai & Harrison, 2000). Thus, therapeutic strategies that can preserve EDD and NO bioavailability with ageing, while inhibiting oxidative stress have important implications for the prevention and treatment of age-associated CVD.
Habitual aerobic exercise is associated with enhanced EDD in middle-aged and older adults (DeSouza et al. 2000; Taddei et al. 2001; Eskurza et al. 2004, 2005; Seals et al. 2008). Previous observations in humans and rats suggest that this influence is mediated by increased NO bioavailability and, perhaps, reduced oxidative stress (Taddei et al. 2001; Spier et al. 2004; Eskurza et al. 2005; Franzoni et al. 2005). However, at present there is no direct evidence that exercise suppresses or prevents the development of oxidative stress in arteries with ageing. Moreover, little is known about the cellular and molecular mechanisms by which aerobic exercise exerts these beneficial vascular effects with ageing.
The experimental goal of the present study was to obtain direct evidence for exercise-associated suppression of arterial oxidative stress with ageing and to gain insight into mechanisms that may be involved. In particular, we sought to determine the possible roles of increases in superoxide dismutase (SOD), a key antioxidant enzyme system (Faraci & Didion, 2004; Wassmann et al. 2004), and inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a major oxidant-producing enzyme (Bedard & Krause, 2007; Wadsworth, 2008), in exercise-mediated reductions in vascular oxidative stress and enhancement of EDD and its NO component with ageing. A secondary aim was to determine if the hypothesized improvement in NO-mediated EDD in this setting is associated with activation of endothelial NO synthase (eNOS), the enzyme responsible for production of NO in the vascular endothelium.
To address these aims, we used a recently established mouse model of age-associated vascular endothelial dysfunction (Lesniewski et al. 2009). Voluntary wheel running (VR) was employed to simulate the effects of voluntary aerobic exercise in humans.
Methods
Animals
B6D2F1 mice were obtained from the National Institute on Aging rodent colony. All mice were housed in an animal care facility at the University of Colorado at Boulder on a 12 h : 12 h light–dark cycle. Thirty-four young (4–7 months) and 36 old (29–32 months, age at approximately 50% survival) male B6D2F1 mice were fed normal rodent chow ad libitum and housed in standard mouse cages or in cages fitted with running wheels for 10–14 weeks prior to killing. Running distance was monitored daily. Food intake was monitored weekly. All animal procedures conformed to the Guide to the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised 1996) and were approved by the UCB Animal Care and Use Committee.
Systemic vascular endothelial function
Mice (n= 5–6 per group) were surgically implanted with indwelling carotid artery and jugular vein catheters (Micro-renathane 0.025″ O.D. × 0.012″ I.D., Braintree Scientific Inc., Braintree, MA, USA) while under anaesthesia (80 mg kg−1 ketamine HCL, 16 mg kg−1 xylazine, 0.5 mg kg−1 acepromazine maleate, i.p.) and allowed to recover for 24 h. Carotid artery blood pressure and heart rate were monitored in the conscious mouse using a saline-filled external pressure transducer (Becton Dickinson, Franklin Lakes, NJ, USA) coupled to a Gould amplifier and Windaq data acquisition system (DATAQ Instruments, Inc, Akron, OH, USA). Measurements were taken pre-infusion and in response to intravenous infusion of ACh (0.05, 0.1, 0.15, 0.25, 0.5, 0.75 mg kg−1) and sodium nitroprusside (SNP: 0.05, 0.1, 0.2, 0.4, 0.6, 0.8 mg kg−1), administered via jugular catheters using a Harvard pump (KD Scientific Inc., Holliston, MA, USA) (15 μl min−1) as previously described (Lesniewski et al. 2009). The magnitude of the reductions in mean arterial pressure from baseline in response to ACh were taken as a measure of systemic EDD, whereas the reductions in arterial pressure in response to SNP were used as a measure of systemic endothelium-independent dilatation (EID) (Cernadas et al. 1998; Lesniewski et al. 2009).
Carotid artery endothelial function
Mice (n= 5–10 per group) were killed via exsanguination by cardiac puncture while under isoflourane anaesthesia. Right and left carotid arteries were excised, placed in a myograph chamber (DMT, Inc., Atlanta, GA, USA) containing EDTA-buffered physiological salt solution (PSS), and cannulated onto glass micropipettes with nylon (11-0) suture. Arteries were warmed to 37°C, pressurized to 50 mmHg intraluminal pressure (Blackwell et al. 2004; Lesniewski et al. 2009) and allowed to equilibrate for 60 min (Lesniewski et al. 2009). Because the carotid artery does not develop spontaneous tone, arteries were submaximally preconstricted with phenylephrine (2 μmol l−1) prior to vasodilator dose responses (d’Uscio et al. 2003; Lesniewski et al. 2009). After preconstriction, increases in luminal diameter were measured in response to the cumulative addition of ACh (1 × 10−9 to 1 × 10−4 mol l−1) and SNP (1 × 10−10 to 1 × 10−4 mol l−1). To determine the contribution of NO to dilatation, responses to ACh were repeated in the presence of NG-nitro-l-arginine methyl ester (l-NAME) (0.1 mmol l−1, 30 min). To determine if superoxide contributes to age- and VR-associated changes in EDD, dose responses were repeated in the contralateral vessel after a 60 min incubation in the presence of the SOD mimetic, 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL; 1 mmol l−1). Finally, to determine if superoxide production via NADPH oxidase contributed to age- and VR-associated changes in EDD, a second cohort of mice (n= 5–7 per group) was used to complete dose responses after pre-incubation with apocynin (1 mmol l−1, 60 min), a NADPH oxidase inhibitor.
Vessel segments were imaged and diameters measured by MyoView software (DMT, Inc., Atlanta, GA). All dose response data are presented on a percentage basis. Percentage preconstriction was calculated as percentage of maximal diameter according to the following formula:
Because of increased maximal carotid artery diameter with advancing age, active responses were recorded as actual diameters and expressed as a percentage of maximal response according to the following formula:
Where Dm is maximal inner diameter at 50 mmHg, Ds is the steady-state inner diameter recorded after addition of drug and Db is the steady state inner diameter following preconstriction before the first addition of drug. Sensitivity (IC50) was defined as the concentration of vasoactive agent (ACh, SNP) that yielded 50% of the maximal response. NO bioavailability was determined from the maximal EDD in the absence or presence of l-NAME according to the following formula:
 |
Arterial protein expression and enzyme activities
The carotid arteries could not be used to determine protein expression and enzyme activities because both carotid arteries from each mouse were used to assess functional measures of EDD and, thus, were treated with either the NOS inhibitor, l-NAME, or l-NAME and either TEMPOL or apocynin. The large amount of carotid arteries required (because of the small tissue volume per artery, sets of arteries from multiple animals would need to be pooled for a single adequate sample), the cost and availability of older mice, and the judicious use of animals, together, precluded us from killing mice solely to obtain carotid artery tissue for protein measures. Accordingly, we utilized the thoracic aorta, another large elastic artery, from the same mice as a surrogate artery to assess protein expression and enzyme activities. This approach has been used previously by our laboratory (Lesniewski et al. 2009) and other investigators (Didion et al. 2002; d’Uscio et al. 2007), as EDD responses are similar in carotid arteries and aorta of mice (Bonthu et al. 1997; Faraci et al. 1998; Lamping et al. 2000).
