Abstract
Cardiac utilisation of very-low-density lipoprotein (VLDL) and chylomicrons (CM) was investigated in the ZDF rat model of type 2 diabetes, in order to define the role of triacylglycerol (TAG) metabolism in the development of contractile dysfunction. Hearts from obese diabetic and lean littermate control rats were perfused with VLDL and CM from diabetic and control rats. Metabolic fate of the lipoprotein TAG and contractile function were examined. Myocardial utilisation of both VLDL- and CM-TAG was increased in the diabetic state. Diabetic hearts oxidised diabetic lipoprotein-TAG to a greater extent than control lipoproteins; glucose oxidation was decreased. There was no difference in lipoprotein-TAG assimilation into diabetic heart lipids; diabetic lipoproteins were, however, a poor substrate for control heart tissue lipid accumulation. Although the proportion of exogenous lipid incorporated into tissue TAG was increased in diabetic hearts perfused with control lipoproteins, this effect was not seen in diabetic hearts perfused with diabetic lipoproteins. Myocardial heparin-releasable lipoprotein lipase (LPL) activity was moderately increased in the diabetic state, and diabetic lipoproteins increased tissue-residual LPL activity. Cardiac hydraulic work was decreased only in diabetic hearts perfused with diabetic CM. Compositional analysis of diabetic variant lipoproteins indicated changes in size and apoprotein content. Alterations in cardiac TAG-rich lipoprotein metabolism in type 2 diabetes are due to changes in both the diabetic myocardium and the diabetic lipoprotein particle; decreased contractile function is not related to cardiac lipid accumulation from TAG-rich lipoproteins but may be associated with changes in TAG-fatty acid oxidation.
Diabetes is characterized by dyslipidaemia, with raised plasma TAG concentrations due to increased circulating triacylglycerol-rich lipoproteins (TGRLPs) including VLDL and chylomicrons (Howard, 1994; Lewis & Steiner, 1996; Steiner et al. 1998). The increased plasma TAG is due to both a decreased plasma TAG clearance associated with tissue-specific decreased lipoprotein lipase (LPL) activity (Taskinen, 1987; Tavangar et al. 1992) and to increased hepatic VLDL synthesis and secretion (Tomkin & Owens, 2001). In addition, the composition of both lipid and apolipoprotein components of TGRLP particles is altered in diabetes (Howard, 1994; Goldberg, 1996; McEneny et al. 2000; Tomkin & Owens, 2001) and this may make these lipoproteins poorer substrates for tissue uptake mechanisms, which besides LPL-mediated core TAG hydrolysis (Augustus et al. 2003) include lipoprotein receptor-mediated endocytotic routes (Kamataki et al. 2002). The plasma lipid clearance defect is limited to certain tissues only (e.g. adipose tissue), with some tissues able to assimilate sufficient, and possibly excess, lipid for energetic needs. Diabetes is also accompanied by tissue dysfunction, which may be related to the changes in energy provision (Belke et al. 2000; Aasum et al. 2002; Lopaschuk, 2002; Young et al. 2002b; Severson, 2004; An & Rodrigues, 2006; How et al. 2006). Recently, diabetic cardiomyopathy has been recognised as a significant factor in diabetic morbidity and mortality, distinct from and unrelated to the atherosclerosis that also occurs in insulin deficiency/resistance. The pathoaetiology of this cardiac contractile dysfunction is uncertain, but may be related to changes in lipid metabolism: intracellular accumulation of lipids, particularly TAG (Paulson & Crass, 1982; Zhou et al. 2000), but also other fatty acid (FA)-derived lipids including sphingolipids (Unger & Orci, 2002), is observed in the diabetic myocardium, and this ‘lipotoxicity’ has been suggested as a cause for the heart failure associated with the diabetic state (Unger & Orci, 2002).
Cardiac energy provision is principally (∼70%) derived from fatty acid oxidation, the remainder being obtained mostly from carbohydrates (van der Vusse et al. 2000). In diabetes, this proportion is increased further, with > 90% myocardial ATP synthesised from fatty acids. The fatty acid can be sourced from the plasma as non-esterified (‘free’) fatty acid (NEFA) or from the lipolysis of TAG contained in plasma TGRLPs. Myocardial preference for FAs from these various sources has only recently been elucidated (most studies to date have examined only NEFA utilisation): TAG is a major provider of FA, and hence energy, to the myocardium, and both VLDL and CM support cardiac work, with VLDL-TAG and CM-TAG avidly assimilated and oxidised by working myocardium (Hauton et al. 2001; Mardy et al. 2001; Augustus et al.2003; Neitzel et al. 2003; Teusink et al. 2003; Niu et al. 2004). Indeed, TGRLP-FA supply to the heart may be more important than NEFA, which are limited in concentration in plasma and hence availability, even in diabetes. However, recent work suggests that VLDL and CM are assimilated differently by myocardium, undergo differential intracellular metabolic channelling and partitioning, and may serve different functions in energy homeostasis and intracellular lipid provision in the physiological state (Niu et al. 2004). These relationships may be altered in the diabetic state and this may contribute to the abnormalities of lipid metabolism and cardiac mechanical function observed in diabetes.
The purpose of the present study was therefore to examine metabolism of normal and diabetic variant VLDL and chylomicrons by the normal and diabetic heart, and to relate myocardial lipid handling to cardiac function. Experiments were performed in Zucker diabetic fatty rats – these animals are obese and hyperglycaemic with insulin resistance (Carley & Severson, 2005; Chen & Wang, 2005) and commonly used as a model of type 2 (insulin resistant) diabetes.
Methods
Ethical approval
The investigation was performed in accordance with the Home Office Guidance On The Operation Of The Animals (Scientific Procedures) 1986 Act published by HMSO, London, UK, under Home Office Project Licence PPL 30/2139.
Animals
Male Zucker diabetic fatty (ZDF/Leprfa/fa) obese rats (12 weeks of age) and age-matched male lean littermates (controls) were obtained from Charles River Laboratories (Margate, Kent, UK). Homozygous rats at this age are hyperglycaemic and exhibit insulin resistance; lean littermate rats were used as controls. Animals were fed ad libitum on a chow diet (comprising by weight approximately 52% carbohydrate, 21% protein and 4% fat; the residue was non-digestible material (Special Diet Services, Witham, Essex, UK)) with free access to drinking water, and were maintained at an ambient temperature of 20 ± 2°C with a 12 h light–12 h dark cycle (light from 07.30 h).
Chemicals
[9,10(n)-3H]oleic acid, glycerol tri[9,10(n)-3H] oleate and [U-14C] glucose were obtained from Amersham Biosciences UK (Amersham, Bucks, UK). Waymouth's medium was purchased from Gibco BRL (Life Technologies, Paisley, UK); biochemicals were obtained from Sigma Chemical Co. (Poole, Dorset, UK).
Experimental protocol
To investigate the effects of diabetes on cardiac lipoprotein metabolism and lipoprotein preference, non-diabetic (control) VLDL, diabetic VLDL, non-diabetic (control) CM and diabetic CM were prepared from lean and obese ZDF rats, and used to perfuse hearts from lean control and obese diabetic rats.
Preparation of lipid substrates
Preparation of VLDL
VLDL containing 3H-labelled triolein were prepared by the rat liver perfusion technique: 3H-labelled sodium oleate (specific activity 485 mCi mmol−1) was prebound to fatty acid- and endotoxin-free bovine serum albumin (BSA) (5% w/v) and added to liver perfusate for production of VLDL containing [3H]triolein as described previously (Bennett et al. 2000a). Rats (diabetic or control) were anaesthetised with intraperitoneal sodium pentobarbitone (60 mg (kg body weight)−1) and the portal vein and thoracic inferior vena cava were rapidly cannulated; the abdominal inferior vena cava was ligated. Heparin was not used. The liver was perfused in situ for 8 h with a recirculating solution comprising Waymouth's synthetic tissue culture medium supplemented with amino acids (glutamine, serine and alanine) and glucose. Washed red cells were added to give a final haematocrit of 10% (v/v) and the perfusate was gassed with O2–CO2 95–5% (v/v) at 37°C; [3H]oleate (1.0 mm final concentration) prebound to fatty acid-free albumin was added to the perfusate prior to liver perfusion and subsequently also infused into the perfusate for the first 4 h of the perfusion to maintain the circulating NEFA concentration at about 0.4 mm for the first half of the procedure. After the perfusion, the perfusate was filtered through an ultrafilter with molecular weight cut-off at 30 000 Da (Amicon, Stonehouse, Gloucestershire, UK) then ultracentrifuged at 144 500 g to separate the layer that was less dense than 1.006 g ml−1. Thin-layer chromatography of radio-labelled VLDL showed that > 95% of the [3H]-label was in the triacylglycerol band. VLDL was suspended in fatty acid-free bovine serum albumin (5% w/v), TAG content was assayed with enzymatic colorimetric test kit (Randox Laboratories Ltd, Crumlin, Co. Antrim, UK). The [3H]-labelled VLDL was subsequently added to the heart perfusate to give a final concentration of 0.4 mm TAG.
