Abstract
Oestradiol modulates paracellular permeability and tight junction (TJ) function in endothelia and reproductive tissues, but whether the ovarian hormones and cycle affect the paracellular pathway in the intestinal epithelium remains unclear. Oestrogen receptors (ERs) are expressed in intestinal epithelial cells, and oestradiol regulates epithelium formation. We examined the effects of oestrous cycle stage, oestradiol benzoate (EB), and progesterone (P) on colonic paracellular permeability (CPP) in the female rat, and whether EB affects expression of the TJ proteins in the rat colon and the human colon cell line Caco-2. In cyclic rats, CPP was determined through lumen-to-blood 51Cr-labelled EDTA clearance, and in Ussing chambers for dextran permeability. CPP was also examined in ovariectomized (OVX) rats treated with P or EB, with and without the ER antagonist ICI 182,780, or with the selective agonists for ERα (propyl pyrazole triol; PPT) or ERβ (diarylpropionitrile; DPN). In oestrus rats, CPP was reduced (P < 0.01) relative to dioestrus. In OVX rats, EB dose-dependently decreased CPP, an effect mimicked by DPN and blocked by ICI 182,780, whereas P had no effect. Oestradiol increased occludin mRNA and protein in the colon (P < 0.05), but not zona occludens (ZO)-1. Further, EB and DPN enhanced occludin and junctional adhesion molecule (JAM)-A expression in Caco-2 cells without change in ZO-1, an effect blocked by ICI 182,780. These data show that oestrogen reinforces intestinal epithelial barrier through ERβ-mediated up-regulation of the transmembrane proteins occludin and JAM-A determining paracellular spaces. These findings highlight the importance of the ERβ pathway in the control of colonic paracellular transport and mucosal homeostasis.
One critical function of intestinal epithelium is to provide a protective barrier of the internal milieu against adverse luminal factors. This physical barrier is restricted by the integrity of the apical intercellular tight junctions (TJs) sealing paracellular spaces between epithelial cells (Turner, 2006). Increased paracellular permeability has been implicated in the pathogenesis of chronic mucosal inflammation in animals and humans (Meddings, 1997; Cenac et al. 2004; Resta-Lenert et al. 2005; Shen & Turner, 2006; Moriez et al. 2007). However, the intestinal epithelial barrier is not impermeable in normal conditions, permitting fluid transport (Masyuk et al. 2002) and communication between the mucosal immune system and the commensal flora, the latter playing a major role in antigen sampling and the development of tolerance (Artis, 2008). Although there is growing evidence that oestrogens play a role in the architectural maintenance of intestinal epithelium, driving cell differentiation and proliferation (Wada-Hiraike et al. 2006a), the influence of the changing hormonal milieu during the ovarian cycle on paracellular permeability has received no attention. Of note, Homma et al. (2005) pointed out an improved epithelial barrier function in pro-oestrus rats compared with males, and a decreased intestinal permeability in males following oestradiol supplementation. This suggested a mechanistic link between the absolute levels of plasma oestrogens and the regulation of paracellular spaces, which remains to be explored.
Both ER α and β are expressed in the gastrointestinal tract (Enmark et al. 1997; Campbell-Thompson et al. 2001; Konstantinopoulos et al. 2003; Kawano et al. 2004), with ERβ as the predominant ER in the colon, mainly located in epithelial cells (Konstantinopoulos et al. 2003). Recent studies in ERβ–/– mice showed an irregular and abnormal shape of the lateral surface contacts between colonic epithelial cells, underlying changes in the standard features of TJs (Wada-Hiraike et al. 2006a,b). These results suggest that oestrogen affects colonic permeability through regulation of TJ integrity. Tight junctions are composed of transmembrane proteins, claudins, occludin and junctional adhesion molecules (JAMs), interacting with cytoplasmic proteins such as ZO-1 to maintain dynamic structures with the cell cytoskeleton, thus determining paracellular spaces. In human endothelial and ectocervical epithelial cells, oestradiol has been shown to regulate paracellular permeability through modulation of occludin expression (Ye et al. 2003; Kang et al. 2006; Gorodeski, 2007). In the colon, no information is available about the interactions between oestrogens and TJ proteins in regulating the epithelial barrier permeability.
The present in vivo and in vitro studies were designed to investigate the influence of the oestrous cycle on basal paracellular permeability in the rat colon. We also examined the effects of oestradiol and progesterone, an ER antagonist and stimulation by specific ERα and β agonists on colonic paracellular permeability (CPP) in ovariectomized (OVX) rats. We show that CPP varied during the sexual cycle, decreasing under plasma oestrogen dominance in the follicular phase, an effect mimicked by ERβ agonist in OVX rats. To address the question of whether oestrogens affect expression of key TJs proteins, we examined occludin and ZO-1 expression in the colon of oestradiol-treated OVX rats. We demonstrated a link between oestrogen-mediated decrease in CPP and modulation of occludin mRNA and protein in the colon. In further support of the notion that epithelial cells are targeted by oestradiol, we found that ERβ stimulation of the human cell line Caco-2 cells up-regulated the transmembrane proteins occludin and JAM-A, both have pivotal functions in the control of paracellular permeability.
Methods
Animals and treatments
Adult female Wistar rats (Janvier, Le Genest St Isle, France) were housed in cages with free access to food and water under a 12 : 12 h light–dark cycle. All protocols were approved by the local Institutional Animal Care and Use Committee in compliance with the European laws on the protection of animals (86/609/EEC).
In a first series of experiments, oestrous cycle stages were assessed through daily vaginal smears. Two groups of rats were used for in vivo comparison of CPP for 24 h during the follicular and the luteal phase of the sexual cycle, that is, from pro-oestrus to oestrus (n= 14), and from metoestrus to dioestrus (n= 11), respectively. Two other groups of rats were killed by decapitation in oestrus (n= 13) or dioestrus (n= 9), and used for in vitro evaluation of CPP in Ussing chambers.
In a second series of experiments, bilateral ovariectomies (OVX) or sham operations were performed on rats anaesthetized with a single intraperitoneal bolus dose of ketamine hydrochloride (150 mg kg−1, Imalgene 500, Rhône Mérieux, Lyon, France). After a 6 day recovery period for complete depletion of endogenous sex hormones, OVX rats were re-anaesthetized and Silastic implants (medical grade tubing; Dow Corning, Midland, MI, USA) filled with oestradiol benzoate (EB) (1,3,5[10]-estratriene-3, 17β-diol-3-benzoate; Sigma, St Louis, MO, USA), progesterone (P) (4-pregnen-3,20-dione; Sigma) or empty implants (controls) were positioned under the skin of the neck for 5 days as already described (Houdeau et al. 2007). According to Vongher and Frye (1999), the wall thickness and length of the implants (EB: 1.57 mm I.D./3.18 mm O.D., 24 mm; P: 3.36 mm I.D./4.65 mm O.D., 10 mm) determined physiological plasma levels of oestradiol and progesterone. Animals were assigned to the following groups: (1) untreated sham operated females killed in oestrus (sham oestrus, n= 3), (2) control OVX with empty implants (OVX, n= 16), (3) OVX rats with EB implants (OVX+EB, n= 14), (4) OVX rats with P implants (OVX+P, n= 14), and (5) OVX rats with EB implants receiving daily subcutaneous injections of the pure ER antagonist ICI 182,780 (2 mg (kg body weight (BW))−1/day in olive oil; Tocris, Bristol, England) (OVX+EB+ICI, n= 5). At the end of treatment, rats were killed by decapitation, and distal colons were dissected, washed in Krebs–Henseleit solution (Sigma) and used for CPP measurements in Ussing chambers. Additional tissue segments were simultaneously frozen in liquid nitrogen until protein extraction for Western blot analyses.