In the present study, the thoracic aorta was excised, cleared of surrounding tissues while maintained in 4°C physiological salt solution (PSS), and frozen in liquid nitrogen. Whole artery lysates were prepared as previously described (Lesniewski et al. 2009). For measures of protein expression in aortic lysates, 15 μg of protein with 2 mol l−1 dithiothreitol were loaded into polyacrylamide gels, separated by electrophoresis and transferred onto a nitrocellulose membrane. The membrane was blocked in 5% non-fat dry milk in Tris-buffered saline with 0.05% Tween (TBS-T) overnight at 4°C. After blocking, the membrane was washed with TBS-T and incubated overnight at 4°C in primary antibody. eNOS (1 : 1000; 140 kDa; BD Biosciences, San Jose, CA, USA), Ser1177-phosphorylated eNOS (peNOS, 1 : 1000; 140 kDa; Cell Signalling, Danvers, MA, USA), manganese (Mn) SOD (1 : 2000; 25 kDa; Stressgen, Ann Arbor, MI, USA), copper zinc (CuZn) SOD (1 : 2000; 19, 23 kDa; Stressgen), extracellular (ec) SOD (1 : 500; 35, 31 kDa; Sigma-Aldrich, St Louis, MO, USA), p67phox-NADPH oxidase (p67, 1 : 1000; 67 kDa; BD Biosciences, San Jose, CA, USA) and xanthine oxidase (1 : 1000; 146 kDa; Abcam, Cambridge, MA, USA) expression and nitrotyrosine (1 : 1000; 25, 55, 160 kDa; Abcam) abundance were measured by standard Western blotting techniques using an HRP-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) and Supersignal ECL (Pierce, Rockford, IL, USA). Bands were visualized using a digital acquisition system (ChemiDoc-It, UVP, Upland, CA, USA) and quantified using ImageJ software (NIH, Bethesda, MD, USA). To account for differences in protein loading, expression is presented normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH 1 : 1000; 37 kDa; Cell Signalling) expression. Data are presented normalized to the mean of the young control group of an individual blot. Representative blots show bands from the same blot and exposure and GAPDH was assessed on the same blot after stripping.
Total SOD activity was determined in aortic lysates (1 μg protein) using the SOD Activity Assay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instructions. MnSOD activity was determined in the same lysates using the SOD Activity Assay kit in the presence of 1 mmol l−1 potassium cyanide to block CuZnSOD and ecSOD activities (MacMillan-Crow et al. 1996). CuZnSOD and ecSOD activity was calculated as the difference between total and MnSOD activity for each sample.
NADPH oxidase and xanthine oxidase activities were determined in aortic lysates (10 μg protein) using the Amplex Red Xanthine/Xanthine Oxidase Assay kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions with NADPH (200 μmol l−1 per reaction) and xanthine (200 μmol l−1 per reaction) as the reaction substrates. Skeletal muscle citrate synthase activity was determined in quadriceps muscle homogenate as previously described (Srere, 1969; Delp & Duan, 1996).
Statistics
For animal and vessel characteristics and Western blotting, maximal vasodilatation and sensitivity, group differences were determined by one-way analysis of variance (ANOVA). Least squares difference post hoc tests were used where appropriate. Data are presented as means ±s.e.m. Significance was set at P < 0.05.
Results
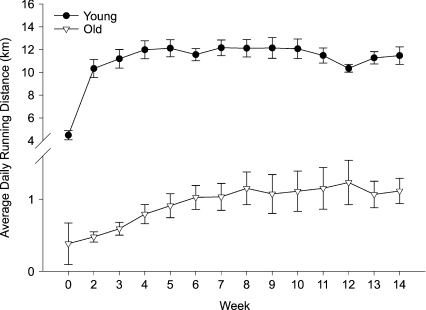
Both young and old mice voluntarily ran when housed in a cage equipped with a running wheel, with old mice running a much shorter distance per day (Fig. 1). In response to VR, both young (P < 0.05) and old (P= 0.05) mice demonstrated an increase in quadriceps muscle citrate synthase activity compared with age-matched cage control mice (Table 1). Body mass was lower in old compared with young mice (P < 0.05; Table 1); 10–14 weeks of VR was associated with lower body mass in young (P < 0.01) but not old mice. Food intake did not differ among the groups (Table 1). Absolute heart mass was greater in old compared with young mice (P < 0.01) and was unchanged by VR (Table 1). VR had no effect on the heart to body mass ratio in either group (Table 1). Absolute gastrocnemius muscle mass was lower in old compared with young mice (P < 0.01) and was unchanged by VR. The ratio of gastrocnemius muscle mass to body mass was greater with VR in young (P= 0.01) but not old mice. Maximal carotid artery diameter was greater in old compared with young cage control mice (P < 0.01), but was not affected by VR in either group (Table 1).
Figure 1. Daily running distance (n= 14–16 per group) of young and old mice.
Values are means ±s.e.m.
Table 1.
Animal characteristics
| YCC | OCC | YVR | OVR | |
|---|---|---|---|---|
| Body Mass (g) | 36.4 ± 0.5 | 34.7 ± 0.5* | 30.5 ± 0.5* | 33.4 ± 1.5* |
| Food Intake (g day−1) | 4.8 ± 0.1 | 5.0 ± 0.1 | 5.5 ± 0.2 | 4.8 ± 0.3 |
| HT (mg) | 184 ± 3 | 223 ± 6* | 186 ± 5 | 211 ± 7* |
| HT:BW (g/g × 100) | 0.48 ± 0.02 | 0.77 ± 0.16* | 0.61 ± 0.02 | 0.65 ± 0.03 |
| GAST (mg) | 207 ± 9 | 149 ± 5* | 197 ± 10 | 146 ± 7* |
| GAST:BW (g/g × 100) | 0.52 ± 0.02 | 0.40 ± 0.02* | 0.65 ± 0.03* | 0.41 ± 0.03* |
| CS (mol min−1 g−1 wet wt) | 19.8 ± 1.0 | 20.5 ± 1.4 | 22.9 ± 1.0* | 23.8 ± 1.3*† |
| Carotid artery Dm (μm) | 403 ± 4 | 432 ± 8* | 410 ± 6 | 420 ± 4* |
Mass and mass to body weight (BW) ratios of the heart (HT), gastrocnemius muscle (GAST), quadriceps muscle citrate synthase activity (CS) and carotid artery maximum diameter (Dm) in young (Y) and old (O) cage control (CC: n= 40) and voluntary running (VR: n= 30) mice.