Preparation of chylomicrons
Chylomicrons containing 3H-labelled triolein were prepared using a rat thoracic duct cannulation technique (Bezman-Tarcher et al. 1965). Briefly, anaesthetised rats (diabetic or control) had a polyethylene catheter inserted into the lower thoracic duct via an extra-peritoneal loin incision and externalised to continuously collect chyle; a gastrostomy was also performed. Heparin was not used. The animals were maintained in a restraining cage with free access to food and water, but were given additional intragastric fluid replacement. Triolein/[3H]triolein (1.0 g; 22 mCi) was administered into the stomach, and chyle collected for the subsequent 12 h. Radio-labelled chylomicrons were isolated by washing with bovine serum albumin solution and centrifugation. Thin-layer chromatography of the radiolabelled chylomicrons showed that > 95% of the [3H]-label was in the triacylglycerol band. Chylomicrons were suspended in fatty acid-free bovine serum albumin (5% w/v) and TAG was assayed with an enzymatic colorimetric test kit (as above). 3H-labelled chylomicrons were subsequently added to the heart perfusate to give a final concentration of 0.4 mm TAG.
In separate experiments, a group of fed obese ZDF and lean littermate rats were anaesthetised, and blood and heart tissue samples taken. Plasma was centrifuged to separate ‘native’ CM and VLDL which were analysed for lipid and apoprotein content (see below). Hearts were rapidly freeze-clamped in liquid N2 and subsequently analysed for total (unlabelled) lipid content (see below).
Analysis of TAG-rich lipoprotein composition
VLDL and CM samples were delipidated as described previously (Scanu & Edelstein, 1971). Apolipoprotein components of lipoproteins were determined by electrophoresis on SDS-PAGE gradient gels as described previously (Irwin et al. 1984). A molecular weight marker ladder and albumin standard were co-run in the same gel. The gel was stained with Coomassie blue solution, then scanned (GeneSnap, Synoptics Ltd, Cambridge, UK). By referring to the molecular weight marker ladder, bands of different apolipoproteins were determined. The optical density (OD) of each band was calculated by commercially available software for gel analysis (GeneTools, Synoptics Ltd). By calibrating with albumin standard bands, the amount of protein in each sample band was calculated.
Lipid components of lipoproteins were determined by enzymatic colorimetric test kits (TAG and cholesterol: Randox Laboratories Ltd; phospholipids (PL) and NEFA: Wako Chemical GmbH, Neuss, Germany). All assays were conducted in duplicate.
Isolated perfused ‘working’ heart preparation
Hearts were perfused through the left atrium in ‘working’ mode by the method of Taegtmeyer et al. (1980). Fed rats were anaesthetised with intraperitoneal sodium pentobarbitone (60 mg (kg body weight)−1). The heart was rapidly excised and briefly placed in ice-cold Krebs–Henseleit bicarbonate saline; it was then cannulated via the aorta (< 2 min from excision) and perfused retrogradely through the coronary arteries in ‘Langendorff’ mode whilst lung, mediastinal and peri-cardiac brown adipose tissue were excised, right pulmonary arteriotomy performed, and the left atrium separately cannulated after which the apparatus was switched to ‘working’ mode and cardiac perfusion maintained through the left atrium. A recirculating Krebs–Henseleit bicarbonate buffer solution containing 1.3 mmCaCl2, 11 mm glucose and 1% (w/v) endotoxin- and fatty acid-free bovine serum albumin was filtered through a 5 μm cellulose nitrate filter (Millipore, Bedford, MA, USA) and gassed with O2–CO2 (95–5%) at 37°C. The first 50 ml of coronary effluent was discarded to free the circuit of blood cells; final perfusate volume was 100 ml. Afterload was maintained at 100 cmH2O and preload (atrial filling pressure) at 15 cmH2O. After an initial 15 min stabilisation period, lipid was added slowly (2 min) to the reservoir (time ‘0’). [U-14C] glucose (specific activity 3.23 mCi mol−1) in aqueous solution was also added to the perfusate. Peak systolic pressure (PSP) and heart rate (HR) were measured by calibrated pressure transducer (Druck Ltd, Groby, Leicestershire, UK) connected to a side arm of the aortic cannula. Aortic flow rate (AFR) was measured by a timed collection of perfusate ejected through the aortic line, and coronary flow rate (CFR) was measured by a timed collection of perfusate effluent dripping from the heart. Measurements were made at time 0 and at 10 min intervals for 60 min. Cardiac output (CO) was calculated as (CFR + AFR). Rate–pressure product (RPP) was calculated as (HR × PSP). Hydraulic work (HW) was calculated as (CO × mean aortic pressure/heart wet wt). After the final measurements at 60 min, 5 IU ml−1 heparin (Leo Laboratories Ltd, Princes Risborough, Bucks, UK) was added to the perfusate, and after a further 2 min the heart was rapidly excised, freeze-clamped in light alloy tongs cooled in liquid N2, and weighed. A duplicate sample of the post-heparin perfusate was also frozen in liquid N2.
Measurement of lipoprotein-TAG oxidation rate
TAG-fatty acid oxidation rate was estimated by measuring 3H2O production from [3H]triolein in the perfusate (Hauton et al. 2001); at 10 min intervals, aliquots of perfusate (1.0 ml) were removed and subjected to Folch lipid extraction with chloroform : methanol (2 : 1, v/v), and water. An aliquot of the water phase was removed and counted for radioactivity.
TAG uptake (disappearance from the perfusate) was measured by assay of TAG in the organic infranatant phase of the Folch extracts of the timed perfusate using an enzymatic colorimetric assay test kit (see above).
Incorporation of exogenous lipoprotein-TAG into myocardial lipid
Myocardial 3H-lipid content was estimated by grinding frozen myocardium to powder under liquid N2 and extracting the lipids from an aliquot with chloroform–methanol (Folch). After repeated washing, the lipids were resolubilised in chloroform and separated by thin-layer chromatography using a hexane–diethylether–acetic acid system with standards co-run. [3H]-radioactivity was measured in the various lipid bands after visualization with rhodamine 6G under ultraviolet light.
Lipoprotein-TAG utilization was the sum of TAG oxidation and tissue lipid incorporation.
Measurement of glucose oxidation rate
Glucose oxidation rate was determined by measuring 14CO2/H14CO3− production (Belke et al. 2000). [U-14C]glucose (3.23 mCi mol−1) in aqueous solution was added to the perfusate. Hearts were then perfused in a closed system that allowed quantitative collection of gaseous and perfusate 14CO2. Perfusate and gaseous samples were collected at 10 min intervals throughout the perfusion. The 14CO2 liberated in the gaseous state was trapped in Optisorb ‘1’ carbon dioxide absorbent/liquid scintillant (Wallac UK, Milton Keynes, Bucks, UK) in the gas outlet line. Samples of this solution were counted directly for radioactivity. Perfusate samples were immediately injected into the middle well of sealed Ehrlenmeyer flasks containing H2SO4, with Optisorb CO2 absorbent/scintillant in the outer well. The flasks were gently agitated for 1 h and the absorbent removed and counted for radioactivity (H14CO3− production).
Lipoprotein lipase activity
LPL activity was estimated in duplicate samples by using a 3H-labelled triolein substrate emulsion containing fasted rat serum as a source of apolipoprotein-CII to maximise LPL detection (Hauton et al. 2001); the serum was pretreated by heating to 56°C to inactivate non-specific plasma lipases. Radioactivity in evolved fatty acids was counted following extraction in methanol–chloroform–heptane. Heparin-releasable LPL activity was measured by adding post-heparin perfusate taken at 62 min directly in the above assay system without modification (expressed as nmol fatty acid released (min)−1 (g wet wt of heart)−1). Tissue residual LPL activity was measured in acetone–diethyl ether-dried tissue powders ground from the working hearts frozen in liquid N2; a duplicate sample of frozen heart tissue was weighed, dried down with acetone–ether in parallel with the samples and re-weighed to correct expression of activity (from nmol of fatty acid released (min)−1 (mg of acetone dried powder)−1 to nmol of fatty acid released (min)−1 (g wet wt of heart)−1).
