Abstract
We performed these experiments to determine if repeated exposure to episodic hypoxia induces long term facilitation (LTF) in anaesthetized spontaneously breathing rats. A previous study in spontaneously breathing rats was unable to demonstrate evidence of LTF with repeated hypoxia, but this may have been due to the low number of hypoxic episodes used. We hypothesized that with sufficient exposure, episodic hypoxia LTF of genioglossus (GG), hyoglossus (HG) and diaphragm (Dia) activities would be elicited. Experiments were performed in 24 anaesthetized spontaneously breathing rats with intact vagi. Peak and tonic GG, HG and Dia EMG activities were recorded before, during and for 1 h following exposure to eight (n= 8) or three (n= 8) episodes of isocapnic hypoxia (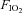 = 0.1) each of 3 min duration. A third time control series was also performed with exposure to normoxia alone (
= 0.1) each of 3 min duration. A third time control series was also performed with exposure to normoxia alone (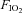 = 0.28, n= 8). Short-term potentiation of GG and HG muscle activity developed during the early period after repeated exposure to eight and three hypoxic episodes. LTF, however, occurred only after eight hypoxic episodes. This manifested as an increase in peak GG and Dia inspiratory muscle activity and tonic HG activity. LTF of respiratory breathing frequency was also induced, reflected by a reduction in inspiratory and expiratory time. These findings support our initial hypothesis that LTF in the anaesthetized, spontaneously breathing rat is dependent on the number of exposures to hypoxia and show that the responses to repetitive hypoxia are composed of both short and long-term facilitatory changes.
= 0.28, n= 8). Short-term potentiation of GG and HG muscle activity developed during the early period after repeated exposure to eight and three hypoxic episodes. LTF, however, occurred only after eight hypoxic episodes. This manifested as an increase in peak GG and Dia inspiratory muscle activity and tonic HG activity. LTF of respiratory breathing frequency was also induced, reflected by a reduction in inspiratory and expiratory time. These findings support our initial hypothesis that LTF in the anaesthetized, spontaneously breathing rat is dependent on the number of exposures to hypoxia and show that the responses to repetitive hypoxia are composed of both short and long-term facilitatory changes.
Repeated exposure to episodic hypoxia or carotid sinus nerve stimulation has been shown to induce a long lasting enhancement of respiratory motor output to chest wall and upper airway (UA) muscles in a number of different species, referred to as long-term facilitation (LTF) (Millhorn et al. 1980; Fregosi & Mitchell, 1994; Turner & Mitchell, 1997; Cao et al. 1992; Fuller, 2005). Respiratory LTF in response to repetitive exposure to hypoxia is proposed to be important for the protection of respiratory homeostasis during sleep, especially in adults who suffer from recurrent obstructive sleep apnoea (OSA) (Suratt et al. 1988). LTF of hypoglossal nerve activity could increase UA muscle tone helping to maintain airway patency. Fuller (2005) recently demonstrated in the anaesthetized, vagotomized, artificially ventilated rat that hypoxia-induced LTF is reflected as an increase in inspiratory neural drive to both tongue protrudor and retractor muscle groups.
Airway obstruction is also accompanied by the generation of negative transmural pressure below the site of obstruction. We, and others, have shown in an anaesthetized rat model that inspiratory co-activation of UA dilator and retractor tongue muscles occurs under baseline conditions (Fuller et al. 1998, 1999; Bailey & Fregosi, 2004) and is further recruited by upper airway negative pressure (UANP) (Ryan et al. 2001). There is general agreement that UANP is an important and sensitive respiratory signal indicating that airway patency may be compromised. Obstructive apnoeas during sleep will lead to repeated episodes of UANP as well as repeated exposures to hypoxia. We do not know if repeated exposure to UANP results in LTF of UA muscle activity, nor how repeated exposure to UANP might interact with or modulate the LTF induced by repeated exposure to hypoxia.