P < 0.05 vs. YCC;
P < 0.05 vs. OCC.
VR prevents age-associated reductions in EDD and NO bioavailability
Systemic EDD
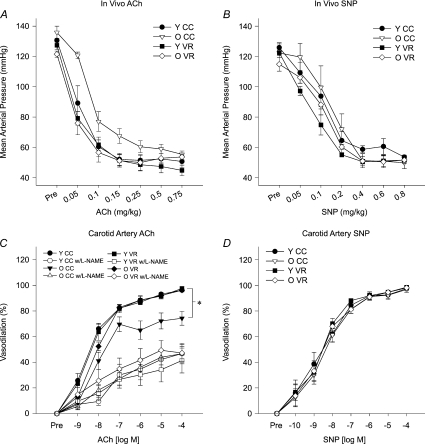
The maximal reduction in blood pressure in response to the intravenous infusion of ACh was not different between young and old mice (Fig. 2A), but old age was associated with reduced in vivo sensitivity to ACh (P < 0.05; Table 2). VR increased this systemic sensitivity to ACh in old (P < 0.01) but not young mice. VR did not affect the maximal reduction in blood pressure in either group (Fig. 2A). There were no differences in either the maximal reduction in blood pressure (Fig. 2B) or sensitivity (Table 2) to SNP among the groups. Heart rate responses to the infusion of both ACh and SNP were similar among the groups (data not shown, all P > 0.10).
Figure 2. Systemic (in vivo) EDD to ACh (n= 6 per group) (A) and EID to sodium nitroprusside (SNP) (B), carotid artery EDD to ACh in the absence or presence of the NOS inhibitor, l-NAME (n= 6–10 per group) (C), and carotid artery EID to SNP in young (Y) and old (O) cage control (CC) and voluntary wheel running mice (VR) (D).
Values are means ±s.e.m.*P < 0.05 vs. YCC.
Table 2.
Systemic (in vivo) (n= 5–6 per group) and carotid artery (n= 5–9 per group) sensitivity (IC50) to ACh (CNT), to ACh after incubation with TEMPOL or apocynin (APO), or to sodium nitroprusside (SNP) in young (Y) and old (O) cage control (CC) and voluntary wheel running (VR) mice
| YCC | OCC | YVR | OVR | |
|---|---|---|---|---|
| In vivo | ||||
| ACh (mg kg−1) | 0.05 ± 0.01 | 0.09 ± 0.01* | 0.04 ± 0.01 | 0.04 ± 0.01† |
| SNP (mg kg−1) | 0.12 ± 0.02 | 0.13 ± 0.02 | 0.09 ± 0.03 | 0.10 ± 0.02 |
| Carotid artery | ||||
| CNT (×10−9m) | 6.9 ± 2.7 | 7.7 ± 1.9 | 7.9 ± 2.8 | 8.9 ± 2.4 |
| TEMPOL (×10−9m) | 8.3 ± 2.9 | 4.6 ± 0.7 | 8.0 ± 5.0 | 7.1 ± 3.3 |
| APO (×10−9m) | 8.4 ± 4.5 | 12.3 ± 4.6 | 7.1 ± 2.7 | 6.9 ± 1.7 |
| SNP (×10−9m) | 7.2 ± 3.1 | 7.2 ± 2.6 | 3.0 ± 1.1 | 2.7 ± 0.8 |
P < 0.05 vs. YCC;
P < 0.05 vs. OCC.
Carotid artery EDD
Baseline (preconstricted) diameter was greater in old compared with young carotid arteries (P < 0.05; Table 3) and was not affected by VR in either age group. Percentage preconstriction was not different among groups before ACh alone (CNT; Table 3). Maximal EDD to ACh was reduced in isolated carotid arteries of old vs. young cage control mice (P < 0.01; Fig. 2C), whereas sensitivity to ACh was not different (Table 2).
Table 3.
Carotid artery preconstricted diameter (μm) and percentage preconstriction (%) prior to dose response to ACh with and without l-NAME in the absence (CNT) or presence of pre-treatment with TEMPOL (TMP) or apocynin (APO) (n= 4–10 per group) in young (Y) and old (O) cage control (CC) and voluntary wheel running (VR) mice
| YCC |
OCC |
YVR |
OVR |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ACh alone | ACh +l-NAME | ACh alone | ACh +l-NAME | ACh alone | ACh +l-NAME | ACh alone | ACh +l-NAME | ||
| μm | CNT | 334 ± 4 | 292 ± 10# | 362 ± 10* | 318 ± 14# | 338 ± 13 | 293 ± 6# | 355 ± 11 | 323 ± 11 |
| TMP | 354 ± 7‡ | 334 ± 11‡ | 384 ± 6* | 371 ± 7*‡ | 360 ± 6 | 320 ± 9#‡ | 369 ± 5 | 349 ± 9 | |
| APO | 331 ± 7 | 330 ± 9‡ | 362 ± 11* | 314 ± 14# | 306 ± 15 | 300 ± 15 | 350 ± 6 | 325 ± 5# | |
| % | CNT | 17.1 ± 1.2 | 28.4 ± 2.8# | 16.3 ± 1.7 | 26.6 ± 2.7# | 17.7 ± 3.3 | 28.1 ± 2.6 | 15.5 ± 2.6 | 23.2 ± 2.6 |
| TMP | 13.6 ± 1.7 | 18.9 ± 2.4‡ | 13.8 ± 1.2 | 15.8 ± 1.0‡ | 13.3 ± 1.9 | 23.0 ± 2.3# | 13.2 ± 1.4 | 17.7 ± 2.3 | |
| APO | 21.0 ± 2.2 | 21.7 ± 2.7 | 18.6 ± 2.9 | 29.2 ± 3.5# | 21.1 ± 3.8 | 20.6 ± 3.7 | 14.3 ± 2.9 | 20.2 ± 2.9 | |
P < 0.05 vs. YCC within treatments;
P < 0.05 vs. OCC within treatments;
P < 0.05 vs. ACh after l-NAME within the same group and treatment;
P < 0.05 vs. CNT treatment within dose response.
Incubation with l-NAME reduced carotid artery preconstricted diameter in all groups (all P < 0.05; Table 3) and tended to reduce absolute diameter in old VR (P= 0.06), resulting in a greater percent preconstriction in arteries from both young (P < 0.01) and old (P < 0.01) cage control mice (Table 3). l-NAME tended to increase percentage preconstriction to phenylephrine in both young (P= 0.06) and old (P= 0.06) VR mice. l-NAME reduced maximal EDD in the carotid arteries by 55 ± 6% in young (P < 0.01 vs. ACh alone) and by 28 ± 8% in old mice (P < 0.01 vs. ACh alone; Fig. 2C); the reduction in the old mice was smaller than in young mice (P < 0.01).