Analysis of myocardial lipid content
Frozen hearts from obese ZDF and lean littermate controls were ground in liquid N2 and subjected to Folch extraction, separation of lipid classes by thin-layer chromatography (see above) and quantitation of lipid bands by enzymatic colorimetric test kits (TAG and total cholesterol: Randox Laboratories; phospholipid and NEFA: Wako Chemicals GmbH).
Statistics
Results are expressed as mean ±s.e.m. Statistical analysis was performed by one-way ANOVA for repeated measurements and Tukey's test, or by Student's t test with Bonferroni correction for multiple comparisons, where appropriate. Statistical significance was set at P < 0.05.
Results
General characteristics of obese ZDF and lean control rats
Body weight, heart weight and blood glucose level in the two groups were consistent with previously published data on ZDF rats (Chatham & Seymour, 2002; Wang et al. 2005). At 12 weeks of age ZDF rats showed significant obesity (body weight 373 ± 3 g (n= 20) versus 322 ± 5 g (n= 20) for lean controls; P < 0.05) and hyperglycaemia (blood glucose 26.5 ± 0.8 mmol l−1 (n= 20) versus 8.2 ± 0.1 mmol l−1 (n= 20); P < 0.01), characterising the type 2 diabetic state. However, the heart weight of ZDF rats (1.52 ± 0.06 g (n= 20)) was not significantly different to that of control lean rats (1.38 ± 0.08 g (n= 20)).
Myocardial lipid composition
Unperfused hearts from a group of ZDF rats (body wt: 388 ± 14 g; heart wt: 1.55 ± 0.10 g (n= 5)) and lean littermate controls (body wt: 309 ± 12 g (P < 0.01); heart wt: 1.39 ± 0.07 g (NS) (n= 5)) were analysed for total lipid content (Table 1). TAG content was significantly increased in the obese ZDF rat hearts but there were no significant differences in other lipid classes (Table 1).
Table 1.
Myocardial tissue lipid content
| Lean control | Obese ZDF | |
|---|---|---|
| Triacylglycerol (μmol FA (g wet wt)−1) | 4.3 ± 0.6 | 10.3 ± 1.4* |
| Total cholesterol (μmol (g wet wt)−1) | 9.8 ± 1.3 | 8.7 ± 2.2 |
| Phospholipid (μmol (g wet wt)−1) | 41.2 ± 6.8 | 49.5 ± 7.6 |
| Non-esterified fatty acid (μmol (g wet wt)−1) | 0.37 ± 0.07 | 0.61 ± 0.09 |
Results are expressed as means ±s.e.m. (n= 5 per group). FA, fatty acid. Animals were fed ad libitum, and following blood sampling the heart was removed, frozen in liquid N2 and ground to powder; lipids were extracted and analysed for lipid content. For further details see text. Statistically significant differences between obese ZDF rats and lean littermates are indicated: *P < 0.05.
Lipoprotein composition analysis in control and diabetes
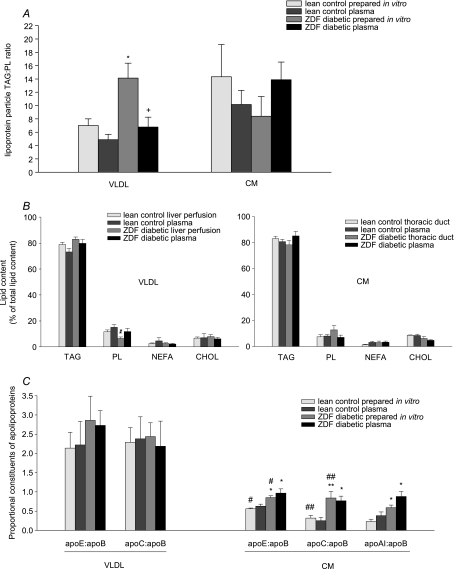
Lipoproteins were prepared and analysed both from plasma and from in vitro liver perfusions (VLDL) and thoracic duct cannulations (CM). In order to estimate particle size, the TAG : PL ratio was calculated as an approximation to the lipoprotein core-to-surface ratio (Kalogeris & Story, 1992). On this basis, lean control CM was larger than lean control VLDL, as expected; however, ZDF-diabetic VLDL was significantly larger than control VLDL, whilst ZDF-diabetic CM tended to have a decreased TAG : PL ratio compared to control CM, indicating a smaller particle, though this was not statistically significant (Fig. 1A). There was no difference in lipid composition of the various lipoproteins, except a relatively decreased PL content in diabetic VLDL (Fig. 1B). As each lipoprotein particle contains one copy of apo-B, the apo-B content represents the number of lipoprotein particles (Elovson et al. 1988). Therefore, the ratio of other major apolipoproteins to apo-B content was used to represent the relative proportion of each apolipoprotein in the VLDL and CM particle. Type 2 diabetes had no significant effects on apolipoproteins in VLDL particles, but diabetic CM had significantly increased apo-E, apo-C and apo-AI contents, relative to apo-B (Fig. 1C). Native plasma TAG-rich lipoproteins were similar in composition to those prepared in vitro except for the TAG : PL ratio in diabetic VLDL – diabetic rat plasma VLDL had a significantly lower TAG : PL ratio than VLDL prepared from diabetic rat liver perfusion (Fig. 1A).
Figure 1. Lipoprotein particle compositional analysis in lean and ZDF rats.
Lipoproteins were obtained from rat plasma or prepared in vitro from rat liver perfusion (VLDL) or rat thoracic duct cannulation (CM). Lipid contents were determined using enzymatic colorimetric assay test kits; apolipoprotein contents were determined by SDS-PAGE. A, lipoprotein particle TAG : PL ratio; B, lipid composition, expressed as percentage of total lipid content; C, apolipoproteins, expressed as a proportion of apo-B content, to correct for particle numbers. For further details see text. Results are expressed as means ±s.e.m. (n= 4–5 per group). VLDL, very-low-density lipoprotein; CM, chylomicrons; TAG, triacylglycerol; PL, phospholipid; NEFA, non-esterified fatty acid; CHOL, total cholesterol; ZDF, Zucker diabetic fatty. Statistically significant differences between ZDF rats and lean controls are indicated: *P < 0.05, **P < 0.01; significant differences between VLDL and CM particles are indicated: #P < 0.05, ##P < 0.01.
Metabolism of VLDL and CM by isolated hearts from ZDF rats
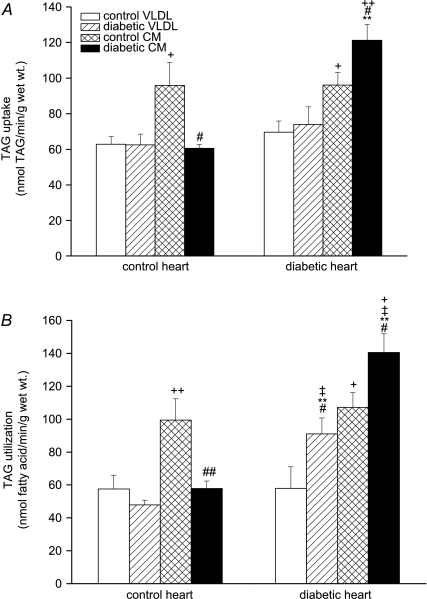
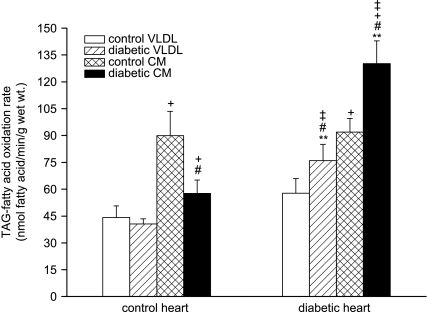
Metabolism of lipoprotein-[3H]TAG was examined by measuring its oxidation rate and deposition into tissue [3H]-labelled lipids. In control rat hearts, uptake of TAG from control CM was greater than from control VLDL, as previously noted (Niu et al. 2004); however, whilst diabetic VLDL-TAG was taken up to a similar extent as control VLDL-TAG in these hearts, diabetic CM-TAG was taken up to a significantly lesser extent than control CM-TAG (Fig. 2A). Diabetic rat hearts took up VLDL-TAG to the same extent as control hearts, but in contrast to control hearts took up significantly more diabetic CM-TAG (Fig. 2A) – therefore diabetic CM was a good substrate for diabetic hearts but a relatively poor substrate for control hearts. When lipoprotein assimilation was measured as TAG utilisation (total recovered TAG-derived label following uptake and metabolism), a similar pattern was found, except diabetic VLDL was a better substrate for diabetic heart (Fig. 2B). Lipoprotein-TAG oxidation rates (Fig. 3) reflected TAG uptake: control CM-TAG oxidation was about 2-fold higher than control VLDL-TAG oxidation in control hearts, again consistent with previous findings (Hauton et al. 2001; Niu et al. 2004). Control hearts oxidised diabetic VLDL-TAG to the same extent as control VLDL-TAG, but oxidised diabetic CM-TAG significantly less than control CM-TAG (Fig. 3). The TAG oxidation rates of both control lipoproteins were similar in diabetic hearts to control hearts, but diabetic hearts oxidised diabetic lipoprotein-TAG to a greater extent than control hearts. Therefore control lipoproteins were similar oxidative substrates for control and diabetic hearts, but diabetic lipoproteins were significantly better substrates for diabetic hearts: the TAG oxidation rates of diabetic VLDL and CM in diabetic hearts increased significantly compared to that of control VLDL and CM in control hearts (Fig. 3).