An investigation of the interaction between hypoxia and UANP represents a challenge in experimental design. LTF has been most extensively investigated in anaesthetized, paralysed, artificially ventilated, vagotomized rats as it is under these conditions that the phenomenon is most clearly expressed, manifest as a persistent augmentation of phrenic (Baker & Mitchell, 2000; Fuller et al. 2000; Bavis & Mitchell, 2003) and hypoglossal nerve activities (Bach & Mitchell, 1996; Fuller et al. 2001a; Fuller, 2005). However, we have shown that the reflex UA motor response to UANP is markedly reduced in paralysed ventilated rats (Ryan et al. 2003) but is clearly expressed in anaesthetized spontaneously breathing animals. This means that the optimal experimental conditions for the study of LTF are very poor experimental conditions for the study of UANP. Furthermore, the ability to detect LTF even within the same species is variable under different conditions (Mitchell et al. 2001b). For example, Olson et al. (2001) recently demonstrated ventilatory LTF in unanaesthetized spontaneously breathing rats, but LTF was not found in anaesthetized, spontaneously breathing rats following exposure to intermittent hypoxia (Janssen & Fregosi, 2000). Failure to induce LTF in the latter may have been attributed to the low number (3 × 5 min episodes) of repeated exposures to hypoxia. McGuire et al. (2002) showed that the number (greater than 5 episodes) and severity (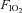 = 0.1) of hypoxic exposure is critical in determining the magnitude of LTF, at least in the awake rat.
= 0.1) of hypoxic exposure is critical in determining the magnitude of LTF, at least in the awake rat.
This is a preliminary investigation with vagi intact, as opposed to the more conventional anaesthetized, vagotomized, paralysed and artificially ventilated model This model was chosen as we have shown that the reflex UA motor response to UANP is markedly reduced in paralysed ventilated rats (Ryan et al. 2003) but is clearly expressed in anaesthetized spontaneously breathing animals. Therefore, given the known utility of the anaesthetized spontaneously breathing rat with intact vagi for studies of the reflex response to UANP (Ryan et al. 2001) we set out to determine the conditions under which hypoxic LTF could be induced in this animal model. We hypothesized that exposure to sufficient numbers of episodic hypoxia would induce LTF of tongue protrudor (genioglossus, GG) and retractor (hyoglossus, HG) muscles and the diaphragm in anaesthetized spontaneously breathing rats with intact vagi. We tested this hypothesis by examining the effect of exposure to eight episodes of hypoxia (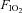 = 0.1), each of 3 min duration, on the activity of these muscle groups. We compared the responses obtained with those following exposure to three 3 min episodes of hypoxia.
= 0.1), each of 3 min duration, on the activity of these muscle groups. We compared the responses obtained with those following exposure to three 3 min episodes of hypoxia.
Methods
General procedures
Experiments were conducted on 24 adult male Wistar rats (weight ranging from 280 to 350 g). The work was performed under license from the Department of Health and Children, Ireland, and conformed to national and university guidelines regarding animal experimentation. Surgical anaesthesia was induced with sodium pentobarbitone (60 mg kg−1i.p.; Sagatal, Rhone Merieux, Ireland) and maintained with intravenous infusion of Saffan (alphaxalone/alphadalone, 4–8 mg ml−1 h−1i.v.) to maintain a stable systemic arterial pressure and respiratory rate as well as to suppress reflex withdrawal, arterial pressure and respiratory responses to paw pinch. The rats were placed in the supine position on a thermostatically controlled heating blanket (Harvard homeothermic blanket system, Harvard Apparatus, Edenbridge, UK) to maintain rectal temperature close to 37°C. The right femoral artery and vein were cannulated to record systemic arterial pressure (Statham P23Dd, Heto Rey, PR, USA) and for injection of drugs, respectively. The left femoral vein was cannulated to allow infusion of Saffan anaesthetic.