In a dose-dependent experiment, OVX rats were given daily for 5 days a subcutaneous (s.c.) injection of EB (0.001, 0.005, 0.05, 0.5, or 5 mg (kg BW)−1 day−1) dissolved in olive oil (n= 5–8 per group) for CPP measurements in Ussing chambers. For the effects of ER selective agonists, 21 OVX rats were divided into three groups, and used for CPP measurements in Ussing chambers: each group was s.c. treated for 5 days with 10% DMSO in olive oil (vehicle), propylpyrazole triol (PPT, an ERα-specific agonist; 4 mg (kg BW)−1 day−1; Tocris), or diarylpropionitrile (DPN, an ERβ-specific agonist; 4 mg (kg BW)−1 day−1; Tocris). The selective ligand activity of these compounds has been previously described using competitive binding and transcription assays (Stauffer et al. 2000; Meyers et al. 2001). The dose used herein for PPT was as effective as EB stimulation to elicit a full uterotrophic response as already reported (Harris et al. 2002; Frasor et al. 2003), while DPN at the daily dose used was appropriate to investigate ERβ activities in vivo (Lee et al. 2005; Weiser et al. 2009), and did not evoke uterotrophic activity in the present study. Additionally, to determine a genomic activity for EB, a last group of OVX rats received a single injection of EB (5 mg (kg BW)−1, s.c.; n= 6), with or without ICI 182,780 (2 mg (kg BW)−1, s.c.; n= 5). Control rats received the vehicle, olive oil (Ve, n= 6). Eight hours after treatment, rats were killed, and colons subjected to RNA extraction.
In vivo measurement of colonic paracellular permeability
Lumen to blood clearance for 24 h of 51Cr-labelled ethylenediamine tetra-acetic acid (51Cr-EDTA; Perkin Elmer Life Sciences, Paris, France) was used to assess CPP during the sexual cycle. Animals were anaesthetized as above, and surgically equipped with an intracolonic catheter 1 week before experimentation, as previously described (Ait-Belgnaoui et al. 2005), then placed in individual metabolic cages 24 h before intracolonic injection of 51Cr-EDTA (0.7 μCi) diluted in 250 μl saline. Faeces and urine were collected separately for 24 h, and total radioactivity found in urine was measured with a gamma counter (Cobra II, Packard, Meriden, CT, USA). Colonic permeability to 51Cr-EDTA was expressed as the percentage of total administered radioactivity recovered in urine.
Ussing chamber experiments
Immediately after killing, distal colons were removed and cut along the mesenteric border, and three colonic strips from each rat were mounted in Ussing chambers (Easymount, Hamden, CT, USA) having a flux area of 0.5 cm2. Both sides of each colonic sheet were bathed in 5 ml of circulating oxygenated Krebs–Henseleit solution (Sigma), and maintained at 37°C. Colonic paracellular permeability was assessed by measuring mucosal-to-serosal flux of fluorescein isothiocyanate (FITC)-labelled 4 kDa dextran (Sigma) across the colonic strip. In brief, after a 20 min equilibrium period, 500 μl of buffer solution on the mucosal side was replaced by 500 μl solution of FITC-dextran (2.2 mg ml−1 as final concentration). After 1 h, fluorescence was measured in the serosal buffer with a fluorimeter (Tecan Infinite M200, Austria). Results were expressed as the flux of FITC-dextran crossing 1 cm2 of epithelium per hour (nmol cm−2 h−1), and are the means of measurements done in triplicates. Trans-epithelial resistance (TER), an indicator of tissue viability and paracellular ion exchange, was expressed as Ohm cm2.
Cell culture and treatments
Caco-2 cells were grown at 37°C in 5% CO2 humidified atmosphere in phenol-red free Dulbecco's modified Eagle's medium (DMEM; MidiMed, Boussens, France), with 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and non-essential amino acids (MidiMed), and augmented with 10% fetal bovine calf serum (FCS; MidiMed) pretreated with dextran coated charcoal. Charcoal stripped FCS was prepared by mixing 500 ml FCS with 10 g activated charcoal (Sigma) overnight at 4°C. Following centrifugation to remove charcoal, the FCS was filtered at 0.22 μm, then added to the DMEM. Cells were plated in a 24-well plate coated with collagen type 1 (5 μg cm2; BD Biosciences, Le Pont de Claix, France) or on collagen type 1-coated round glass coverslips at a density of 5 × 104 cells per cm2 for 3 days, then exposed in triplicate to EB (10 nm), PPT (100 nm) and DPN (100 nm) for 8 h in charcoal stripped FCS. For treatment with the ER antagonist, ICI 182,780 (10 μm) was added in the medium 1 h before EB or DPN treatment. All chemicals were pre-dissolved in 100% ethanol at a concentration of 10 μm, then diluted with the medium to bring the final concentration, while control cells were exposed to vehicle ethanol (final dilution < 0.1%).
Immunofluorescence labelling
Caco-2 monolayers on coverslips were fixed and permeabilized in methanol–acetic acid (95/5%) for 20 min at −20°C, then blocked for 30 min with phosphate-buffered saline (PBS)–0.1% bovine serum albumin (BSA) at room temperature (RT). Cells were incubated overnight at 4°C with rabbit anti-occludin polyclonal antibody (Zymed Laboratories, South San Francisco, CA, USA), diluted 1 : 100 in PBS, or rabbit anti-JAM-A (Zymed Laboratories), diluted 1 : 25 in PBS. After washing, secondary detection was performed using Alexa fluor 488-conjugated IgG donkey anti-rabbit (1 : 2000 in PBS; Molecular Probes/Invitrogen, Cergy Pontoise, France) for 30 min. Caco-2 monolayers were washed and mounted in Prolong mounting medium (Invitrogen), and the labelling was examined under a Nikon 90i fluorescence microscope. The z-axial images were collected using an Olympus FV5-101 confocal laser scanning, and Fluoview software (Olympus).