Figure 2. Uptake (A) and utilisation (B) of triacylglycerol from very-low-density lipoprotein or chylomicrons by isolated working hearts from lean and obese Zucker diabetic fatty (ZDF) rats perfused with TAG-rich lipoproteins.
Isolated rat hearts were perfused with very-low-density lipoprotein (VLDL) or chylomicrons (CM) (0.4 mm TAG) prepared from control or diabetic rats together with [14C]glucose (11 mm). The concentration of glucose was 11 mm. Triacylglycerol (TAG) uptake was estimated from disappearance of TAG from perfusate. TAG utilisation was calculated as the sum of TAG oxidation and tissue lipid deposition; for further details see text. Results are expressed as means ±s.e.m. (n= 7 per group). Statistically significant differences between diabetic and control hearts are indicated: **P < 0.01; significant differences between diabetic and control lipoproteins are indicated: #P < 0.05, ##P < 0.01; significant differences between VLDL and CM are indicated: +P < 0.05, ++P < 0.01; significant differences between diabetic hearts perfused with diabetic lipoproteins and control hearts perfused with control lipoproteins are indicated: ‡P < 0.05.
Figure 3. Oxidation rate of lipoprotein triacylglycerol-fatty acid in isolated working hearts from lean and obese ZDF rats perfused with triacylglycerol (TAG)-rich lipoproteins.
Isolated rat hearts were perfused with very-low-density lipoprotein (VLDL) or chylomicrons (CM) (0.4 mm TAG) prepared from lean control or obese diabetic rats together with [14C]glucose (11 mm); for further details see text. Results are expressed as means ±s.e.m. (n= 7 per group). Statistically significant differences between diabetic and control hearts are indicated: **P < 0.01; significant differences between diabetic and control lipoproteins are indicated: #P < 0.05; significant differences between VLDL and CM are indicated: +P < 0.05; significant differences between diabetic hearts perfused with diabetic lipoproteins and control hearts perfused with control lipoproteins are indicated: ‡P < 0.05.
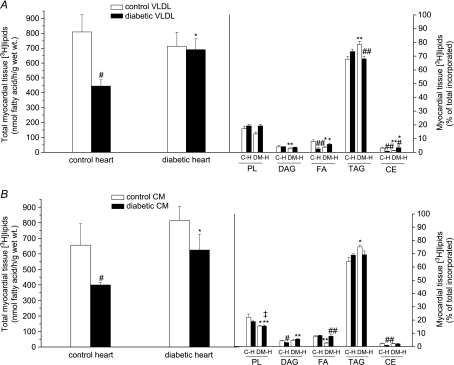
There was no significant difference in myocardial tissue [3H]lipid deposition into control or diabetic hearts from control VLDL (Fig. 4A) and control CM (Fig. 4B); however, whilst diabetic VLDL-TAG and CM-TAG were incorporated into diabetic heart lipids to a similar extent as control lipoproteins, significantly less TAG from both diabetic lipoproteins was incorporated into control heart lipids: hence diabetic lipoproteins were relatively poor substrates for control heart tissue deposition (Figs 2B and 4). No significant alteration in tissue lipid deposition in diabetic hearts perfused with diabetic lipoproteins compared to control hearts perfused with control lipoproteins was found (Fig. 4). Most (more than two thirds) of the exogenous lipoprotein-TAG recovered in myocardial lipids was esterified into tissue TAG (Fig. 4), but patterns of distribution of exogenous lipid into tissue lipids differed between groups. With control VLDL as substrate, diabetic hearts incorporated proportionately more VLDL-TAG into tissue TAG and relatively less into other tissue lipids, compared to control hearts; conversely, with diabetic VLDL as substrate, diabetic heart incorporated proportionately less diabetic TAG into tissue TAG and more into the minor tissue lipids. There was no difference in tissue lipid distribution between control hearts perfused with control VLDL and diabetic hearts perfused with diabetic VLDL. With control CM as substrate, diabetic heart again incorporated proportionately more CM-TAG into tissue TAG, as with VLDL, and less into tissue phospholipids and fatty acids; however, with diabetic CM as substrate, diabetic heart showed similar incorporation of exogenous TAG into tissue lipids as control CM (except into fatty acids; Fig. 4B). Diabetic hearts incorporated proportionately less CM-TAG into phospholipids, regardless of the origin of the chylomicrons: hence, diabetic hearts perfused with diabetic CM incorporated less PL into tissue lipids compared to control hearts perfused with control CM (Fig. 4B).
Figure 4. Incorporation of VLDL (A) and chylomicrons (B) [3H]triolein into myocardial tissue lipids.
Isolated rat hearts were perfused with very-low-density lipoprotein (VLDL) or chylomicrons (CM) (0.4 mm TAG) prepared from lean control or obese diabetic rats together with [14C]glucose (11 mm). Lipids were extracted from myocardial tissue and separated by thin-layer chromatography according to lipid class to estimate incorporation of VLDL- or CM-[3H]triolein into tissue [3H]lipid. TAG, triacylglycerol; DAG, diacylglycerol; FA, fatty acid; PL, phospholipid; CE, cholesterol ester. Results are expressed as total mass of [3H]lipid incorporated, and proportion incorporated into each lipid class; for further details see text. Results are expressed as means ±s.e.m. (n= 7 per group). Statistically significant differences between diabetic and control hearts are indicated: *P < 0.05, **P < 0.01; significant differences between diabetic and control lipoproteins are indicated: #P < 0.05, ##P < 0.01; significant differences between diabetic hearts perfused with diabetic lipoproteins and control hearts perfused with control lipoproteins are indicated: ‡P < 0.05.
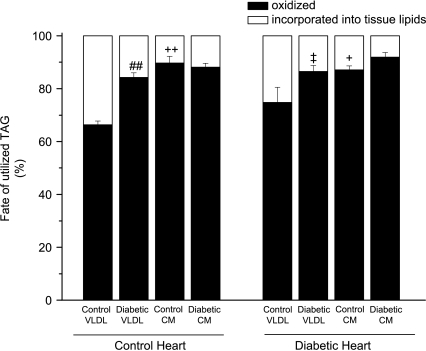
Partitioning of assimilated lipoprotein-TAG between its principal metabolic fates, oxidation and tissue lipid deposition was calculated (Fig. 5). In control hearts, the proportion of control VLDL-TAG oxidised was about 65% (slightly higher in these littermate-matched controls than previous observations in Wistar rats (Niu et al. 2004)). However, although diabetic VLDL-TAG was oxidised to a significantly greater extent than control VLDL-TAG only by diabetic hearts and not by control hearts (Fig. 3), the proportion of diabetic VLDL-TAG oxidised compared to tissue lipid deposition was greater in control hearts (and not different in diabetic hearts; Fig. 5). Diabetic VLDL-TAG was oxidised to a proportionately greater extent by diabetic heart than was control VLDL-TAG by control hearts. Chylomicron-TAG was mostly (> 85%) oxidised, regardless of conditions; there was no difference in the proportion of CM-TAG (control or diabetic) oxidised between control and diabetic hearts. Therefore, the diabetic state of the heart had no significant effect on the distribution of lipoprotein-TAG between oxidation and tissue lipid deposition (Fig. 5).