Animals were tracheostomized and the recurrent laryngeal nerves were preserved bilaterally. The animals were allowed to breathe spontaneously through the tracheal cannula, which was attached to a T-piece. A gas-mixing apparatus, comprising a bank of rotameters, one each for O2, N2 and CO2, delivered the inspired gas mix to one arm of this T-piece at a total gas flow of approximately 1 l min−1, and exhaled gases and excess fresh gas flowed out through the other arm of the T-piece. An oxygen-rich mix (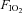 = 0.28) was normally delivered to the T-piece to maintain normal arterial oxygenation. Where required, animals could be exposed to isocapnic hypoxia by reducing the flow of O2 and adding CO2 to the inspired gas mix. We determined in a preliminary series of experiments that simultaneously reducing
= 0.28) was normally delivered to the T-piece to maintain normal arterial oxygenation. Where required, animals could be exposed to isocapnic hypoxia by reducing the flow of O2 and adding CO2 to the inspired gas mix. We determined in a preliminary series of experiments that simultaneously reducing 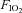 to 0.1 and increasing
to 0.1 and increasing 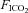 to 0.1 reliably reduced
to 0.1 reliably reduced 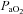 to approximately 40 mmHg while maintaining a
to approximately 40 mmHg while maintaining a 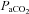 of approximately 45 mmHg. We used a standard hypoxic exposure of 3 min duration followed by a return to normoxic conditions for a 5 min period.
of approximately 45 mmHg. We used a standard hypoxic exposure of 3 min duration followed by a return to normoxic conditions for a 5 min period.
Electromyography (EMG) recording
All EMG recordings were obtained using disposable concentric EMG needles (diameter 0.6 mm/23 G, length 75 mm, SLE Diagnostics, Surrey, UK). This included recordings of the right genioglossus (GG), hyoglossus (HG) and diaphragm (Dia) muscle activities in all animals (n= 24). Activity was amplified (Neurolog NL100AK pre-amplifier and NL104 amplifier, Digitimer, Welwyn Garden City, UK), filtered (0.3–2 kHz, NL 125, Digitimer), processed by a leaky integrator (time constant 100 ms, NL 703, Digitimer) and fed to an audio monitor and an oscilloscope. Amplification settings were similar for all EMG recordings. EMG activity and with systemic arterial pressure and UA pressure were recorded and stored on computer using a CED micro1401 interface and Spike 2 software (CED, Cambridge, UK).
Experimental protocols
After all surgical procedures were complete, a period of at least 30 min was allowed to stabilize the preparation. Three series of experiments were performed involving 24 animals in total. Baseline EMG activity was first established in normoxic normocapnic conditions (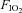 = 0.28, see Table 1 for blood gas data). Eight rats were exposed to 3 × 3 min episodes of isocapnic hypoxia separated by 5 min normoxia exposure. Eight rats were exposed to 8 × 3 min episodes of isocapnic hypoxia separated by 5 min normoxia exposure as described above. During each 3 min hypoxic episode, GG, HG and Dia EMG activity was measured at 150 s into the hypoxic challenge.
= 0.28, see Table 1 for blood gas data). Eight rats were exposed to 3 × 3 min episodes of isocapnic hypoxia separated by 5 min normoxia exposure. Eight rats were exposed to 8 × 3 min episodes of isocapnic hypoxia separated by 5 min normoxia exposure as described above. During each 3 min hypoxic episode, GG, HG and Dia EMG activity was measured at 150 s into the hypoxic challenge.
Table 1.
Mean arterial blood gases
| Baseline | Episodic hypoxia | LTF hour early | LTF hour middle | LTF hour late | ||
|---|---|---|---|---|---|---|
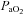 |
Normoxia | 107.4 ± 0.8 | 108.1 ± 1 | 106.8 ± 0.8 | 106.3 ± 0.8 | 108.7 ± 0.3 |
| 3 × 3 Hypoxia | 106.4 ± 3.1 | 41.9 ± 1.2 | 109.5 ± 1.7 | 108.7 ± 1.8 | 110.5 ± 1.6 | |
| 8 × 3 Hypoxia | 112.5 ± 3.3 | 38.6 ± 0.4 | 118.9 ± 2.5 | 121.2 ± 3.2 | 116.6 ± 3.2 | |
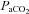 |
Normoxia | 44.6 ± 1.7 | 45.7 ± 0.7 | 44.0 ± 1.7 | 45.8 ± 1.7 | 44.7 ± 0.6 |
| 3 × 3 Hypoxia | 46.1 ± 2.2 | 45.8 ± 1 | 46.3 ± 1.4 | 43.5 ± 2.3 | 45.4 ± 1.6 | |
| 8 × 3 Hypoxia | 44.4 ± 1.2 | 43.3 ± 0.6 | 44.4 ± 1.2 | 43.8 ± 1.2 | 43.8 ± 1 | |
| pH | Normoxia | 7.32 ± 0.01 | 7.34 ± 0.01 | 7.32 ± 0.02 | 7.31 ± 0.025 | 7.32 ± 0.02 |
| 3 × 3 Hypoxia | 7.31 ± 0.01 | 7.32 ± 0.17 | 7.32 ± 0.01 | 7.35 ± 0.01 | 7.34 ± 0.01 | |
| 8 × 3 Hypoxia | 7.32 ± 0.01 | 7.33 ± 0.01 | 7.34 ± 0.01 | 7.34 ± 0.02 | 7.34 ± 0.01 |
Group mean (±s.e.m.) data showing partial pressure of arterial carbon dioxide (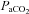 ), partial pressure of arterial oxygen (
), partial pressure of arterial oxygen (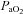 ), and pH during experimental protocols where spontaneously breathing anaesthetized rats were exposed to eight (n= 8) or three (n= 8) 3 min episodes of intermittent hypoxia or normoxia alone (n= 8).