Protein extraction and Western blot
Total proteins were extracted from colons and Caco-2 cells in RIPA buffer containing 1% Igepal, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulphate in Tris-buffered saline (TBS) 1×, and a complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany), before clarification at 10 000 g for 10 min (4°C). Protein concentrations were measured using the BC Assay Uptima kit (Interchim, France). Equal amounts of protein per lane were separated by SDS-PAGE, then transferred onto nitrocellulose membranes (Optitran, Schleicher 1 Schuell Biosciences, Dassel, Germany). Membranes were blocked with 5% dry milk in 0.1% tween in TBS (TBST) for 2 h at RT, and then incubated overnight at 4°C with primary antibodies. Immunoblotting was performed using polyclonal rabbit anti-occludin antibody (Zymed Laboratories) diluted 1 : 500 in 5% dry milk in TBST, and polyclonal rabbit anti-ZO-1 (Zymed Laboratories) or anti-JAM-A (Abcam, Cambridge, UK) diluted 1 : 1000 and 1 : 500 in 3% BSA in TBST, respectively. After washing in TBST–milk, membranes were incubated for 1 h at RT with horseradish peroxidase-conjugated secondary antibody (Visualizer Detection Kit, Upstate, Lake Placid, NY, USA) diluted 1 : 20 000 in TBST, and washed. Bands were identified using SuperSignal West Femto (Thermo Scientific, Rockford, IL, USA). Relative values of the band density were estimated using ImageJ software (NIH, Bethesda, MD, USA), and are presented as the mean of blot determination in 5 to 7 animals.
RNA extraction and RT-PCR
Total RNA was prepared from colons or Caco-2 cells with QIAzol (Qiagen, Courtaboeuf France) by standard isopropanol–chloroform precipitation. The resulting RNA pellets were washed with 75% ethanol and resuspended in RNase free water. RNA quality was confirmed by analysis of the 260 : 280 nm absorbance ratio. cDNA was synthesized from 1 μg RNA using an Omniscript RT kit (Qiagen) and 5 μmol l−1 random primers (Invitrogen, France) according to the manufacturer's instructions on an automated Applied Biosystems 9700 PCR System. cDNA was diluted 5 times before PCR amplification. Primer sets were as follows: for rat occludin (forward 5′GCT-CAG-GGA-ATA-TCC-ACC-TAT-CA3′, reverse 5′CAC-AAA-GTT-TTA-ACT-TCC-CAG-ACG3′), annealing temperature 62°C; human occludin (forward 5′TCA-GGG-AAT-ATC-CAC-CTA-TCA-CTT-CAG3′, reverse 5′CAT-CAG-CAG-CAG-CCA-TGT-ACT-CTTCAC3′), annealing temperature 53°C; rat ZO-1 (forward 5′CGG-AAC-TAT-GAC-CAT-CGC-CAT-C3′, reverse 5′GCC-TGT-ACC-TGT-TGT-GCA-CC3′), annealing temperature 62°C; human ZO-1 (forward 5′CGG-TCC-TCT-GAG-CCT-GTA-AG3′, reverse 5′GGA-TCT-ACA-TGC-GAC-GAC-AA3′), annealing temperature 46°C; human JAM-A (forward 5′GGT-CAA-GGT-CAA-GCT-CAT3′, reverse 5′CTG-AGT-AAG-GCA-AAT-GCA-G3′), annealing temperature 48°C; rat GAPDH (forward 5′ATC-ACC-ATC-TTC-CAG-GAG-CG3′, reverse 5′TTC-TGA-GTG-GCA-GTG-AGG-GC3′), annealing temperature 50°C; human GAPDH (forward 5′TTC-ATT-GAC-CTC-AAC-TAC-AT3′, reverse 5′GTG-GCA-GTG-ATG-GCA-AGG-AC3′), annealing temperature 48°C. The 50 μl PCR reaction mixtures contained 2.5 units of HotStarTaq DNA polymerase (QIAgen), 0.2 μm of each primer, 200 μm of each dNTP and cDNA corresponding to 1 μg of total RNA. The PCR products (10 μl of each PCR reaction) were separated on a 2% agarose gel and were visualized by SYBR Gold staining under UV light. Control experiments were also performed in which RNA was omitted from the reverse transcription reactions. Under these conditions, no bands were seen on agarose gels for any of the genes studied (data not shown).
Statistical analysis
All data are presented as means ±s.e.m. Statistical significance was assessed by Student's t-test or one-way ANOVA followed by Bonferroni's post hoc test for multiple comparisons where appropriate. Analyses were performed by running Prism 4 software (GraphPad, San Diego, CA, USA). P < 0.05 was considered significant.
Results
Oestrous cycle-dependent variations of colonic paracellular permeability
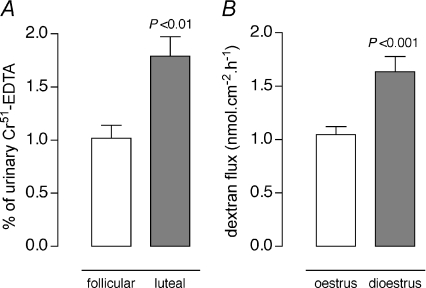
The initial study focused on determining the in vivo parameters of CPP to intracolonic 51Cr-EDTA in cyclic rats. In animals in follicular phase, CPP, expressed as the percentage of 51Cr-EDTA that crosses the colonic barrier, showed a significant decrease compared with rats in luteal phase (−44%, 1.0 ± 0.1 vs. 1.8 ± 0.2% of total 51Cr-EDTA recovered in urine, respectively; P < 0.01) (Fig. 1A). Similar differences in paracellular fluxes were observed in Ussing chambers, as assessed by a decreased CPP to FITC-dextran in colonic segments from oestrus rats compared with those of dioestrus rats (−38%, 1.0 ± 0.1 vs. 1.6 ± 0.1 nmol cm−2 h−1, respectively; P < 0.001) (Fig. 1B), associated with an increased baseline TER (Table 1).
Figure 1. Effect of oestrous cycle on paracellular permeability.
A, in vivo51Cr-EDTA recovery in urine 24 h after injection into the colon of rats in follicular and luteal phase. B, in vitro paracellular FITC-dextran flux measured for 1 h in Ussing chambers in colon segments from oestrus and dioestrus rats. Bars are means ±s.e.m. Statistical significance was assessed by Student's t-test.
Table 1.
Colonic trans-epithelial resistance (TER) in cyclic rats, and OVX rats with or without oestradiol benzoate, progesterone and ICI 182,780
| Animals | TER (Ω cm2) | n |
|---|---|---|
| Cyclic | ||
| Dioestrus | 84.5 ± 5.5 | 4 |
| Oestrus | ||
| 114.0 ± 10.9* | 4 | |
| OVX | ||
| Ve | 90.3 ± 2.8 | 9 |
| EB | 109.3 ± 5.5a | 10 |
| P | 75.6 ± 4.7ns | 5 |
| EB+ICI | 95.6 ± 9.6ns | 5 |
Data are means ±s.e.m. of triplicate measurements in Ussing chambers.
P < 0.05 vs. dioestrus rats;
P < 0.05, and ns (not significant) vs. control Ve.