Figure 5. Metabolic fate of lipoprotein [3H]TAG utilised by isolated working hearts from lean and obese Zucker diabetic fatty (ZDF) rats perfused with triacylglycerol (TAG)-rich lipoproteins.
Isolated rat hearts were perfused with very-low-density lipoprotein (VLDL) or chylomicrons (CM) (0.4 mm TAG) prepared from control or diabetic rats together with [14C]glucose (11 mm); for further details see text. Results are expressed as means ±s.e.m. (n= 7 per group). Statistically significant differences between diabetic and control lipoproteins are indicated: ##P < 0.01; significant differences between VLDL and CM are indicated: +P < 0.05, ++P < 0.01; significant differences between diabetic hearts perfused with diabetic lipoproteins and control hearts perfused with control lipoproteins are indicated: ‡P < 0.05.
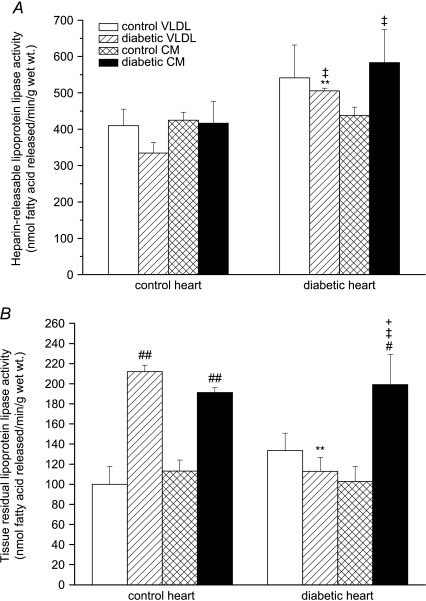
Lipoprotein lipase activity
Cardiac LPL activity (both heparin-releasable and tissue-residual) was measured after 1 h of heart perfusion. HR-LPL activity tended to be higher in diabetic hearts compared to control hearts (Fig. 6A); HR-LPL activity was significantly increased in hearts from obese ZDF rats perfused with diabetic lipoproteins compared to hearts from lean controls perfused with lean control lipoproteins (Fig. 6A). Interestingly, perfusion of control hearts with diabetic lipoproteins stimulated tissue-residual LPL activity, and this effect was also seen in diabetic hearts perfused with diabetic chylomicrons (Fig. 6B).
Figure 6. Heparin-releasable (A) and tissue-residual (B) lipoprotein lipase activity in isolated working hearts from lean and obese Zucker diabetic fatty (ZDF) rats perfused with triacylglycerol (TAG)-rich lipoproteins.
Isolated rat hearts were perfused with very-low-density lipoprotein (VLDL) or chylomicrons (CM) (0.4 mm TAG) prepared from control or diabetic rats together with [14C]glucose (11 mm); for further details see text. Results are expressed as means ±s.e.m. (n= 7 per group). Statistically significant differences between diabetic and control hearts are indicated: **P < 0.01; significant differences between diabetic and control lipoproteins are indicated: #P < 0.05, ##P < 0.01; significant differences between VLDL and CM are indicated: +P < 0.05; significant differences between diabetic hearts perfused with diabetic lipoproteins and control hearts perfused with control lipoproteins are indicated: ‡P < 0.05.
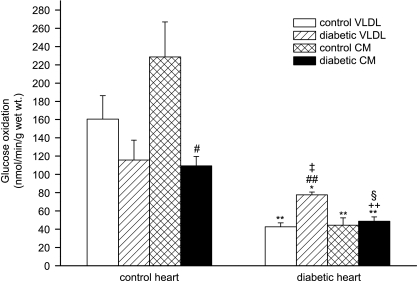
Glucose oxidation
Myocardial glucose oxidation rate was significantly decreased in hearts from obese ZDF rats compared to lean controls (Fig. 7). Diabetic CM suppressed glucose oxidation in control hearts but did not further suppress the already low glucose oxidation rate in diabetic hearts. However, diabetic hearts showed increased glucose oxidation when perfused with diabetic VLDL compared to control VLDL; despite this, glucose oxidation rate under these conditions was still less than rates in control hearts perfused with control lipoproteins (Fig. 7).
Figure 7. Glucose oxidation in isolated working hearts from lean and obese Zucker diabetic fatty (ZDF) rats perfused with triacylglycerol (TAG)-rich lipoproteins.
Isolated rat hearts were perfused with very-low-density lipoprotein (VLDL) or chylomicrons (CM) (0.4 mm TAG) prepared from control or diabetic rats together with [14C]glucose (11 mm); for further details see text. Results are expressed as means ±s.e.m. (n= 7 per group). Statistically significant differences between diabetic and control hearts are indicated: *P < 0.05, **P < 0.01; significant differences between diabetic and control lipoproteins are indicated: #P < 0.05, ##P < 0.01; significant differences between VLDL and CM are indicated: ++P < 0.01; significant differences between diabetic hearts perfused with diabetic lipoproteins and control hearts perfused with control lipoproteins are indicated: ‡P < 0.05, §P < 0.01.
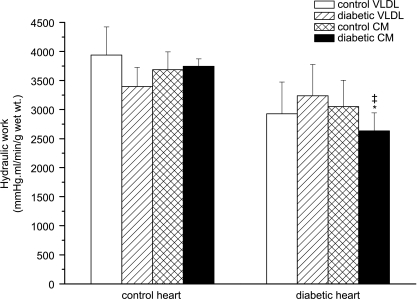
Cardiac mechanical function in ZDF rats
In the present study, hearts from 12-week-old obese diabetic rats demonstrated a generally lower cardiac mechanical performance compared to control hearts, regardless of the source of lipoproteins used in the perfusion; however, this was only statistically significant when diabetic hearts were perfused with diabetic CM (Fig. 8). This result agrees well with previous studies reporting impaired cardiac function in ZDF rats (Wang et al. 2005).
Figure 8. Cardiac hydraulic work in isolated working hearts from lean and obese Zucker diabetic fatty (ZDF) rats perfused with triacylglycerol (TAG)-rich lipoproteins.
Isolated rat hearts were perfused with very-low-density lipoprotein (VLDL) or chylomicrons (CM) (0.4 mm TAG) prepared from lean control or obese diabetic rats together with [14C]glucose (11 mm). Afterload was maintained at 100 cmH2O and atrial filling pressure at 15 cmH2O; for further details see text. Results are expressed as means ±s.e.m. (n= 7 per group). Statistically significant differences between diabetic and control hearts are indicated: *P < 0.05; significant differences between diabetic hearts perfused with diabetic lipoproteins and control hearts perfused with control lipoproteins are indicated: ‡P < 0.05.
Discussion
Diabetes has become a major global epidemic. The World Health Organization estimates that approximately 170 million people suffer from the condition worldwide, with this figure likely to more than double by 2030. Most diabetic patients (approximately 90%) have type 2 diabetes, characterised by insulin resistance with hyperinsulinaemia and euglycaemia prior to the onset of overt hyperglycaemia, and clearly distinct from the rapid onset of type 1 (insulin deficient) diabetes (Chatham & Seymour, 2002; Wang & Chatham, 2004). Type 2 diabetes is associated with obesity and deranged lipid metabolism and this may underlie many of its complications: characteristically, plasma TGRLPs are increased and abnormal in composition (Howard, 1994; Lewis & Steiner, 1996; McEneny et al. 2000; Tomkin & Owens, 2001). Therefore, knowledge of cardiac lipid metabolism is essential for understanding the significance of exogenous and endogenous substrate utilisation in diabetes and its relationship to the development of cardiovascular complications, especially diabetic cardiomyopathy. Cardiovascular abnormalities have been identified as leading causes of mortality in diabetes. Diabetic cardiomyopathy has been recognised as a significant complication, distinct from other cardiovascular risk factors such as atherosclerosis and hypertension (Lopaschuk, 1996; Belke et al. 2001; Aasum et al. 2003; Severson, 2004). The pathogenesis of diabetic cardiomyopathy is probably multifactorial and complex: several factors, such as changes in protein synthesis, calcium handling and contractile machinery could contribute. However, some of the earliest changes are at the level of myocardial substrate utilisation (Chatham & Seymour, 2002), and it has been suggested that the metabolic alterations may be causally related to the subsequent development of cardiomyopathy (Belke et al. 2000; Aasum et al. 2003; Carley & Severson, 2005; An & Rodrigues, 2006). Increased myocardial dependence on fatty acids for energy production concomitant with decreased glucose oxidation is the dominant feature of diabetic cardiac metabolism (Lopaschuk, 2002; Carley & Severson, 2005; An & Rodrigues, 2006), and this may underlie the failure of cardiac function. However, the extent to which the diabetic heart utilises ‘free’ fatty acids, or triacylglycerols supplied as TAG-rich lipoproteins, is unknown. In earlier work we established that heart utilises both VLDL and CM to a significant extent, that LPL is a major (but not sole) route of uptake, and that FAs derived from the two lipoproteins undergo different metabolic fates, with consequences on cardiac systolic function (Niu et al. 2004). One previous study (Neitzel et al. 2003) examined utilisation by db/db diabetic mouse hearts of control chylomicrons isolated from rat, but examination of species- and disease-specific TGRLP by hearts in diabetes has not been previously attempted.