), and pH during experimental protocols where spontaneously breathing anaesthetized rats were exposed to eight (n= 8) or three (n= 8) 3 min episodes of intermittent hypoxia or normoxia alone (n= 8).
Activity was also recorded on the first and fifth minute during the 5 min of normoxia between successive hypoxic challenges. After the final hypoxic challenge, inspired gases were returned to normoxic inspired mixtures and EMG activity was measured 1 min later and every 10 min thereafter for 1 h to determine if LTF was induced. In the third series of experiments (n= 8) EMG activity was recorded under control conditions (sham hypoxia) in which normoxia was maintained throughout the protocol and EMG activity was measured at similar time points to that in the other two series.
Blood gases were taken at the commencement of the experimental protocol, immediately before each hypoxic exposure, approximately 150 s into each hypoxic exposure, and at 5, 20, 40 and 60 min into the post-hypoxia hour. Arterial blood samples were withdrawn in 200 μl aliquots and were analysed immediately after sampling for blood gases and pH. The maximum number of samples per animal was 21. When a sample for arterial blood gas analysis was withdrawn, approximately 300 μl of normal saline was administered to maintain intravascular volume.
Data analysis
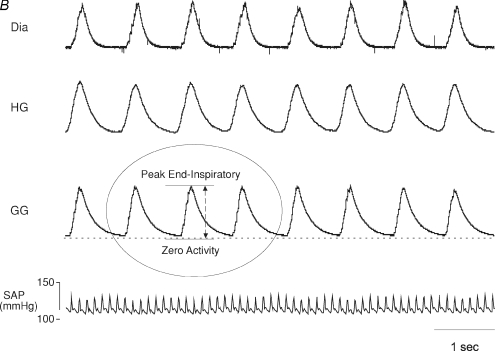
The activity of all muscles was quantified in arbitrary units. Peak activity was quantified, as this has been the most commonly chosen measurement in previous studies of LTF and was calculated as the difference between peak activity reached during end-inspiration and zero activity measured at the end of each experiment after the animal died (Fig. 1B). Peak activity of GG, HG and Dia inspiratory EMG activities were calculated under baseline conditions, after 150 s into each 3 min hypoxic episode with comparative timed breaths used in control experiments where animals were exposed only to normoxia, at 1 and 5 min in the intervening normoxic period between successive hypoxic challenges, and finally after completion of all hypoxic episodes at 1, 10, 20, 30, 40, 50 and 60 min in the post-hypoxic hour. Peak activity was then expressed as a percentage change from baseline activity. Tonic EMG activity was also measured from GG and HG recordings as the difference between zero and end-expiratory activity on the breath analysed.
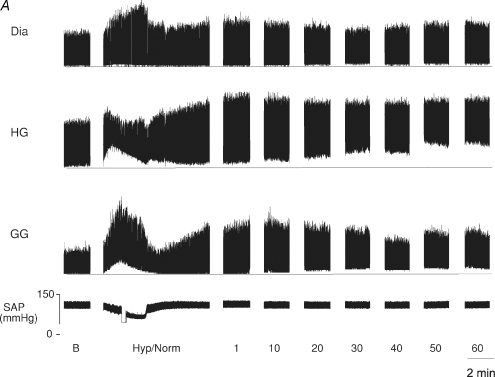
Figure 1. A, effect of episodic hypoxia on upper airway (UA), and respiratory pump muscle activities in anaesthetized spontaneously breathing rat.