Effects of ovariectomy, progesterone, oestradiol and ER specific ligands on colonic paracellular permeability
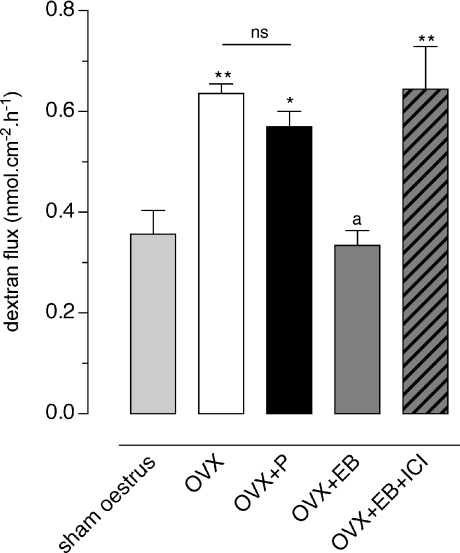
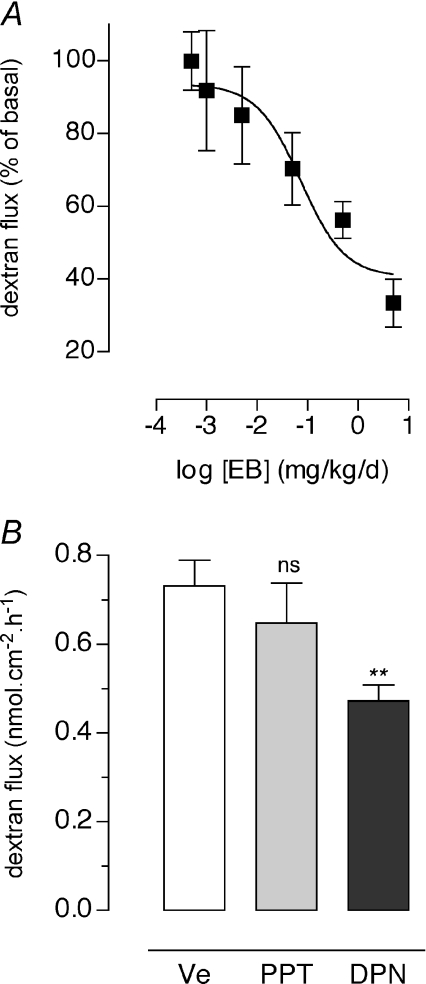
Compared to sham oestrus rats, bilateral ovariectomy induced a marked increase in CPP monitored in Ussing chambers (0.36 ± 0.05 vs. 0.64 ± 0.02 nmol cm−2 h−1, respectively; P < 0.01) (Fig. 2). Treatment with P did not change CPP and TER (Fig. 2 and Table 1). In contrast, EB replacement elicited a 49% decrease of FITC-dextran fluxes compared to OVX controls (0.33 ± 0.03 vs. 0.64 ± 0.02 nmol cm−2 h−1, respectively; P < 0.001) (Fig. 2), correlated with a significant increase in TER (P < 0.05), reaching baseline values observed in oestrus rats (Table 1). When the ER antagonist, ICI 182,780, was co-administered with EB in OVX rats, both CPP and TER returned to baseline values similar to those observed in OVX controls (Fig. 2 and Table 1). We also examined the effects of various doses of EB injected daily for 5 days (10 μg to 5 mg (kg BW)−1 day−1) on CPP to FITC-dextran. As shown in Fig. 3A, a dose-dependent decrease of dextran flux throughout colonic strips was observed (P < 0.05), with a median effective dose (ED50) of 72 μg (kg BW)−1 day−1, and maximal inhibition at 5 mg (kg BW)−1 day−1.
Figure 2. Effect of ovariectomy, progesterone, oestradiol and ICI 182,780 on paracellular permeability.
FITC-dextran flux measurements for 1 h in Ussing chambers in colon segments from sham operated rats (sham oestrus), and ovariectomized rats treated for 5 days with empty s.c. implants (OVX) or with implants filled with P (OVX+P) or EB (OVX+EB) or EB with daily s.c. injection of the ER antagonist ICI 182,780 (2 mg (kg BW)−1 day−1) (OVX+EB+ICI). Bars are means ±s.e.m. P < 0.001, ANOVA; *P < 0.05, **P < 0.01 vs. sham oestrus, aP < 0.001 vs. OVX or P group of rats; ns: not significant (Bonferroni post hoc test).
Figure 3. Dose effect of oestradiol on paracellular permeability in OVX rats and involvement of ERα and ERβ.
A, dose–response study of oestradiol benzoate (EB, 1 μg to 5 mg (kg BW)−1 day−1 for 5 days, s.c.) on paracellular FITC-dextran flux measured for 1 h in colon segments mounted in Ussing chambers. Values are means ±s.e.m. of triplicate measurements in 5–8 rats per group (P < 0.05, ANOVA). B, effects of a 5 day treatment with the selective ligand agonist for ERα (PPT, 4 mg (kg BW)−1 day−1, s.c.), ERβ (DPN, 4 mg (kg BW)−1 day−1, s.c.) on colonic paracellular permeability in Ussing chambers. OVX rats treated with vehicle DMSO in olive oil (Ve) were used as controls. Values are means ±s.e.m. of triplicate measurements in 7 rats per group. **P < 0.01 and ns (not significant) vs. Ve.
To determine the ER subtype mediating oestradiol regulation of CPP, OVX rats were treated with DPN (a selective ERβ agonist), PPT (a selective ERα agonist) or vehicle (controls) for 5 days. In Ussing chambers, the flux of FITC-dextran was reduced by 36% in DPN treated rats compared with controls (0.47 ± 0.03 vs. 0.73 ± 0.06 nmol cm−2 h−1, respectively; P < 0.05), whereas no significant changes were observed following PPT stimulation (Fig. 3B).
Oestradiol stimulates occludin expression but not ZO-1 in the rat colon
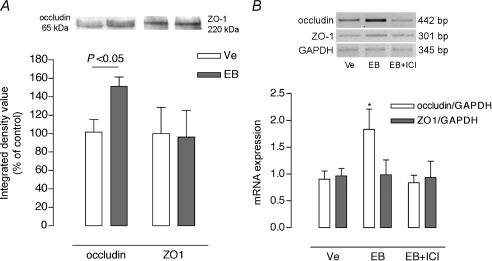
We further analysed whether oestradiol-induced decrease in CPP resulted from changes in the in vivo expression of the tight junction proteins, occludin and ZO-1. OVX rats were treated for 5 days with either vehicle (olive oil) or EB at the dose (5 mg (kg BW)−1 day−1) producing maximal inhibition on CPP as observed in the prior dose–response study. As shown in Fig. 4A, there was no significant difference in the ZO-1 protein expression compared to control OVX, whereas EB treatment induced a sharp increase in occludin protein expression (+49%, P < 0.05). To further examine the signalling mechanisms by which EB increases occludin amount, OVX rats were treated with a single injection of EB (5 mg (kg BW)−1 day−1) and killed at 8 h after treatment, a time point compatible with a genomic effect of EB on occludin expression in other epithelia (Kang et al. 2006). Colons from animals treated with EB alone exhibited a marked increase in occludin mRNA, blocked by ICI 182,780, whereas no change was observed for ZO-1 mRNA (Fig. 4B).