VR increased maximal carotid artery EDD (P < 0.01) and restored the NO component of EDD in old mice to that of young animals (Fig. 2C), but had no effect on sensitivity to ACh (Table 2). There were no differences in maximal carotid artery dilatation (Fig. 2D) or sensitivity (Table 2) to SNP among the groups.
VR suppresses vascular oxidative stress, increases SOD activity and down-regulates NADPH oxidase in old mice
Nitrotyrosine
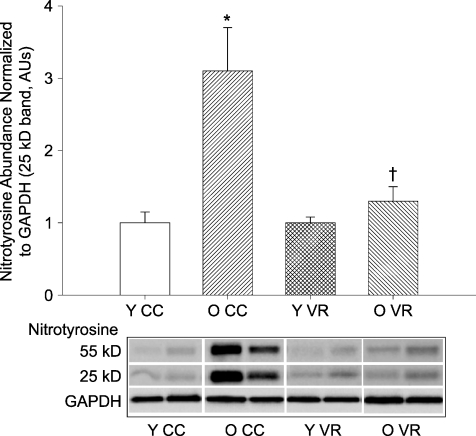
Old age was associated with an increase in the cellular oxidative marker nitrotyrosine in the aorta (P < 0.01) and this was ameliorated by VR (Fig. 3). VR had no effect on nitrotyrosine abundance in the aorta of young mice.
Figure 3. Nitrotyrosine abundance in aortas from young (Y) and old (O) cage control (CC) and voluntary wheel running (VR) mice (n= 7–9 per group).
Nitrotyrosine abundance is expressed relative to GAPDH to account for differences in protein loading and shown normalized to the YCC mean. Values are means ±s.e.m. Representative blots shown below the summary graph. *P < 0.05 vs. YCC; †P < 0.05 vs. OCC.
Superoxide dismutase
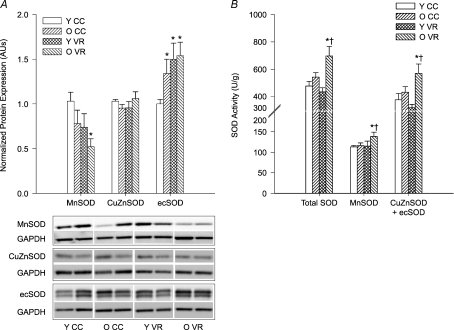
Protein expression of Mn- and CuZnSOD did not differ in the aortas of old compared with young mice, but ecSOD protein expression was higher in old animals (P < 0.05; Fig. 4A). VR had no effect on the protein expression of either Mn- or CuZnSOD isoforms in young mice, but tended to decrease MnSOD in old (P= 0.06) mice (Fig. 4A). VR increased ecSOD expression in aortas of young mice (P < 0.01) and tended to increase expression in old animals (P= 0.07). VR increased total SOD (P < 0.01), MnSOD (P= 0.05) and CuZnSOD + ecSOD (P= 0.05) activities in old mice, without affecting the SOD activities of young mice (Fig. 4B).
Figure 4. Aortic SOD (A) protein expression (n= 7–13 per group) and (B) enzyme activity (n= 7–10 per group) in young (Y) and old (O) cage control (CC) and voluntary wheel running (VR) mice.
Protein expression is expressed relative to GAPDH to account for differences in protein loading and shown normalized to YCC mean. Representative blots shown below the summary graph. Values are means ±s.e.m.*P < 0.05 vs. YCC; †P < 0.05 vs. OCC; ecSOD expression (A) P= 0.07 OVR vs. OCC; MnSOD expression (A) P= 0.06 OVR vs. OCC.
Oxidant enzymes
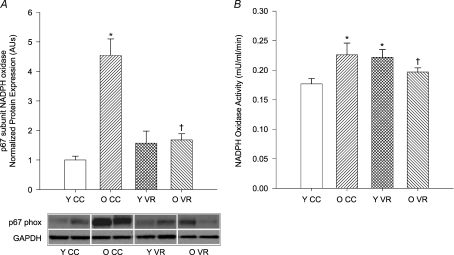
Old cage control mice demonstrated greater aortic expression of the p67 subunit of NADPH oxidase (P < 0.01) and this was ameliorated by VR (P < 0.01; Fig. 5A). VR did not alter expression of p67 in the aortas of young mice. NADPH oxidase activity also was higher in aortas from old cage control mice (P= 0.01) and was reduced by VR (P= 0.05; Fig. 5B). In contrast, VR increased NADPH oxidase activity in young mice (P < 0.05). Neither ageing nor VR altered the aortic protein expression or enzyme activity of xanthine oxidase (Table 4).
Figure 5. Aortic NADPH oxidase (A) p67 subunit protein expression (n= 4–8 per group) and (B) enzyme activity (n= 7–10 per group) in young (Y) and old (O) cage control (CC) and voluntary wheel running (VR) mice.
Protein expression is expressed relative to GAPDH to account for differences in protein loading and shown normalized to YCC mean. Representative blots shown below the summary graph. Values are means ±s.e.m.*P < 0.05 vs. YCC; †P < 0.05 vs. OCC.
Table 4.