Original record shows genioglossus (GG), hyoglossus (HG), and diaphragm (Dia) muscle activities and systemic arterial pressure (SAP) under baseline conditions (B), during a single 3 min hypoxic episode followed by 5 min normoxia (Hyp/Norm), and at separate time points over 1 h following exposure to eight hypoxic episodes. The horizontal line in each muscle recording represents zero activity; amplification settings were similar for all EMG recordings. B, method used to measure peak muscle activity This expanded time base demonstrates the muscle recordings obtained and the method used to measure peak activity. Peak muscle activity was calculated as the difference between peak end-inspiratory activity and zero activity (as measured after the animal had died). Genioglossus (GG), hyoglossus (HG), and diaphragm (Dia) muscle activities and systemic arterial pressure (SAP) under baseline conditions.
Inspiratory time (TI) was recorded as the interval between the onset of diaphragm EMG activity and the beginning of its sharp decline at the end of inspiration. Total respiratory cycle time (TTOT) was measured as the interval between the onset of diaphragm inspiratory activity and the onset of the inspiratory burst of the next respiratory cycle. We calculated expiratory time (TE) by subtracting TI from TTOT. Respiratory frequency was measured as the average number of breaths during a 1 min period.
Data were normalized by expression as a percentage change from baseline, as is conventional for studies of LTF and to allow direct comparison with other studies. We also statistically analysed the raw data and this analysis did not change any of the conclusions of our study. Analysis of variance (ANOVA) for repeated measures was used to test statistical hypothesis. The Student–Newman–Keuls and Dunnett's tests were used for post hoc multiple comparisons. Data were log transformed when required to eliminate skewness or to homogenize variance across groups. P < 0.05 was accepted as indicating a statistically significant effect.
Results
The group mean (±s.e.m., n= 24) 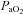 ,
, 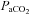 , and pH values are shown in Table 1 for each of the three series of experiments performed. The values obtained in our anaesthetized, spontaneously breathing rats are comparable to other studies using either spontaneously breathing or paralysed, vagotomized artificially ventilated anaesthetized rats (Janssen & Fregosi, 2000; Fuller, 2005). There was no statistically significant difference in the
, and pH values are shown in Table 1 for each of the three series of experiments performed. The values obtained in our anaesthetized, spontaneously breathing rats are comparable to other studies using either spontaneously breathing or paralysed, vagotomized artificially ventilated anaesthetized rats (Janssen & Fregosi, 2000; Fuller, 2005). There was no statistically significant difference in the 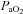 ,
, 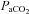 , or pH recorded in animals exposed to three episodes of hypoxia when compared to those exposed to eight.
, or pH recorded in animals exposed to three episodes of hypoxia when compared to those exposed to eight.
Figure 1A is an example of an original record taken from one animal following exposure to eight episodes of hypoxia. We observed three general phenomena, which can be seen in this original record and are further demonstrated in the group mean data. First, there were immediate changes in respiratory muscle activity in response to hypoxia. Specifically, there was a marked increase in phasic and peak GG activity and a smaller increase in tonic discharge. In contrast, hypoxia produced a large increase in tonic HG activity with little or no change, in peak and a reduction in phasic activity. Peak Dia activity also increased during hypoxia. Second, there were short-term changes in the activity of all three muscles in the minutes following each hypoxic exposure. Third, where animals were exposed to eight hypoxic challenges, there were long-term changes (LTF) in UA and Dia muscle activities that developed and were maintained for up to 1 h (Fig. 1A).
Effect of episodic hypoxia on genioglossus activity
Baseline GG EMG activity was similar in all three protocols performed. Hypoxia significantly increased peak GG activity with similar changes in activity obtained during each episode whether animals were exposed to three or eight hypoxic episodes (P < 0.0001, Figs 1A and 2). Between each hypoxic episode, during the intervening 5 min of normoxia, short-term potentiation of inspiratory GG EMG activity was detectable on the first (except after the first hypoxic episode in animals exposed to 8 × 3 min hypoxic episodes) and fifth minute of normoxia (P < 0.001, Figs 1A and 2). There was no significant difference between the activity at these two times points in any post-hypoxic 5 min period.