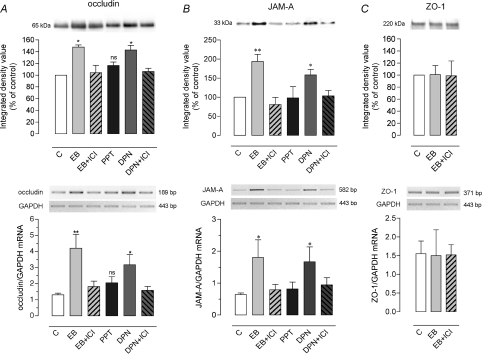
Figure 4. Effects of oestradiol on occludin and ZO-1 expression in the colon of OVX rats.
A, representative Western blot lanes for occludin and ZO-1 from the same protein extract, and quantitative representation of corresponding protein contents from 7 independent experiments. Note that EB significantly increased occludin protein without change in ZO-1 amount. B, RT-PCR results for occludin, ZO-1, and GAPDH mRNA using total RNA from colon lysates of 6 independent experiments. In OVX rats 8 h after a single EB injection (5 mg kg−1), EB significantly increased occludin mRNA, blocked by ICI 182,780 (EB+ICI), without change in the expression of mRNA for ZO-1. Values are means ±s.e.m. Ve: control vehicle. *P < 0.05 vs. corresponding control (Ve).
Effects of oestradiol, PPT and DPN on occludin, JAM-A and ZO-1 expression in Caco-2 cells
To evaluate whether the EB-mediated increase in occludin expression specifically involved ERβ expressed by epithelial cells, and whether it could affect other transmembrane proteins involved in TJ function, we assessed occludin and JAM-A mRNA and protein levels in human Caco-2 cell line stimulated with EB, PPT or DPN, with or without the ER antagonist ICI 182,780. We found that DPN, like EB, up-regulated occludin (+43%; P < 0.05) and JAM-A (+58%; P < 0.05) protein expression by Caco-2 cells, while PPT had no significant effect (Fig. 5A and B). Pre-treatment of EB- or DPN-stimulated Caco-2 cells with ICI 182,780 resulted in reducing occludin as well as JAM-A protein amount to an extent comparable to that observed in controls (Fig. 5A and B). At the transcription level, Caco-2 cells showed a significant increase in occludin mRNA when treated with EB (P < 0.01) or DPN (P < 0.05), and these effects were blocked by the pre-treatment with ICI 182,780 (Fig. 5A). Similar EB and DPN effects were found for JAM-A mRNA (Fig. 5B). Compared to control cells, no significant change in both occludin and JAM-A mRNA levels was observed following PPT stimulation (Fig. 5A and B). Further, as observed in the rat colon (Fig. 4), no EB-related changes could be discerned on mRNA and protein level of ZO-1 (Fig. 5C).
Figure 5. Effects of oestradiol, ERα and β agonists, and ICI 182,780 on occludin, JAM-A and ZO-1 expression in Caco-2 cells.
The expression of occludin (A), JAM-A (B), and ZO-1 (C) proteins and mRNA were examined by Western blotting (upper panels) and RT-PCR (lower panels), with representative band images and corresponding densitometric analysis. Caco-2 cells were treated for 8 h with ethanol vehicle (C), or with EB (10 nm), DPN (100 nm) or PPT (100 nm), in the presence or absence of ER antagonist ICI 182,780 (ICI, 10 μm). Note that EB like DPN, but not PPT, significantly increased mRNA and protein levels of occludin (A) and JAM-A (B), without effect on ZO-1 expression (C). Both EB and DPN effects on occludin and JAM-A expression were blocked by ICI. Each bar represents mean ±s.e.m. from 6 to 7 independent experiments. P= 0.01, ANOVA; **P < 0.001, *P < 0.05, and ns (not significant) (Bonferroni post hoc test) vs. control cells (C).
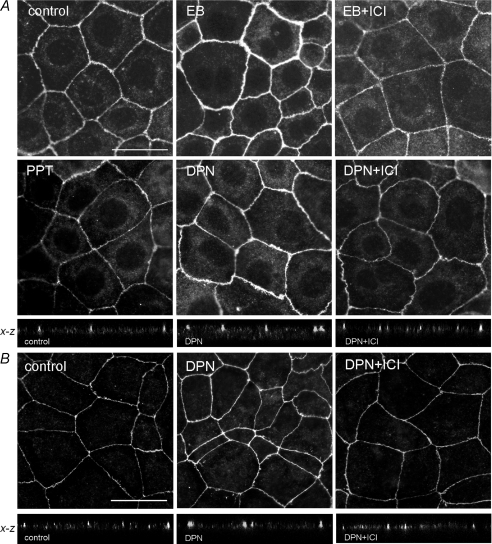
To test whether EB and DPN affect the architecture of TJs, Caco-2 monolayers were immunostained by fluorescent antibodies to occludin and JAM-A. In control cells, occludin and JAM-A immunofluorescence were distributed as distinct continuous bands along the cell borders, and cell morphology did not differ among treatment groups (Fig. 6). In EB- and DPN-treated monolayers, immunostaining for occludin was markedly increased at the TJ level, whereas no change was detected after PPT treatment compared to control cells (Fig. 6A). Similar DPN effect was observed for JAM-A staining at the apical cell-to-cell contact (Fig. 6B), while PPT had no effect (not shown). Pretreatment with ICI 182,780 prevented the DPN-induced increase in occludin and JAM-A immunostaining (Fig. 6).
Figure 6. Immunofluorescence detection of occludin and JAM-A in Caco-2 cells.
Cell monolayers were exposed for 8 h to ethanol vehicle (Control), EB (10 nm), DPN (100 nm), or PPT (100 nm), in the presence or absence of ER antagonist ICI 182,780 (ICI, 10 μm). A, occludin: note that DPN like EB, but not PPT, increases occludin staining at the level of epithelial cell membranes, and ICI added 1 h before EB or DPN treatment blocked this effect. In confocal images in the x–z plane, note that DPN enhances occludin immunostaining at the apical cell-to-cell contact of epithelial cells compared to control cells, and this effect was abrogated in the presence of ICI 182,780. B, JAM-A, representative images of immunostaining with or without DPN treatment and ICI 182,780, and x–z plane confocal images showing modulation of epithelial JAM-A staining at apical sites. Scale bars = 25 μm.
Discussion
A major route of transport in gut epithelia is the paracellular pathway, regulated by TJs that form part of the apical junctional complex between epithelial cells (Turner, 2006). In the absence of pathological disorders, epithelial cells are not totally sealed and allow transport of water and solutes, as well as microbial sampling for maintaining intestinal immune hoemostasis (Masyuk et al. 2002; Shen & Turner, 2006; Artis, 2008). The present study in female rats shows that colonic paracellular permeability was not static in basal conditions, but fluctuated depending on stages of the oestrous cycle. Here we present evidence of a physiological link between cycle-dependent permeability changes, circulating oestrogens and ERβ-mediated increase in expression of occludin and JAM-A, two TJ transmembrane proteins with pivotal functions in the maintenance of epithelial intercellular spaces.