Aortic expression (n= 4–8 per group) and activity (n= 7–10 per group) of xanthine oxidase (XO) in young (Y) and old (O) cage control (CC) and voluntary wheel running (VR) mice
| YCC | OCC | YVR | OVR | |
|---|---|---|---|---|
| Expression (norm to YCC AUs) | 1.00 ± 0.07 | 0.93 ± 0.12 | 0.96 ± 0.19 | 0.90 ± 0.10 |
| Activity (×100 mU ml−1 min−1) | 2.6 ± 0.5 | 2.5 ± 0.2 | 2.7 ± 0.5 | 2.3 ± 0.1 |
Pharmacological increases in superoxide scavenging and inhibition of NADPH oxidase improve EDD and NO bioavailability in old cage control but not old VR mice
TEMPOL
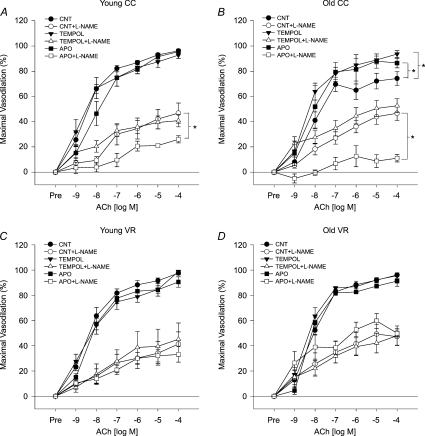
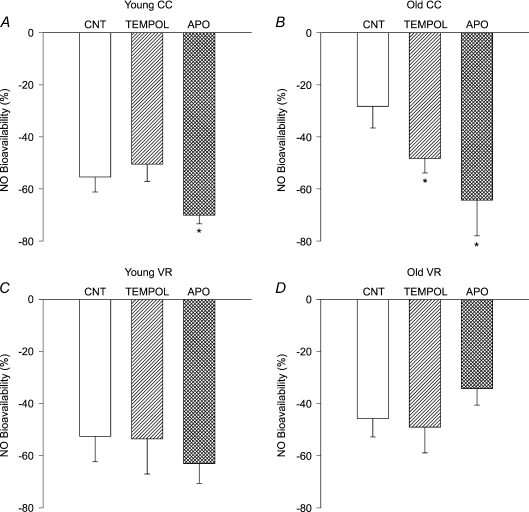
Incubation with the SOD mimetic, TEMPOL, increased preconstricted carotid artery diameter in young mice (P < 0.01 vs. ACh alone; Table 3) and tended to increase diameter in old animals (P= 0.06). Preconstricted diameter after TEMPOL was not different from ACh alone in the VR groups, nor was percentage preconstriction in carotid arteries from any group (Table 3). Treatment with TEMPOL restored maximal carotid artery EDD (P < 0.01) and tended to increase carotid artery sensitivity to ACh (P= 0.07; Table 2) in old cage control mice, but had no effect in young mice (Fig. 6A and B, Table 2). TEMPOL selectively increased the NO component of EDD in carotid arteries from old cage control mice (P < 0.05; Fig. 7). In contrast, TEMPOL had no effect on maximal EDD (Fig. 6C and D), the NO component of EDD (Fig. 7C and D) or sensitivity to ACh (Table 2) in either young or old VR mice.
Figure 6. Dilatation of carotid arteries to ACh alone (CNT) or to ACh after pre-treatment with TEMPOL or apocynin (APO) in the absence or presence of the NOS inhibitor, l-NAME, in (A) young (Y) cage control (CC), (B) old (O) CC, (C) Y voluntary wheel running (VR) and (D) OVR mice (n= 6–10 per group).
Values are means ±s.e.m.*P < 0.05 vs. matched CNT maximal dilatation.
Figure 7. Nitric oxide (NO) component of EDD (NO bioavailability) in carotid arteries from (A) young (Y) cage control (CC), (B) old (O) CC, (C) Y voluntary wheel running (VR) and (D) OVR mice in response to ACh alone (CNT) or after pre-treatment with TEMPOL or apocynin (APO) (n= 6–10 per group).
NO bioavailability (%) = Maximum DilatationACh+L−NAME– Maximum DilatationACh. *P < 0.05 vs. matched CNT.
Apocynin
Incubation with apocynin did not affect carotid artery preconstricted diameter or percentage preconstriction in any group (Table 3). Inhibition of NADPH oxidase by apocynin improved maximal EDD (P < 0.05; Fig. 6B) and markedly increased the NO component of EDD (P < 0.01; Fig. 7B) in carotid arteries of old cage control mice, while not affecting maximal EDD of young cage control mice (Fig. 6A). In contrast to old cage control mice, apocynin did not influence maximal EDD (Fig. 6D) or the NO component of EDD (Fig. 7D) in old VR mice and did not affect maximal EDD in young VR mice (Fig. 6C). Apocynin had no effect on sensitivity to ACh in any group (Table 2).
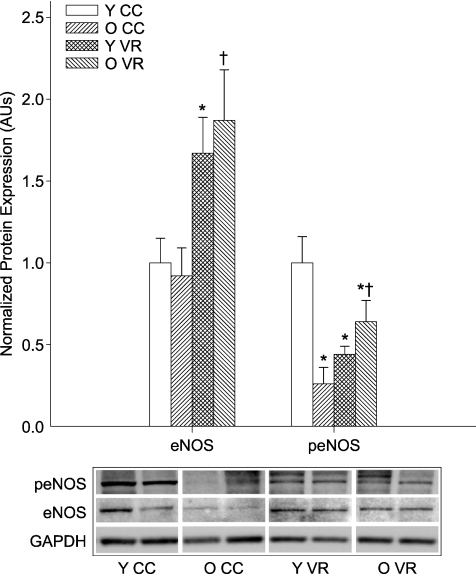
VR increases eNOS protein expression and activation in old mice
Aortic eNOS protein expression was not different in young and old cage control (Fig. 8). VR increased eNOS protein expression in the aortas of old (P < 0.05) and young (P < 0.05) mice (Fig. 8). eNOS phosphorylation at Ser1177 was lower in old compared with young cage control mice (P < 0.01; Fig. 8). VR increased eNOS phosphorylation at Ser1177 in old mice (P < 0.01), but decreased levels in young mice (P < 0.01; Fig. 8).
Figure 8. Total and Ser1177-phosphorylated eNOS (peNOS) in aortas from young (Y) and old (O) cage control (CC) and voluntary wheel running (VR) mice (n= 5–7 per group).
Protein expression is expressed relative to GAPDH to account for differences in protein loading and shown normalized to the YCC mean. Values are means ±s.e.m. Representative blots shown below the summary graph. *P < 0.05 vs. YCC; †P < 0.05 vs. OCC.
Discussion
EDD
In the present study, we show that VR completely restores systemic and carotid artery EDD in old male B6D2F1 mice, without affecting endothelium-independent dilatation as indicated by unchanged vasodilatation in response to SNP. Remarkably, the improvement in EDD occurred even though the old mice ran only ∼1 km per day during their peak activity, which was only ∼10% of the VR performed by young mice (Fig. 1). The reduction in VR distance with ageing noted here has been observed previously in both mice (Ingram et al. 1981; Valentinuzzi et al. 1997) and rats (Seo et al. 2006), although we know of no previous data on rodents as old as those studied here. VR was sufficient to increase muscle citrate synthase activity, a commonly used marker of adaptation to habitual exercise (Sexton, 1995; Delp & Duan, 1996), in our old mice, but did not alter total body, heart or skeletal muscle mass (Table 1). Interestingly, the present results are consistent with earlier findings from our laboratory in humans showing that moderate daily walking restored the forearm blood flow responses to ACh in previously sedentary middle-aged and healthy older men to levels observed in young men (DeSouza et al. 2000). Thus, voluntary habitual aerobic exercise appears to be a potent physiological stimulus for enhancing EDD in old male B6D2F1 mice as well as older humans. Our data using a recently established model of systemic and isolated carotid artery EDD (Lesniewski et al. 2009) also support previous observations in humans (DeSouza et al. 2000; Taddei et al. 2001; Eskurza et al. 2004; Franzoni et al. 2005), indicating that improvements in EDD with exercise in older adults are not limited to the exercising limbs, but rather appear to be adaptations to some type of systemic (perhaps shear stress-related) stimulus (DeSouza et al. 2000; Tanaka et al. 2006; Seals et al. 2008).