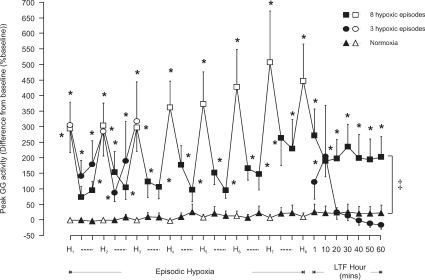
Figure 2. Group mean (±s.e.m., n= 8) effect of the number of hypoxic episodes on peak genioglossus muscle activity in anaesthetized rats.
The change in peak genioglossus (GG) muscle activity expressed as a percentage of baseline activity, after 150 s into each 3 min hypoxic episode (H, open symbols), on the first and fifth minute during the intervening 5 min period of normoxia between successive hypoxic challenges (dashed lines, filled symbols), and at separate time points over one hour following exposure to the last hypoxic challenge in animals exposed either to eight (squares) or three (circles) hypoxic episodes or to normoxia alone (triangles). *Significant change in peak GG activity from baseline (P < 0.05). ‡Significant difference at all time points during LTF hour from normoxia alone.
LTF of GG muscle activity was induced following exposure to eight hypoxic episodes with peak inspiratory GG EMG activity being significantly greater than baseline at all times (P < 0.01, Figs 1A, 2 and 7A). This facilitation of GG activity was early in onset and was maintained for 1 h without significant attenuation. GG activity was significantly greater than baseline (pre-hypoxic) activity and also significantly greater than that recorded at comparable time points in animals exposed only to normoxia (P < 0.001, Figs 2 and 7A). In contrast, exposure to three episodes of hypoxia failed to induce LTF of GG activity with the only significant change in activity measured at 10 min following the last hypoxic episode (P= 0.028, Figs 2 and 7A).
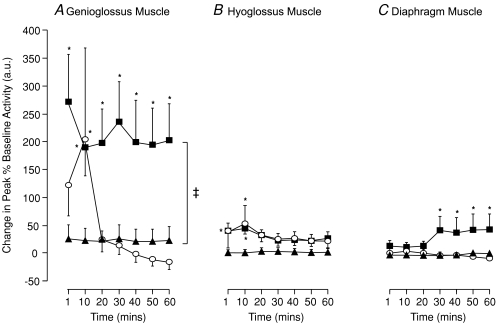
Figure 7. Effect of hypoxic number on long-term facilitation (LTF) of upper airway and respiratory pump muscle activity (group means ±s.e.m.) in anaesthetized spontaneously breathing rats.
The change in peak genioglossus (A), hyoglossus (B) and diaphragm (C) muscle activities, expressed as a percentage of baseline, during the 60 min following exposure to eight (filled squares, n= 8), or three (open circles, n= 8) episodes of intermittent hypoxia or normoxia alone (filled triangles, n= 8). *Significant difference from baseline (P < 0.05). ‡Significant difference at all time points during LTF hour from normoxia alone.
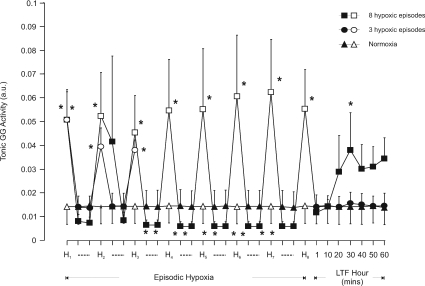
Tonic GG activity at baseline was similar in all three experimental protocols (Fig. 3). Hypoxia significantly increased tonic GG EMG activity (P < 0.05) and this increase was similar during each hypoxic challenge (Fig. 3). Between hypoxic episodes tonic activity was significantly reduced below baseline on both the first and fifth minute following the third and subsequent hypoxic episode in those animals exposed to eight episodes (P < 0.05, Fig. 3). Following eight hypoxic exposures there was a trend for tonic GG activity to increase over the next 60 min but this increase failed to reach statistical significance except for that measured at 30 min (P= 0.024). No effect on tonic activity was obtained in the 60 min after exposure to three hypoxic challenges (Fig. 3, n= 8).