There is growing evidence that sex steroids influence gut physiology. Both oestrogen and progesterone receptors are expressed in the GI tract under normal conditions (i.e. without tumour formation or developing inflammation) (Enmark et al. 1997; Campbell-Thompson et al. 2001; Konstantinopoulos et al. 2003; Kawano et al. 2004; Xiao et al. 2005), and sex steroids have been shown to influence gastric motility (Günal et al. 2004), colonic transit time (Xiao et al. 2005; Cong et al. 2007), chloride ion secretion (Condliffe et al. 2001; O’Mahony et al. 2007), and epithelium formation (Wada-Hiraike et al. 2006a). In contrast, the literature is less abundant regarding influence of the natural cyclic shift from oestrogen to progesterone plasma dominance on gut function. Our data in cyclic rats show, first, that paracellular permeability was lower in oestrus (i.e. oestrogen dominance) than in dioestrus (progesterone dominance); second, that the decrease of paracellular permeability under oestrogen dominance appeared concomitant to an increase in TER, suggesting the reinforcement of TJ function during the follicular phase, an effect which is lost in the subsequent luteal phase. Our statement that these variations occurred in relation to changes in plasma oestrogen is supported by the following findings. First, progesterone did not influence epithelial permeability in OVX rats, while oestradiol evoked an increase in TER, and a dose-related decrease in paracellular flux. Similarly, Mullick et al. (2001) also documented a reduced permeability following oestradiol treatment, but not progesterone, in rat carotid arteries. Second, the oestradiol effects on colonic paracellular flux and TER were blocked by the ER antagonist ICI 182,780, demonstrating that these responses are mediated by ERs. These findings reinforce the hypothesis that oestradiol is able to limit TJ opening in the colon during the reproductive cycle, thus able to limit the passage of potentially harmful luminal components. Consistent with this suggestion, Homma et al. (2005) demonstrated sexual dimorphism in rat ileal permeability, with a female gut in pro-oestrus stage (i.e. oestrogen peak) better preserved than the male intestine under hypoxia and acidosis, a sex difference abrogated by oestradiol pretreatment in males. A protective role of oestradiol in decreasing paracellular permeability enlarges its beneficial effects on intestinal barrier function, since oestradiol was thought to have primarily anti-inflammatory activities in the gut, by decreasing neutrophil infiltration and cytokine production in colitis (Verdu et al. 2002; Günal et al. 2003; Harnish et al. 2004; Houdeau et al. 2007).
Gut epithelial cells contain functional ERs (Thomas et al. 1993), and oestrous cycle-related changes in
ER expression have been reported in the mouse intestine (Kawano et al. 2004). Our findings emphasize a multifaceted role of oestrogen in the physiological control of intestinal epithelium. For instance, oestradiol appeared to inhibit chloride ion exchange in distal colonic cells, an anti-secretory response also found to be oestrous cycle-dependent (O’Mahony & Harvey, 2008). These authors postulated that the anti-secretory effect of oestradiol may enhance salt and water retention in females, as commonly observed in oral contraceptive users with high oestrogen dosage, or during natural periods of elevated plasma oestrogen (Oelkers, 1996; Fruzzetti et al. 2007). Because water molecules can be driven passively by paracellular flux additional to the transcellular pathway (Masyuk et al. 2002), our data support the hypothesis that the oestrogen-mediated decrease of paracellular permeability may act in combination with inhibition of chloride ion channels to modulate fluid movement throughout the intestinal epithelium. Such a mechanism may compensate the natriuretic and blood pressure lowering effect of endogenous progesterone during the luteal phase, thus contributing to body water homeostasis throughout the menstrual cycle (Oelkers, 1996). According to O’Mahony & Harvey (2008), the body fluid-retaining effects of oestrogen during the reproductive cycle may allow for volume expansion of the uterus in preparation for embryo implantation, a water transport also leading to reduction in viscosity of the uterine luminal fluid (Jablonski et al. 2003; Richard et al. 2003).
Oestrogens interact with at least two receptors ERα and ERβ (Heldring et al. 2007). Herein we report that the EB-mediated decrease in epithelial permeability was mimicked by the ERβ agonist DPN, but not by the ERα agonist PPT. Although both ERs were expressed in the colon, ERβ predominates in normal colonic mucosa (Campbell-Thompson et al. 2001), consistent with the effect observed only through DPN stimulation in the present study. It is of note that DPN acts as an agonist on both ERs, but in support of the present data, DPN has a 70-fold higher binding affinity and a 170-fold higher potency in transcription assays with ERβ than with ERα (Meyers et al. 2001), and appeared as effective as EB stimulation to decrease intestinal permeability in our study, in contrast to PPT, thus confirming this effect only attributed to ERβ activation. In the colon, ERβ was mainly found in epithelial cells (Konstantinopoulos et al. 2003), suggesting that these cells are the main targets for the oestrogen-induced decrease in paracellular permeability. To address this question, we compared the effect of oestradiol in the rat colon and in a human intestinal cell line on the expression of TJ proteins that control the intercellular spaces. Tight junction proteins are composed of cytoplasmic and transmembrane proteins. Among them, occludin and ZO-1 contribute to the rate-limiting step for paracellular passage in intestinal epithelium (Turner, 2006). In human endothelial cell lines, it has been shown that the amount of occludin is inversely correlated to paracellular permeability, the latter being decreased when occludin expression is increased, a modulation mediated by oestradiol (Ye et al. 2003; Kang et al. 2006; Sumanasekera et al. 2007). In the rat colon, we report here that EB stimulation is associated with an increase of occludin protein, without changes in the amount of ZO-1. In addition, consistent with a transcription activity, the mechanisms involved an increase in occludin mRNA, an effect blocked by the ER antagonist ICI 182,780. A similar activation of occludin by oestradiol has been described in the mouse brain, where oestrogens also participate in the control of paracellular diffusion in the blood–brain barrier (Kang et al. 2006). Furthermore, the effect of EB was reproduced on cultured Caco2 cells in the present study, demonstrating that EB acts directly on epithelial cells to enhance occludin protein and mRNA. As reported in the colon, ERα and β were co-expressed in the human cell line Caco-2, with ERβ the predominant subtype (Campbell-Thompson et al. 2001), and our data using the selective agonist DPN confirm that the ERβ pathway is the intrinsic mechanism regulating occludin expression in colonic epithelial cells. To further explore the effects of oestradiol and DPN on Caco-2 cells, we also found increased expression levels of JAM-A, a transmembrane protein apically positioned at TJs with occludin in the colon (Vetrano et al. 2008). Several studies have implicated JAM-A in the regulation of intestinal barrier function. For instance, JAM-A deficiency in mice resulted in enhanced intestinal permeability to FITC-dextran and decreased TER (Laukoetter et al. 2007), and silencing of JAM-A in Caco-2 cells also resulted in increased epithelial permeability (Vetrano et al. 2008). Consistently, Vetrano et al. (2008) showed a dramatic loss of epithelial JAM-A that correlates with epithelial barrier defect in Crohn's disease and ulcerative colitis, the two major forms of inflammatory bowel disorders in humans. Interestingly, it has been proposed that JAM-A plays a pivotal role in the assembly of the TJ protein complex, by interacting with ZO-1 and stabilizing occludin at the junctions (Bazzoni et al. 2000). Hence, it is likely that an increased amount of epithelial JAM-A together with occludin ameliorates TJ integrity, thus improving the epithelial barrier function under oestrogen dominance. However, we cannot exclude that oestradiol may also target other proteins involved in the epithelial TJ complex, and not investigated in the present study, mainly the claudins, a large family of intercellular adhesion molecules (Van Itallie & Anderson, 2006), of which up-regulation by various factors, for instance solutes and nutrients, has been shown to enhance paracellular sealing in the colon (Li et al. 2004; Amasheh et al. 2009; Suzuki & Hara, 2009).