In the present study, we therefore examined cardiac utilisation of VLDL and CM in a ZDF rat model of obese type 2 diabetes. The ZDF rat is one of the leptin receptor mutation murine models which shows obesity and extreme insulin resistance (Carley & Severson, 2005; Chen & Wang, 2005). Male homozygous ZDF rats before 6 weeks of age are hyperinsulinaemic but euglycaemic; hyperglycaemia develops at about 10 weeks (Carley & Severson, 2005), with a metabolic profile typical of human type 2 diabetes.
Analysis of radiolabelled lipoproteins prepared in vitro demonstrated a similar lipid and apoprotein profile to native VLDL and CM obtained from rat plasma. A smaller particle size (TAG : PL ratio) of plasma VLDL compared to liver perfusion VLDL in diabetes probably reflects the degree of metabolism that native particles undergo in plasma in vivo. Using the lipoproteins prepared in vitro, changes in myocardial lipid metabolism were found in association with type 2 diabetes, and these were due to both cardiac- and lipoprotein-related factors. We found that: (1) both VLDL and CM particles have altered composition in ZDF rats; (2) impaired cardiac mechanical function occurred when the diabetic heart was perfused with ‘diabetic’ chylomicrons; (3) both VLDL-TAG and CM-TAG uptake and utilisation were markedly increased in the diabetic state. This was mainly reflected by the increased oxidative metabolism of VLDL-TAG and CM-TAG by diabetic hearts rather than non-oxidative metabolism, i.e. myocardial tissue deposition of exogenous lipid; (4) there was no significant change in intramyocardial [3H]TAG accumulation in ZDF rat hearts after 1 h perfusion in vitro, although total myocardial TAG content was increased in diabetic hearts prior to perfusion; (5) more assimilated lipoprotein-TAG was oxidised rather than incorporated into tissue lipids in the diabetic state. The proportion of VLDL-TAG oxidised was increased from ∼60% in control rat hearts to ∼90% in diabetic rat hearts; (6) HR-LPL activity was increased, notably in diabetic hearts perfused with diabetic lipoproteins; and (7) as expected, glucose oxidation was markedly decreased.
It has been reported that in 20-week-old ZDF rats cardiac function was significantly depressed (Zhou et al. 2000). The present data suggest that impaired cardiac function may occur at a relatively early stage in these homozygous rats; however, systolic function was only significantly impaired in diabetic hearts perfused with diabetic chylomicrons (Fig. 8), conditions associated with a very high TAG-FA oxidation rate (Fig. 3) – possibly above a ‘toxic’ threshold of utilisation (despite increased TAG content of these diabetic hearts in all substrate groups examined). The contractile dysfunction of diabetic cardiomyopathy also involves a diastolic component (Severson, 2004; An & Rodrigues, 2006); we did not examine diastolic function but it is possible that diastolic dysfunction precedes systolic failure and may correlate with smaller changes in lipoprotein-TAG oxidation. Recently, hydronephrosis has been implicated as a mechanism in cardiovascular dysfunction in ZDF rats (Marsh et al. 2007) and such a mechanism cannot be excluded in the present study.
In the present study, cardiac utilisation of fatty acids derived from VLDL-TAG and CM-TAG were markedly increased in ZDF rat hearts, mostly reflected by increased fatty acid oxidation. Strikingly, diabetic hearts tended to oxidise more of the VLDL-TAG and CM-TAG assimilated rather than esterify them into tissue lipids: the proportion of VLDL-TAG oxidised increased to about 90% in diabetes, whilst the proportion of CM-TAG oxidised was very high and may not have been able to increase further. High control CM-TAG oxidation in control hearts (Fig. 3) was not associated with decreased glucose oxidation (Fig. 7) – the inability of CM to suppress cardiac glucose oxidation in the control state suggests that CM utilisation is metabolically inefficient; possibly cataplerotic mechanisms are involved. Fatty acids are more productive than glucose in terms of ATP yield per mole of substrate. However, calculations of ATP yield per oxygen atom consumed shows that more oxygen is required for ATP production when hearts are oxidizing fatty acids compared to glucose (Carley & Severson, 2005); therefore, they may not be optimal substrates, especially in disease-compromised hearts. Hence increased dependence on fatty acid oxidation in diabetic hearts could result in decreased cardiac efficiency (cardiac ATP production/O2 consumption) (How et al. 2006), which may be at least in part a mechanism for the development of cardiac dysfunction: Vincent et al. (2004) reported that elevating the fatty acid concentrations in working rat heart perfusions reduced cardiac efficiency even though oxygen consumption was unchanged. In contrast, increased palmitate oxidation in hearts from 12-week-old ZDF rats was not accompanied by changes in oxygen consumption (Wang et al. 2005). Young et al. (2002a) reported that in the obese Zucker rat cardiac contractile dysfunction was closely related to impaired long-chain fatty acid oxidation. Furthermore, despite some agents (such as GI-262570, a non-thiazolidinedione PPARγ agonist) which decrease fatty acid oxidation (with a concomitant increase in glucose oxidation) improving contractile function in type 2 diabetes (Golfman et al. 2005), other agents (including BM 17.0744, a peroxisome proliferator-activated receptor α ligand) do not show this effect (Aasum et al. 2002; Carley et al. 2004); thus, there are clearly discrepant observations in terms of the relationship between alterations in cardiac substrate utilisation and cardiac function in diabetes. There is no direct evidence in our data to prove the cause–effect relation between increased fatty acid oxidation and impaired cardiac function found in ZDF rat hearts.
If increased TAG uptake and utilisation is the basis for the impaired systolic function in type 2 diabetes, this may be due to increased LPL activity. We previously demonstrated that LPL-mediated hydrolysis is the principal mechanism for hearts to assimilate lipoprotein-TAG under physiological conditions (Niu et al. 2004). However, it is still uncertain whether this is the case in diabetes. Despite extensive study, the effect of diabetes on cardiac LPL activity is inconclusive: increased (Sambandam et al. 1999), decreased (Rodrigues & Severson, 1993; Liu & Severson, 1995) and unchanged (Tavangar et al. 1992; Rodrigues & Severson, 1993) activities have been reported. In the present study, we found modestly elevated HR-LPL activity in 12-week-old obese ZDF rat hearts after 1 h perfusion (Fig. 6A), but this correlated only poorly with cardiac TAG uptake (Fig. 2). Changes in lipoprotein receptor-mediated uptake may be involved but this was not specifically examined. However, diabetic lipoproteins stimulated TR-LPL; the mechanism for this striking effect is not known. Changes in composition of diabetic lipoprotein particles, changes in the structure of diabetic LPL, and the presence of an LPL inhibitor (Koishi et al. 2002) in diabetic heart may all affect catabolism of the lipoprotein TAG core. Augustus et al. (2006) reported that in heart-specific lipoprotein lipase knock-out mice cardiac function was decreased, presumably because of decreased lipoprotein-TAG utilisation by the heart.
We found that composition of lipoprotein particles from type 2 diabetic rats differed. Based on the TAG : PL ratio, VLDL particles from obese ZDF rats were larger than controls, suggesting that they are better substrates for LPL compared to ‘lean’ control particles (Goldberg, 1996). Conversely, diabetic CM particles tended to be smaller than controls. A similar amount of TAG (0.4 mm) was used for each perfusion, therefore even though all hearts were exposed to the same TAG load, the number of particles, and the other lipoprotein components, would have differed. The increased complement of apolipoproteins seen in diabetic CMs, with their diverse functions including lipase modulation and receptor ligand binding properties, may explain some of the effects of diabetic lipoproteins observed.