Figure 3. Group mean (±s.e.m., n= 8) effect of the number of hypoxic episodes on tonic genioglossus muscle activity in anaesthetized rats.
Tonic genioglossus (GG) muscle activity at baseline, after 150 s into each 3 min hypoxic episode (H, open symbols), on the first and fifth minute during the intervening 5 min period of normoxia (dashed lines, filled symbols), and at separate time points over one hour following the last hypoxic challenge in animals exposed either to eight (squares) or three (circles) hypoxic episodes or to normoxia alone (triangles). *Significant effect (P < 0.05) compared to baseline.
In those animals exposed only to normoxia (n= 8) no significant change in peak GG inspiratory activity or tonic discharge was obtained (P > 0.05, Figs 2, 3 and 7A) at any time point during the experimental protocol.
Effect of episodic hypoxia on hyoglossus activity
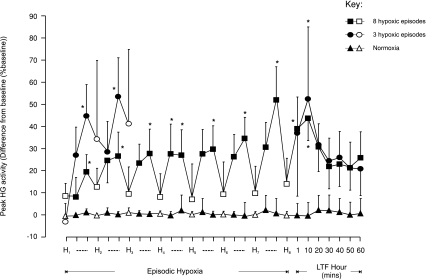
No significant difference between baseline HG activities was detected among the three experimental protocols. Hypoxia tended to increase peak HG activity but this effect was not statistically significant (Fig. 4). Following each episode of hypoxia, a moderate post-hypoxic augmentation of peak inspiratory HG EMG activity developed, which was statistically significant on the fifth minute (P < 0.001, Fig. 4).
Figure 4. Group mean (±s.e.m., n= 8) effect of the number of hypoxic episodes on peak hyoglossus muscle activity in anaesthetized rats.
The change in peak hyoglossus (HG) muscle activity expressed as a percentage of baseline activity, after 150 s into each 3 min hypoxic episode (H, open symbols), on the first and fifth minute during the intervening 5 min period of normoxia between hypoxic challenges (dashed lines, filled symbols), and at separate time points over 60 min following the last hypoxic episode in animals exposed either to eight (squares) or three (circles) hypoxias or to normoxia alone (triangles). *Significant change in peak HG activity from baseline (P < 0.05).
While a small significant post-hypoxic increase in peak HG activity occurred at 1 and 10 min after the last exposure to hypoxia in animals exposed to eight hypoxic episodes (P < 0.0001, Figs 4 and 7B) and at 10 min following three hypoxic challenges (P= 0.0091, Figs 4 and 7B), we did not demonstrate LTF of peak HG activity over the subsequent hour. Mean peak HG activity remained approximately 20% above baseline and time-control levels, but this difference was not statistically significant.
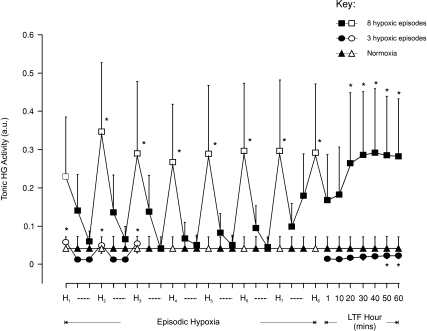
Episodic hypoxia significantly increased (P < 0.05, Figs 1A and 5) tonic HG EMG activity (except on the first hypoxic challenge in those animals exposed to 8 × 3 min hypoxic challenges). LTF of tonic HG discharge was observed (Fig. 1A) in rats exposed to eight episodes of hypoxia. This increase in tonic discharge was statistically significant at 20 min and was sustained for the remainder of that hour following the last episode of hypoxia (P < 0.05, Fig. 5). Tonic HG activity was almost unchanged for most of the hour following three episodes of hypoxia, with only a very small but significant increase occurring at 50 and 60 min (P < 0.05, Fig. 5).