In conclusion, the ERβ-mediated increase of occludin and JAM-A expression in epithelial cells has been identified as the molecular mechanism for the oestrogen modulation of paracellular permeability in the colon. This is in support of the hypothesis that high plasma oestrogen level during the follicular phase of the reproductive cycle limits TJ opening in the normal colon, through up-regulation of transmembrane proteins leading to the reinforcement of the structural integrity of TJs, an effect suppressed during the luteal phase. Because impaired paracellular permeability is a trigger for inflammatory bowel disorders and chronic inflammation in humans (Meddings, 1997; Shen & Turner, 2006), the ERβ pathway may represent a novel target to prevent or limit the epithelial barrier defect in these diseases.
Acknowledgments
We thank Drs H. Guillou and R. Garcia-Villar for helpful comments and H. Tiphaine-Alice for statistical advice. This work was supported by the Agence Nationale de la Recherche, grant ANR-06-PNRA-008-04, and by the Institut National de la Recherche Agronomique.
Glossary
Abbreviations
- CPP
colonic paracellular permeability
- DPN
diarylpropionitrile
- EB
oestradiol benzoate
- ER
oestrogen receptor
- JAM-A
junctional adhesion molecule-A
- OVX
ovariectomized
- P
progesterone
- PPT
propyl pyrazole triol
- TJ
tight junction
- ZO-1
zona occludens
Author contributions
All experiments were done at the Neuro-Gastroenterology & Nutrition Unit, UMR 1054, Institut National de la Recherche Agronomique, Research Center of Toulouse, France. Study concept and design: V.B. and E.H. Analysis and interpretation of data: V.B., M.L., C.B.-B. and E.H. Drafting of the article: V.B. and E.H. Critical revision for important intellectual content: L.B., J.F. and E.H. Final approval of the article: J.F. and E.H.
References
- Ait-Belgnaoui A, Bradesi S, Fioramonti J, Theodorou V, Bueno L. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain. 2005;113:141–147. doi: 10.1016/j.pain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Amasheh S, Milatz S, Krug SM, Bergs M, Amasheh M, Schulzke JD, Fromm M. Na+ absorption defends from paracellular back-leakage by claudin-8 upregulation. Biochem Biophys Res Commun. 2009;378:45–50. doi: 10.1016/j.bbrc.2008.10.164. [DOI] [PubMed] [Google Scholar]
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. J Biol Chem. 2000;275:20520–20526. doi: 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERβ isoforms in colon cancer. Cancer Res. 2001;61:632–640. [PubMed] [Google Scholar]
- Cenac N, Chin AC, Garcia-Villar R, Salvador-Cartier C, Ferrier L, Vergnolle N, Buret AG, Fioramonti J, Bueno L. PAR2 activation alters colonic paracellular permeability in mice via IFN-γ-dependent and -independent pathways. J Physiol. 2004;558:913–925. doi: 10.1113/jphysiol.2004.061721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condliffe SB, Doolan CM, Harvey BJ. 17β-oestradiol acutely regulates Cl– secretion in rat distal colonic epithelium. J Physiol. 2001;530:47–54. doi: 10.1111/j.1469-7793.2001.0047m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong P, Pricolo V, Biancani P, Behar J. Abnormalities of prostaglandins and cyclooxygenase enzymes in female patients with slow-transit constipation. Gastroenterology. 2007;133:445–453. doi: 10.1053/j.gastro.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M, Gustafsson JA. Human estrogen receptor β-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- Frasor J, Barnett DH, Danes JM, Hess R, Parlow AF, Katzenellenbogen BS. Response specific and ligand dose-dependent modulation of estrogen receptor (ER) α activity by ERβ in the uterus. Endocrinology. 2003;144:3159–3166. doi: 10.1210/en.2002-0143. [DOI] [PubMed] [Google Scholar]
- Fruzzetti F, Lazzarini V, Ricci C, Quirici B, Gambacciani M, Paoletti AM, Genazzani AR. Effect of an oral contraceptive containing 30 μg ethinylestradiol plus 3 mg drospirenone on body composition of young women affected by premenstrual syndrome with symptoms of water retention. Contraception. 2007;76:190–194. doi: 10.1016/j.contraception.2007.05.080. [DOI] [PubMed] [Google Scholar]
- Gorodeski GI. Estrogen decrease in tight junctional resistance involves matrix-metalloproteinase-7-mediated remodelling of occludin. Endocrinology. 2007;148:218–231. doi: 10.1210/en.2006-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günal O, Bozkurt A, Deniz M, Sungur M, Yeğen BC. Effect of sex steroids on colonic distension-induced delay of gastric emptying in rats. J Gastroenterol Hepatol. 2004;19:975–981. doi: 10.1111/j.1440-1746.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- Günal O, Oktar BK, Ozçinar E, Sungur M, Arbak S, Yeğen B. Estradiol treatment ameliorates acetic acid-induced gastric and colonic injuries in rats. Inflammation. 2003;27:351–359. doi: 10.1023/b:ifla.0000006703.53427.da. [DOI] [PubMed] [Google Scholar]
- Harnish DC, Albert LM, Leathurby Y, Eckert AM, Ciarletta A, Kasaian M, Keith JC., Jr Beneficial effects of estrogen treatment in the HLA-B27 transgenic rat model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:118–125. doi: 10.1152/ajpgi.00024.2003. [DOI] [PubMed] [Google Scholar]
- Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERα and ERβ, in estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology. 2002;143:4172–4177. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Homma H, Hoy E, Xu DZ, Lu Q, Feinman R, Deitch EA. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointest Liver Physiol. 2005;288:G466–G472. doi: 10.1152/ajpgi.00036.2004. [DOI] [PubMed] [Google Scholar]
- Houdeau E, Moriez R, Leveque M, Salvador-Cartier C, Waget A, Leng L, Bueno L, Bucala R, Fioramonti J. Sex steroid regulation of macrophage migration inhibitory factor in normal and inflamed colon in the female rat. Gastroenterology. 2007;132:982–993. doi: 10.1053/j.gastro.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Jablonski EM, McConnell NA, Hughes FM, Huet-Hudson YM. Estrogen regulation of aquaporins in the mouse uterus: potential roles in uterine water movement. Biol Reprod. 2003;69:1481–1487. doi: 10.1095/biolreprod.103.019927. [DOI] [PubMed] [Google Scholar]
- Kang HS, Ahn HS, Kang HJ, Gye MC. Effect of estrogen on the expression of occludin in ovariectomized mouse brain. Neurosci Lett. 2006;402:30–34. doi: 10.1016/j.neulet.2006.03.052. [DOI] [PubMed] [Google Scholar]