Another possible mechanism in the development of cardiac dysfunction in type 2 diabetes is lipotoxicity. Intracellular TAG accumulation (Paulson & Crass, 1982; Lopaschuk, 1996) is often regarded as a marker of lipotoxicity (An & Rodrigues, 2006): Zhou et al. (2000) reported that the development of cardiac contractile dysfunction in ZDF rats was associated with intracellular TAG accumulation. In the present study, ZDF rats had increased total myocardial TAG content, but myocardial intracellular [3H]TAG derived from exogenous [3H]lipoproteins was not different in diabetic hearts compared to control hearts, although diabetic lipoproteins were a poor substrate for control heart TAG accumulation; therefore, significant intracellular accumulation of TAG substrate did not occur in the diabetic state during the heart perfusion. Lipid accumulation may lead to increased ceramide content and hence cardiomyocyte apoptosis (Unger & Orci, 2002). The obese ZDF rats used in our present work were at a relatively early stage of diabetes, deliberately chosen in an attempt to delineate the relationship between substrate utilisation and the early development of mechanical functional abnormalities. Whilst lipotoxicity resulting from over-utilisation of lipid substrates may have already occurred at this stage, we cannot draw any conclusions regarding its role in the aetiology of cardiac dysfunction.
Extracellular energy storage for heart: putative role of VLDL in cardiac lipid metabolism
VLDL and CM are the two major transporters of TAG in the plasma. Although both can maintain the isolated working heart in good mechanical function, there are several studies which suggest different roles for VLDL and CM in cardiac lipid metabolism (Bennett et al. 2000b; Hauton et al. 2001). CM is clearly a major fatty acid supplier for heart because under different conditions the uptake and oxidation of CM-TAG by hearts is high (Niu et al. 2004). However, the physiological role of VLDL remains unclear. In the present study, we found that the uptake of VLDL-TAG increased significantly in type 2 diabetes (Fig. 3); furthermore, the diabetic heart oxidised the additional VLDL-TAG assimilated rather than incorporate it into cellular lipids – about 80% of VLDL-TAG assimilated was oxidised in hearts from obese ZDF rats, a high proportion compared to the physiological situation. Taking the relatively low proportion of VLDL-TAG oxidised under physiological conditions into account, VLDL can be considered a form of extracellular cardiac energy reserve, which may be accessed under different metabolic stress states, such as diabetes and sepsis/endotoxinaemia (Bennett et al. 2000b), to meet its extra energy requirements in these conditions.
In conclusion, the present study defines myocardial utilisation and metabolic fate of the principal sources of both exogenous (CM) and endogenous (VLDL) TAG by the type 2 diabetic heart. Increased TAG oxidation and decreased glucose oxidation in diabetic hearts reflect the over-utilisation of TAG-rich lipoproteins in such conditions, supportive of the concept of substrate utilisation shift from glucose to lipids in diabetes. However, the relationship between this shift towards TAG utilisation and deleterious effects on myocardial function remains unclear.
Acknowledgments
This work was supported by the Royal Society Sino-British Fellowship Trust, Oxford Cardiac Surgical Sciences Trust Fund, a BJA Project Grant, and a Nuffield Department of Anaesthetics Student Bursary.
References
- Aasum E, Belke DD, Severson DL, Riemersma RA, Cooper M, Andreassen M, Larsen TS. Cardiac function and metabolism in Type 2 diabetic mice after treatment with BM 17. 0744, a novel PPAR-α activator. Am J Physiol Heart Circ Physiol. 2002;283:H949–H957. doi: 10.1152/ajpheart.00226.2001. [DOI] [PubMed] [Google Scholar]
- Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–441. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2006;291:H1489–H1506. doi: 10.1152/ajpheart.00278.2006. [DOI] [PubMed] [Google Scholar]
- Augustus AS, Buchanan J, Park TS, Hirata K, Noh HL, Sun J, Homma S, D’Armiento J, Abel ED, Goldberg IJ. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J Biol Chem. 2006;281:8716–8723. doi: 10.1074/jbc.M509890200. [DOI] [PubMed] [Google Scholar]
- Augustus AS, Kako Y, Yagyu H, Goldberg IJ. Routes of FA delivery to cardiac muscle: modulation of lipoprotein lipolysis alters uptake of TG-derived FA. Am J Physiol Endocrinol Metab. 2003;284:E331–E339. doi: 10.1152/ajpendo.00298.2002. [DOI] [PubMed] [Google Scholar]
- Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–E1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- Belke DD, Larsen TS, Severson DL. Cardiac function in perfused hearts from diabetic mice. Adv Exp Med Biol. 2001;498:241–245. doi: 10.1007/978-1-4615-1321-6_30. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Hauton D, Hole DG, Evans RD. Preparation of radiolabelled very-low-density lipoprotein in high yield by extended rat liver perfusion. Biotechnol Lett. 2000a;22:301–306. [Google Scholar]
- Bennett MJ, Hauton D, Hole DG, Evans RD. Utilization of very low density lipoprotein by rat heart: the effect of endotoxin. Am J Physiol Endocrinol Metab. 2000b;278:E802–E810. doi: 10.1152/ajpendo.2000.278.5.E802. [DOI] [PubMed] [Google Scholar]
- Bezman-Tarcher A, Otway S, Robinson DS. The removal of triglyceride fatty acids from incubation of the supradiaphragmatic portion of the rat. Proc R Soc Lond B Biol Sci. 1965;162:411–426. [Google Scholar]
- Carley AN, Semeniuk LM, Shimoni Y, Aasum E, Larsen TS, Berger JP, Severson DL. Treatment of type 2 diabetic db/db mice with a novel PPARgamma agonist improves cardiac metabolism but not contractile function. Am J Physiol Endocrinol Metab. 2004;286:E449–E455. doi: 10.1152/ajpendo.00329.2003. [DOI] [PubMed] [Google Scholar]
- Carley AN, Severson DL. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochim Biophys Acta. 2005;1734:112–126. doi: 10.1016/j.bbalip.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Chatham JC, Seymour AM. Cardiac carbohydrate metabolism in Zucker diabetic fatty rats. Cardiovasc Res. 2002;55:104–112. doi: 10.1016/s0008-6363(02)00399-1. [DOI] [PubMed] [Google Scholar]
- Chen D, Wang MW. Development and application of rodent models for type 2 diabetes. Diabetes Obes Metab. 2005;7:307–317. doi: 10.1111/j.1463-1326.2004.00392.x. [DOI] [PubMed] [Google Scholar]
- Elovson J, Chatterton JE, Bell GT, Schumaker VN, Reuben MA, Puppione DL, Reeve JR, Jr, Young NL. Plasma very low density lipoproteins contain a single molecule of apolipoprotein B. J Lipid Res. 1988;29:1461–1473. [PubMed] [Google Scholar]
- Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res. 1996;37:693–707. [PubMed] [Google Scholar]
- Golfman LS, Wilson CR, Sharma S, Burgmaier M, Young ME, Guthrie PH, Van Arsdall M, Adrogue JV, Brown KK, Taegtmeyer H. Activation of PPARγ enhances myocardial glucose oxidation and improves contractile function in isolated working hearts of ZDF rats. Am J Physiol Endocrinol Metab. 2005;289:E328–E336. doi: 10.1152/ajpendo.00055.2005. [DOI] [PubMed] [Google Scholar]
- Hauton D, Bennett MJ, Evans RD. Utilisation of triacylglycerol and non-esterified fatty acid by the working rat heart: myocardial lipid substrate preference. Biochim Biophys Acta. 2001;1533:99–109. doi: 10.1016/s1388-1981(01)00146-9. [DOI] [PubMed] [Google Scholar]
- How OJ, Aasum E, Severson DL, Chan WY, Essop MF, Larsen TS. Increased myocardial oxygen consumption reduces cardiac efficiency in diabetic mice. Diabetes. 2006;55:466–473. doi: 10.2337/diabetes.55.02.06.db05-1164. [DOI] [PubMed] [Google Scholar]