In the present study, in vitro maximal carotid artery dilatation, but not sensitivity, to ACh was reduced in old cage control animals as reported recently (Lesniewski et al. 2009), whereas VR selectively restored maximal dilatation in old mice. In contrast, in vivo arterial pressure sensitivity to ACh was reduced in old mice as found previously (Cernadas et al. 1998; Lesniewski et al. 2009) and was restored with VR. These observations are in agreement with previous findings that maximal dilatation and sensitivity to ACh are not consistently related when comparing animals differing in age and/or exercise status (Delp et al. 1993; Johnson et al. 2000; Prisby et al. 2007) and that one or both of these responses can be altered in one circulation or artery and not in another within the same animals (Muller-Delp et al. 2002). The explanation for changes in maximal vasodilatation in isolated arteries and sensitivity changes in the whole animal may involve selective effects of ageing and habitual exercise on arteries that differ in size or location, or in the case of in vivo EDD, interactions between the local vasodilator effects of ACh and systemic baroreflexes.
NO component of EDD
Consistent with previous findings in exercising vs. sedentary older humans (Taddei et al. 2001), in the present study we found that VR normalized carotid artery EDD by restoring the NO component. This was indicated by a greater reduction in EDD after inhibition of NO production by l-NAME in old VR compared with cage control mice such that the difference in EDD between the groups was abolished (Fig. 2C). Although l-NAME did not completely inhibit vasodilatation to acetylcholine, the remaining vasodilatation, which was likely to have been due to prostanoid dilators and endothelium-derived hyperpolarizing factor, was unaffected by either ageing or VR. Again, even the modest VR performed by the old mice was sufficient to restore the NO component of EDD to levels observed in young animals.
Oxidative stress and related mechanisms
Vascular endothelial dysfunction with ageing is mediated, at least in part, by oxidative stress (van der Loo et al. 2000; Csiszar et al. 2002; Blackwell et al. 2004; Donato et al. 2007). In humans, administration of supraphysiological concentrations of the antioxidant ascorbic acid (vitamin C) improves EDD in older sedentary adults without affecting EDD in older adults who regularly perform aerobic exercise (Taddei et al. 2001; Eskurza et al. 2004). This provides support for the idea that exercise preserves vascular endothelial function with ageing by limiting the development of oxidative stress. Surprisingly, however, there is no direct evidence that habitual aerobic exercise reduces oxidative stress in arteries of older adults or animals, nor are there any data on the cellular and molecular mechanisms by which such an effect may be mediated.
Oxidative stress
In the present study, sedentary (cage control) ageing was associated with a marked increase in aortic nitrotyrosine, a cellular marker of oxidative modification of tyrosine residues on proteins (Radi, 2004; Fig. 3), consistent with previous observations in rodents (van der Loo et al. 2000; Csiszar et al. 2002) and recent work from our laboratory in humans (Donato et al. 2007). In striking contrast, in old VR mice, aortic nitrotyrosine was not significantly different than in young cage control or VR mice. To our knowledge, this is the first direct evidence that regular aerobic exercise suppresses the development of oxidative stress in arteries with ageing.
SOD expression and activity
Our results also provide initial insight into the mechanisms that may contribute to this influence of habitual exercise on vascular oxidative stress. One possibility is that exercise increases the expression (Fukai et al. 2000; Rush et al. 2000, 2003; Davis et al. 2003) and/or activity (Rush et al. 2000, 2003) of SOD, a major antioxidant enzyme expressed in arteries that reacts with and reduces the bioavailability of superoxide anion (Faraci & Didion, 2004). In the present study, VR did not significantly influence SOD protein expression in old animals, although MnSOD tended to be lower in the VR compared with cage control old mice (Fig. 4A). Because MnSOD expression is sensitive to changes in the oxidative environment (Xu et al. 1999; Rogers et al. 2001), it is possible that the reductions in MnSOD protein expression with VR in old mice are a consequence and not a cause of the reduction in oxidative stress. However, VR was associated with clear increases in total SOD, MnSOD and combined CuZnSOD + ecSOD activity in the aortas of old mice, while having no effect on young mice (Fig. 4B).
In agreement with an important role for this antioxidant enzyme in mediating the effects of VR, administration of TEMPOL, a SOD mimetic, restored maximal carotid artery EDD by increasing the NO component of EDD in old cage control mice, as reported previously (Csiszar et al. 2002; Lesniewski et al. 2009), but had no effect on these responses in old VR mice or young animals (Figs. 6 and 7). Consistent with this observation, TEMPOL tended to selectively decrease IC50 in old cage control mice in the present study.
Together, these findings provide compelling evidence that increases in SOD activity may be a key mechanism by which habitual aerobic exercise prevents vascular oxidative stress and preserves EDD with ageing by maintaining the NO component of EDD.
Oxidant enzyme expression and activity
We also considered the possibility that exercise may reduce oxidative stress with ageing by suppressing oxidant enzyme expression and/or activity. The two major oxidant-producing enzymes in arteries are NADPH oxidase (Hamilton et al. 2002) and xanthine oxidase (Houston et al. 1999). A previous study found reductions in the p67 subunit of NADPH oxidase in aorta of young swine with exercise training (Rush et al. 2003). In the present study, we found no influence of age or VR on xanthine oxidase protein expression or activity (Table 4). However, NADPH oxidase p67 subunit expression and enzyme activity were increased with age in cage control mice, and VR prevented these changes (Fig. 5). In contrast, in young mice VR had no effect on p67 subunit expression and was associated with an increase in NADPH oxidase activity. Furthermore, apocynin, an inhibitor of NADPH oxidase, improved maximal EDD and the NO component of EDD in old cage control mice, without affecting these responses in old VR animals (Figs. 6 and 7). Collectively, these results support the hypothesis that regular aerobic exercise prevents the development of vascular oxidative stress and restores EDD and its NO component in old mice in part by reducing the expression and activity of NADPH oxidase.