- Kawano N, Koji T, Hishikawa Y, Murase K, Murata I, Kohno S. Identification and localization of estrogen receptor α- and β-positive cells in adult male and female mouse intestine at various estrogen levels. Histochem Cell Biol. 2004;121:399–405. doi: 10.1007/s00418-004-0644-6. [DOI] [PubMed] [Google Scholar]
- Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G, Papavassiliou AG. Oestrogen receptor beta (ERβ) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour's dedifferentiation. Eur J Cancer. 2003;39:1251–1258. doi: 10.1016/s0959-8049(03)00239-9. [DOI] [PubMed] [Google Scholar]
- Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E, Campbell JA, Dermody TS, Nusrat A, Parkos CA. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GS, Kim HJ, Jung YW, Choi KC, Jeung EB. Estrogen receptor α pathway is involved in the regulation of calbindin-D9K in the uterus of immature rats. Toxicol Sci. 2005;84:270–277. doi: 10.1093/toxsci/kfi072. [DOI] [PubMed] [Google Scholar]
- Li N, Lewis P, Samuelson D, Liboni K, Neu J. Glutamine regulates Caco-2 cell tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2004;287:G726–G733. doi: 10.1152/ajpgi.00012.2004. [DOI] [PubMed] [Google Scholar]
- Masyuk AI, Marinelli RA, LaRusso NF. Water transport by epithelia of the digestive tract. Gastroenterology. 2002;122:545–562. doi: 10.1053/gast.2002.31035. [DOI] [PubMed] [Google Scholar]
- Meddings JB. Intestinal permeability in Crohn's disease. Aliment Pharmacol Ther. 1997;11:47–53. doi: 10.1111/j.1365-2036.1997.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Moriez R, Leveque M, Salvador-Cartier C, Barreau F, Theodorou V, Fioramonti J, Bueno L, Eutamene H. Mucosal mast cell proteases are involved in colonic permeability alterations and subsequent bacterial translocation in endotoxemic rats. Shock. 2007;28:118–124. doi: 10.1097/SHK.0b013e3180315ba9. [DOI] [PubMed] [Google Scholar]
- Mullick AE, Walsh BA, Reiser KM, Walsh BA, Reiser KM, Rutledge JC. Chronic estradiol treatment attenuates stiffening, glycoxidation, and permeability in rat carotid arteries. Am J Physiol Heart Circ Physiol. 2001;281:H2204–H2210. doi: 10.1152/ajpheart.2001.281.5.H2204. [DOI] [PubMed] [Google Scholar]
- Oelkers WK. Effects of estrogens and progestogens on the renin-aldosterone system and blood pressure. Steroids. 1996;61:166–171. doi: 10.1016/0039-128x(96)00007-4. [DOI] [PubMed] [Google Scholar]
- O’Mahony F, Harvey BJ. Sex and oestrous cycle-dependent rapid protein kinase signalling actions of estrogen in distal colonic cells. Steroids. 2008;73:889–894. doi: 10.1016/j.steroids.2008.01.021. [DOI] [PubMed] [Google Scholar]
- O’Mahony F, Alzamora R, Betts V, LaPaix F, Carter D, Irnaten M, Harvey BJ. Female gender-specific inhibition of KCNQ1 channels and chloride secretion by 17β-estradiol in rat distal colonic crypts. J Biol Chem. 2007;282:24563–24573. doi: 10.1074/jbc.M611682200. [DOI] [PubMed] [Google Scholar]
- Resta-Lenert S, Smitham J, Barrett KE. Epithelial dysfunction associated with the development of colitis in conventionally housed mdr1a–/– mice. Am J Physiol Gastrointest Liver Physiol. 2005;289:G153–G162. doi: 10.1152/ajpgi.00395.2004. [DOI] [PubMed] [Google Scholar]
- Richard C, Gao J, Brown N, Reese J. Aquaporin water channel genes are differentially expressed and regulated by ovarian steroids during the periimplantation period in the mouse. Endocrinology. 2003;144:1533–1541. doi: 10.1210/en.2002-0033. [DOI] [PubMed] [Google Scholar]
- Shen L, Turner JR. Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. Am J Physiol Gastrointest Liver Physiol. 2006;290:G577–G582. doi: 10.1152/ajpgi.00439.2005. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Sumanasekera WK, Sumanasekera GU, Mattingly KA, Dougherty SM, Keynton RS, Klinge CM. Estradiol and dihydrotestosterone regulate endothelial cell barrier function after hypergravity-induced alterations in MAPK activity. Am J Physiol Cell Physiol. 2007;293:C566–C573. doi: 10.1152/ajpcell.00418.2006. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hara H. Quercetin enhances intestinal barrier function through the assembly of zonula occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J Nutr. 2009;139:965–974. doi: 10.3945/jn.108.100867. [DOI] [PubMed] [Google Scholar]
- Thomas ML, Xu X, Norfleet AM, Watson CS. The presence of functional estrogen receptors in intestinal epithelial cells. Endocrinology. 1993;132:426–430. doi: 10.1210/endo.132.1.8419141. [DOI] [PubMed] [Google Scholar]
- Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- Verdu EF, Deng Y, Bercik P, Collins SM. Modulatory effects of estrogen in two murine models of experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2002;283:G27–G36. doi: 10.1152/ajpgi.00460.2001. [DOI] [PubMed] [Google Scholar]
- Vetrano S, Rescigno M, Cera MR, Correale C, Rumio C, Doni A, Fantini M, Sturm A, Borroni E, Repici A, Locati M, Malesci A, Dejana E, Danese S. Unique role of junctional adhesion molecule-A in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology. 2008;135:173–184. doi: 10.1053/j.gastro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacol Biochem Behav. 1999;64:777–785. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Wada-Hiraike O, Imamov O, Hiraike H, Hultenby K, Schwend T, Omoto Y, Warner M, Gustafsson JA. Role of estrogen receptor β in colonic epithelium. Proc Natl Acad Sci U S A. 2006a;103:2959–2964. doi: 10.1073/pnas.0511271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada-Hiraike O, Warner M, Gustafsson JA. New developments in oestrogen signalling in colonic epithelium. Biochem Soc Trans. 2006b;34:1114–1116. doi: 10.1042/BST0341114. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-β agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150:1817–1825. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao ZL, Pricolo V, Biancani P, Behar J. Role of progesterone signalling in the regulation of G-protein levels in female chronic constipation. Gastroenterology. 2005;128:667–675. doi: 10.1053/j.gastro.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Ye L, Martin TA, Parr C, Harrison GM, Mansel RE, Jiang WG. Biphasic effects of 17-β-estradiol on expression of occludin and transendothelial resistance and paracellular permeability in human vascular endothelial cells. J Cell Physiol. 2003;196:362–369. doi: 10.1002/jcp.10315. [DOI] [PubMed] [Google Scholar]