- Howard BV. Lipoprotein metabolism in diabetes. Curr Opin Lipidol. 1994;5:216–220. doi: 10.1097/00041433-199405030-00009. [DOI] [PubMed] [Google Scholar]
- Irwin D, O’Looney PA, Quinet E, Vahouny GV. Application of SDS gradient polyacrylamide slab gel electrophoresis to analysis of apolipoprotein mass and radioactivity of rat lipoproteins. Atherosclerosis. 1984;53:163–172. doi: 10.1016/0021-9150(84)90192-8. [DOI] [PubMed] [Google Scholar]
- Kalogeris TJ, Story JA. Lymph chylomicron composition and size are modified by level of intestinally infused cholesterol and triglyceride source in rats. J Nutr. 1992;122:1045–1055. doi: 10.1093/jn/122.5.1045. [DOI] [PubMed] [Google Scholar]
- Kamataki A, Takahashi S, Masamura K, Iwasaki T, Hattori H, Naiki H, Yamada K, Suzuki J, Miyamori I, Sakai J, Fujino T, Yamamoto TT. Remnant lipoprotein particles are taken up into myocardium through VLDL receptor – a possible mechanism for cardiac fatty acid metabolism. Biochem Biophys Res Commun. 2002;293:1007–1013. doi: 10.1016/S0006-291X(02)00323-6. [DOI] [PubMed] [Google Scholar]
- Koishi R, Ando Y, Ono M, Shimamura M, Yasumo H, Fujiwara T, Horikoshi H, Furukawa H. Angptl3 regulates lipid metabolism in mice. Nat Genet. 2002;30:151–157. doi: 10.1038/ng814. [DOI] [PubMed] [Google Scholar]
- Lewis GF, Steiner G. Hypertriglyceridemia and its metabolic consequences as a risk factor for atherosclerotic cardiovascular disease in non-insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1996;12:37–56. doi: 10.1002/(SICI)1099-0895(199603)12:1<37::AID-DMR154>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Liu L, Severson DL. Myocardial lipoprotein lipase activity: regulation by diabetes and fructose-induced hypertriglyceridemia. Can J Physiol Pharmacol. 1995;73:369–377. doi: 10.1139/y95-047. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD. Abnormal mechanical function in diabetes: relationship to altered myocardial carbohydrate/lipid metabolism. Coron Artery Dis. 1996;7:116–123. doi: 10.1097/00019501-199602000-00004. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD. Metabolic abnormalities in the diabetic heart. Heart Fail Rev. 2002;7:149–159. doi: 10.1023/a:1015328625394. [DOI] [PubMed] [Google Scholar]
- McEneny J, O’Kane MJ, Moles KW, McMaster C, McMaster D, Mercer C, Trimble ER, Young IS. Very low density lipoprotein subfractions in Type II diabetes mellitus: alterations in composition and susceptibility to oxidation. Diabetologia. 2000;43:485–493. doi: 10.1007/s001250051333. [DOI] [PubMed] [Google Scholar]
- Mardy K, Belke DD, Severson DL. Chylomicron metabolism by the isolated perfused mouse heart. Am J Physiol Endocrinol Metab. 2001;281:E357–E364. doi: 10.1152/ajpendo.2001.281.2.E357. [DOI] [PubMed] [Google Scholar]
- Marsh SA, Powell PC, Agarwal A, Dell’Italia LJ, Chatham JC. Cardiovascular dysfunction in Zucker obese and Zucker diabetic fatty rats: role of hydronephrosis. Am J Physiol Heart Circ Physiol. 2007;293:H292–H298. doi: 10.1152/ajpheart.01362.2006. [DOI] [PubMed] [Google Scholar]
- Neitzel AS, Carley AN, Severson DL. Chylomicron and palmitate metabolism by perfused hearts from diabetic mice. Am J Physiol Endocrinol Metab. 2003;284:E357–E365. doi: 10.1152/ajpendo.00380.2002. [DOI] [PubMed] [Google Scholar]
- Niu YG, Hauton D, Evans RD. Utilization of triacylglycerol-rich lipoproteins by the working rat heart: routes of uptake and metabolic fates. J Physiol. 2004;558:225–237. doi: 10.1113/jphysiol.2004.061473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson DJ, Crass MF., 3rd Endogenous triacylglycerol metabolism in diabetic heart. Am J Physiol Heart Circ Physiol. 1982;242:H1084–H1094. doi: 10.1152/ajpheart.1982.242.6.H1084. [DOI] [PubMed] [Google Scholar]
- Rodrigues B, Severson DL. Acute diabetes does not reduce heparin-releasable lipoprotein lipase activity in perfused hearts from Wistar-Kyoto rats. Can J Physiol Pharmacol. 1993;71:657–661. doi: 10.1139/y93-096. [DOI] [PubMed] [Google Scholar]
- Sambandam N, Abrahani MA, St Pierre E, Al-Atar O, Cam MC, Rodrigues B. Localization of lipoprotein lipase in the diabetic heart: regulation by acute changes in insulin. Arterioscler Thromb Vasc Biol. 1999;19:1526–1534. doi: 10.1161/01.atv.19.6.1526. [DOI] [PubMed] [Google Scholar]
- Scanu AM, Edelstein C. Solubility in aqueous solutions of ethanol of the small molecular weight peptides of the serum very low density and high density lipoproteins: relevance to the recovery problem during delipidation of serum lipoproteins. Anal Biochem. 1971;44:576–588. doi: 10.1016/0003-2697(71)90247-8. [DOI] [PubMed] [Google Scholar]
- Severson DL. Diabetic cardiomyopathy: recent evidence from mouse models of type 1 and type 2 diabetes. Can J Physiol Pharmacol. 2004;82:813–823. doi: 10.1139/y04-065. [DOI] [PubMed] [Google Scholar]
- Steiner G, Tkac I, Uffelman KD, Lewis GF. Important contribution of lipoprotein particle number to plasma triglyceride concentration in type 2 diabetes. Atherosclerosis. 1998;137:211–214. doi: 10.1016/s0021-9150(97)00256-6. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H, Hems R, Krebs HA. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J. 1980;186:701–711. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskinen MR. Lipoprotein lipase in diabetes. Diabetes Metab Rev. 1987;3:551–570. doi: 10.1002/dmr.5610030208. [DOI] [PubMed] [Google Scholar]
- Tavangar K, Murata Y, Pedersen ME, Goers JF, Hoffman AR, Kraemer FB. Regulation of lipoprotein lipase in the diabetic rat. J Clin Invest. 1992;90:1672–1678. doi: 10.1172/JCI116039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teusink B, Voshol PJ, Dahlmans VE, Rensen PC, Pijl H, Romijn JA, Havekes LM. Contribution of fatty acids released from lipolysis of plasma triglycerides to total plasma fatty acid flux and tissue-specific fatty acid uptake. Diabetes. 2003;52:614–620. doi: 10.2337/diabetes.52.3.614. [DOI] [PubMed] [Google Scholar]
- Tomkin GH, Owens D. Abnormalities in apo B-containing lipoproteins in diabetes and atherosclerosis. Diabetes Metab Res Rev. 2001;17:27–43. doi: 10.1002/dmrr.179. [DOI] [PubMed] [Google Scholar]
- Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- Van Der Vusse GJ, van Bilsen M, Glatz JF. Cardiac fatty acid uptake and transport in health and disease. Cardiovasc Res. 2000;45:279–293. doi: 10.1016/s0008-6363(99)00263-1. [DOI] [PubMed] [Google Scholar]
- Vincent G, Bouchard B, Khairallah M, Des Rosiers C. Differential modulation of citrate synthesis and release by fatty acids in perfused working rat hearts. Am J Physiol Heart Circ Physiol. 2004;286:H257–H266. doi: 10.1152/ajpheart.00717.2003. [DOI] [PubMed] [Google Scholar]
- Wang P, Chatham JC. Onset of diabetes in Zucker diabetic fatty (ZDF) rats leads to improved recovery of function after ischemia in the isolated perfused heart. Am J Physiol Endocrinol Metab. 2004;286:E725–E736. doi: 10.1152/ajpendo.00295.2003. [DOI] [PubMed] [Google Scholar]
- Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2005;288:H2102–H2110. doi: 10.1152/ajpheart.00935.2004. [DOI] [PubMed] [Google Scholar]
- Young ME, Guthrie PH, Razeghi P, Leighton B, Abbasi S, Patil S, Youker KA, Taegtmeyer H. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes. 2002a;51:2587–2595. doi: 10.2337/diabetes.51.8.2587. [DOI] [PubMed] [Google Scholar]
- Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation. 2002b;105:1861–1870. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]