The mechanisms by which VR suppressed NADPH oxidase expression and activity selectively in our old mice are not known. This enzyme is regulated by a complex array of factors including circulating hormones, haemodynamic forces and local metabolic influences (Griendling et al. 2000). Several factors that modulate NADPH oxidase were or may have been affected by VR, possibly differently in young and old mice, including shear stress, vascular endothelial growth factor, angiotensin II and cytokines (Ushio-Fukai, 2006). Transient increases in NADPH oxidase activity occur in response to laminar shear in endothelial cells (De Keulenaer et al. 1998; Hwang et al. 2003) and after exercise in canine heart tissue (Sanchez et al. 2008). It is possible that the age-specific effects of VR on the enzyme were influenced by different shear forces produced by the marked differences in VR in the young and older mice (Fig. 1). Alternatively, greater baseline (cage control) levels of NADPH oxidase expression and activity may have resulted in the old animals being more responsive to normalization by VR.
eNOS protein expression and activation
Experimental increases in vascular shear stress (Woodman et al. 2005) and forced treadmill running (Spier et al. 2004) increase EDD and eNOS mRNA and protein expression in soleus feed arteries and arterioles of old rats, respectively. However, the effects of voluntary aerobic exercise on eNOS expression and activation with ageing are unknown. In the present study, we found that eNOS protein expression was unaffected with ageing in cage control mice, whereas Ser1177 phosphorylation of eNOS, an activated form of the enzyme (Dimmeler et al. 1999; Bauer et al. 2003), was reduced (Fig. 8). VR increased eNOS protein in both young and old mice. In contrast, VR increased Ser1177 phosphorylation of eNOS in old mice while decreasing it in the young mice (Fig. 8). These results demonstrate that habitual voluntary aerobic exercise increases eNOS protein independent of age. Our data also provide the first evidence that regular exercise may selectively increase eNOS activation in old mice. This is consistent with the increase in Ser1177-phosphorylated eNOS reported with exercise training in patients with coronary artery disease (Hambrecht et al. 2003) and in shear stress models of exercise (Dimmeler et al. 1999; Fisslthaler, 2000; Boo et al. 2002; Kojda & Hambrecht, 2005; LeBlanc et al. 2008). Together with the reduction in superoxide bioactivity, the net effect of these changes in eNOS protein and its activation with VR is likely to explain the restoration of EDD and enhancement of NO bioavailability observed in old mice.
Ser1177 phosphorylation of eNOS may decrease with VR in young mice because NO bioavailability and EDD already are normal in these animals under cage control conditions. As such, exercise-stimulated increases in eNOS protein might result in excessive NO production in the absence of a corresponding reduction in activation state. However, eNOS activation is a complex process involving multiple activating and inhibiting phosphorylation sites. We examined one activating phosphorylation site (Ser1177) as a marker of eNOS activity, but Ser635, Ser617 and Tyr83 are other known activating sites, whereas Ser113/6 and Thr495 are inhibitory (Dudzinski & Michel 2007). It is possible that the phosphorylation pattern differs between young and old mice and/or with VR and, thus, by measuring only one phosphorylation site we may not have a complete picture of the activation state of the enzyme. For example, because there is no reduction in the NO bioavailability in young VR compared with cage control mice, the lower Ser1177 phosphorylation of eNOS in young VR animals may not be reflecting an actual decrease in enzyme activity.
Limitations
In the present study we sought to gain initial insight into mechanisms by which habitual exercise may preserve EDD with ageing. We focused on selective mechanisms relating to oxidative stress and eNOS. Several other mechanisms not studied here may contribute to reduced oxidative stress with habitual exercise in young and old mice including reductions in mitochondrial superoxide production and/or eNOS uncoupling, and increases in other antioxidant enzymes. Moreover, the results of our in vivo vascular studies suggest that resistance artery EDD also may be improved by VR in old mice. More detailed studies of the microcirculation will be required in the future to confirm that this is the case and to determine if the mechanisms involved are similar to those established for large arteries in the present investigation.
Summary and conclusions
With the changing demographics of ageing leading to unprecedented numbers of older adults now and in the future, it is imperative that we establish the efficacy of strategies to delay, slow and even prevent the development of vascular endothelial dysfunction and CVD with ageing. There is accumulating evidence that habitual aerobic exercise is an effective lifestyle intervention for preserving vascular endothelial function with ageing (Seals et al. 2008). However, we know little about the underlying mechanisms, in part, because of limitations in studying humans (Seals et al. 2008). In the present study, we used a new mouse model of age-associated vascular endothelial dysfunction combined with voluntary wheel running to gain new insight into the mechanisms by which regular aerobic exercise enhances EDD with ageing. We provide the first direct evidence that habitual aerobic exercise reduces oxidative stress in arteries of old animals. Importantly, our results implicate increases in SOD activity and an inhibition of NADPH oxidase as key molecular mechanisms contributing to reduced arterial oxidative stress and normalization of EDD and its NO component in old exercising mice. Finally, our findings demonstrate that VR induces a marked increase in eNOS protein in arteries of old animals and that this is coupled to an increase in eNOS activation. Together, these changes are likely to contribute to the restoration of NO bioavailability and EDD by exercise in old mice. Future studies should explore other mechanisms such as reduced arterial inflammation and the signalling pathways involved in the anti-ageing effects of aerobic exercise on arterial dysfunction.
Acknowledgments
This work was supported by grants from the NIH (AG006537, AG013038, AG029337, AG000279).
Glossary
Abbreviations
- CuZnSOD
copper-zinc superoxide dismutase
- CVD
cardiovascular disease
- ECL
enchanced chemiluminescence
- ecSOD
extracellular superoxide dismutase
- EDD
endothelium-dependent dilatation
- EID
endothelium-independent dilatation
- eNOS
endothelial nitric oxide synthase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- MnSOD
manganese superoxide dismutase
- l-NAME
NG-nitro-l-arginine methyl ester
- peNOS
phosphorylated endothelial nitric oxide synthase
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- TEMPOL
4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl
- VR
voluntary wheel running
Author contributions
J.R.D., D.R.S., A.J.D. and L.A.L. contributed to the conception and design, analysis and interpretation of data, and drafting and revision of the article, and provided final approval of the version to be published. All authors contributed to the interpretation of data and the revision of the manuscript, and provided final approval of the version to be published. All experiments were carried out at the University of Colorado at Boulder.
References
- Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H, Sessa WC. Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem. 2003;278:14841–14849. doi: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: Role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2004;287:H2448–2453. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- Bonetti P, Lerman L, Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- Bonthu S, Heistad DD, Chappell DA, Lamping KG, Faraci FM. Atherosclerosis, vascular remodeling, and impairment of endothelium-dependent relaxation in genetically altered hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 1997;17:2333–2340. doi: 10.1161/01.atv.17.11.2333. [DOI] [PubMed] [Google Scholar]
- Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at ser1179 by Akt-independent mechanisms. Role of protein kinase A. J Biol Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards J, Kaminski P, Wolin M, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- d’Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res. 2003;92:88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- d’Uscio LV, Smith LA, Santhanam AV, Richardson D, Nath KA, Katusic ZS. Essential role of endothelial nitric oxide synthase in vascular effects of erythropoietin. Hypertension. 2007;49:1142–1148. doi: 10.1161/HYPERTENSIONAHA.106.085704. [DOI] [PubMed] [Google Scholar]
- Davis ME, Cai H, McCann L, Fukai T, Harrison DG. Role of c-Src in regulation of endothelial nitric oxide synthase expression during exercise training. Am J Physiol Heart Circ Physiol. 2003;284:H1449–1453. doi: 10.1152/ajpheart.00918.2002. [DOI] [PubMed] [Google Scholar]
- De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state : Role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- Delp MD, McAllister RM, Laughlin MH. Exercise training alters endothelium-dependent vasoreactivity of rat abdominal aorta. J Appl Physiol. 1993;75:1354–1363. doi: 10.1152/jappl.1993.75.3.1354. [DOI] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIa, IId/x, and IIb fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- DeSouza C, Shapiro L, Clevenger C, Dinenno F, Monahan K, Tanaka H, Seals D. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: Relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM, Didion SP. Vascular protection: Superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Sigmund CD, Shesely EG, Maeda N, Heistad DD. Responses of carotid artery in mice deficient in expression of the gene for endothelial NO synthase. Am J Physiol Heart Circ Physiol. 1998;274:H564–570. doi: 10.1152/ajpheart.1998.274.2.H564. [DOI] [PubMed] [Google Scholar]
- Fisslthaler DHBF. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol Scand. 2000;168:81–88. doi: 10.1046/j.1365-201x.2000.00627.x. [DOI] [PubMed] [Google Scholar]
- Franzoni F, Ghiadoni L, Galetta F, Plantinga Y, Lubrano V, Huang Y, Salvetti G, Regoli F, Taddei S, Santoro G, Salvetti A. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am J Hypertens. 2005;18:510–516. doi: 10.1016/j.amjhyper.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, Al-Benna S, Berg G, Dominiczak AF. NAD(P)H oxidase inhibition improves endothelial function in rat and human blood vessels. Hypertension. 2002;40:755–762. doi: 10.1161/01.hyp.0000037063.90643.0b. [DOI] [PubMed] [Google Scholar]
- Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, Freeman BA. Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem. 1999;274:4985–4994. doi: 10.1074/jbc.274.8.4985. [DOI] [PubMed] [Google Scholar]
- Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2– from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- Ingram DK, London ED, Reynolds MA, Waller SB, Goodrick CL. Differential effects of age on motor performance in two mouse strains. Neurobiol Aging. 1981;2:221–227. doi: 10.1016/0197-4580(81)90025-7. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Parker JL, Laughlin MH. Chronic exercise training improves ACh-induced vasorelaxation in pulmonary arteries of pigs. J Appl Physiol. 2000;88:443–451. doi: 10.1152/jappl.2000.88.2.443. [DOI] [PubMed] [Google Scholar]
- Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res. 2005;67:187–197. doi: 10.1016/j.cardiores.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Lakatta E, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lamping KG, Nuno DW, Shesely EG, Maeda N, Faraci FM. Vasodilator mechanisms in the coronary circulation of endothelial nitric oxide synthase-deficient mice. Am J Physiol Heart Circ Physiol. 2000;279:H1906–1912. doi: 10.1152/ajpheart.2000.279.4.H1906. [DOI] [PubMed] [Google Scholar]
- LeBlanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol. 2008;295:H2280–2288. doi: 10.1152/ajpheart.00541.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci. 2009;64:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci U S A. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Prisby RD, Ramsey MW, Behnke BJ, Dominguez JM, Donato AJ, Allen MR, Delp MD. Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J Bone Miner Res. 2007;22:1280–1288. doi: 10.1359/jbmr.070415. [DOI] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RJ, Monnier JM, Nick HS. Tumor necrosis factor-α selectively induces MnSOD expression via mitochondria-to-nucleus signaling, whereas interleukin-1β utilizes an alternative pathway. J Biol Chem. 2001;276:20419–20427. doi: 10.1074/jbc.M008915200. [DOI] [PubMed] [Google Scholar]
- Rush JWE, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol. 2000;279:H2068–2076. doi: 10.1152/ajpheart.2000.279.5.H2068. [DOI] [PubMed] [Google Scholar]
- Rush JWE, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol. 2003;284:H1378–1387. doi: 10.1152/ajpheart.00190.2002. [DOI] [PubMed] [Google Scholar]
- Sanchez G, Escobar M, Pedrozo Z, Macho P, Domenech R, Hartel S, Hidalgo C, Donoso P. Exercise and tachycardia increase NADPH oxidase and ryanodine receptor-2 activity: Possible role in cardioprotection. Cardiovasc Res. 2008;77:380–386. doi: 10.1093/cvr/cvm011. [DOI] [PubMed] [Google Scholar]
- Seals DR, DeSouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol. 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo AY, Hofer T, Sung B, Judge S, Chung HY, Leeuwenburgh C. Hepatic oxidative stress during aging: Effects of 8% long-term calorie restriction and lifelong exercise. Antioxid Redox Signal. 2006;8:529–538. doi: 10.1089/ars.2006.8.529. [DOI] [PubMed] [Google Scholar]
- Sexton WL. Vascular adaptations in rat hindlimb skeletal muscle after voluntary running-wheel exercise. J Appl Physiol. 1995;79:287–296. doi: 10.1152/jappl.1995.79.1.287. [DOI] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere P. Citrate synthase. Methods Enzymol. 1969;13:3–11. [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of no availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Shimizu S, Ohmori F, Muraoka Y, Kumagai M, Yoshizawa M, Kagaya A. Increases in blood flow and shear stress to nonworking limbs during incremental exercise. Med Sci Sports Exerc. 2006;38:81–85. doi: 10.1249/01.mss.0000191166.81789.de. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol Regulatory Integrative Comp Physiol. 1997;273:R1957–1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- Van Der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita J, Keaney J. Endothelial function: A barometer for cardiovascular risk. Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- Wadsworth RM. Oxidative stress and the endothelium. Exp Physiol. 2008;93:155–157. doi: 10.1113/expphysiol.2007.038687. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension. 2004;44:381–386. doi: 10.1161/01.HYP.0000142232.29764.a7. [DOI] [PubMed] [Google Scholar]
- Widlansky M, Gokce N, Keaney J, Vita J. The clinical implications of endothelial dysfunction. J Amer Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Price EM, Laughlin MH. Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries. J Appl Physiol. 2005;98:940–946. doi: 10.1152/japplphysiol.00408.2004. [DOI] [PubMed] [Google Scholar]
- Xu Y, Kiningham KK, Devalaraja MN, Yeh CC, Majima H, Kasarskis EJ, St Clair DK. An intronic NF-κB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-α and interleukin-1β. DNA Cell Biol. 1999;18:709–722. doi: 10.1089/104454999314999. [DOI] [PubMed] [Google Scholar]