Figure 5. Group mean (±s.e.m., n= 8) effect of the number of hypoxic episodes on tonic hyoglossus muscle activity in anaesthetized rats.
Tonic hyoglossus (HG) muscle activity at baseline, 150 s into each 3 min hypoxic episode (H, open symbols), on the first and fifth minute during the intervening five minute period of normoxia (dashed lines, filled symbols), and at separate time points over 60 min following the last hypoxic challenge in animals exposed either to eight (squares) or three (circles) hypoxic episodes or to normoxia alone (triangles). *Significant effect (P < 0.05) compared to baseline.
Peak inspiratory and tonic HG activity did not change significantly during normoxic time-controlled experiments (P > 0.05, Figs 4, 5 and 7B, n= 8).
Effect of episodic hypoxia on peak diaphragm activity and respiratory timing
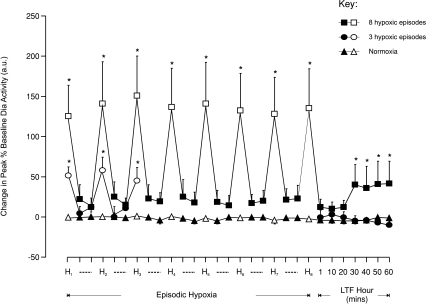
Peak Dia activity under baseline normoxic conditions was similar in each protocol and remained unchanged in those time-control experiments where animals were not exposed to hypoxia. Hypoxia significantly increased peak Dia activity with the change in peak activity being similar during each hypoxic episode whether animals were exposed to eight or three hypoxic episodes (P < 0.001, Fig. 6). While it appears from Fig. 6 that the acute Dia response to hypoxia is greater in those animals exposed to eight hypoxic episodes, there was a large variance in the acute response to hypoxia, and the difference between the means was not significant.
Figure 6. Group mean (±s.e.m., n= 8) effect of the number of hypoxic episodes on peak diaphragm muscle activity in anaesthetized rats.
The change in peak diaphragm (Dia) muscle activity expressed as a percentage of baseline activity, 150 s into each 3 min hypoxic episode (H, open symbols), on the first and fifth minute during the intervening 5 min period of normoxia between hypoxic episodes (dashed lines, filled symbols), and at separate time points over 60 min following the last hypoxic challenge in animals exposed either to eight (squares) or three (circles) hypoxias or to normoxia alone (triangles). *Significant change in peak Dia activity from baseline (P < 0.05).
There were small but non-significant increases in Dia activity during the 5 min normoxic interval between hypoxic episodes. Immediately after exposure to the last of eight episodes of hypoxia, peak Dia activity returned towards baseline levels. However, a significant augmentation in activity developed at 30 min and was maintained for the remainder of that hour (P < 0.036, Figs 6 and 7C). Three 3 min hypoxic exposures, however, had no significant effect on peak Dia EMG activity at any time point after the last episode of hypoxia (Figs 6 and 7C).
Effect of episodic hypoxia on respiratory timing
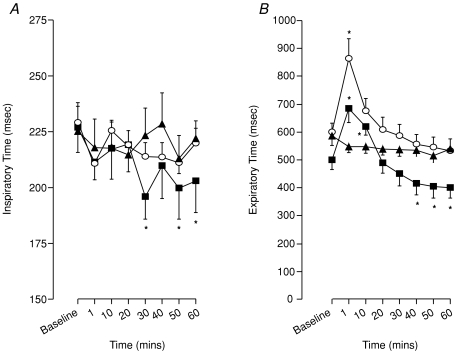
Inspiratory time (TI) was significantly reduced compared to baseline at 30, 50 and 60 min following exposure to eight episodes of hypoxia (P < 0.01, Fig. 8A) with the reduction in inspiratory time at 40 min just outside significance levels (P= 0.051). No effect on TI was detected after exposure to 3 × 3 min episodes of hypoxia (Fig. 8A).
Figure 8. Effect of hypoxic number on ventilatory long-term facilitation (LTF) in anaesthetized spontaneously breathing rats.
Inspiratory time (A) and expiratory time (B) following exposure to eight (squares) and three (circles) episodes of intermittent hypoxia or normoxia alone (triangles). *Significant difference from baseline (P < 0.05).
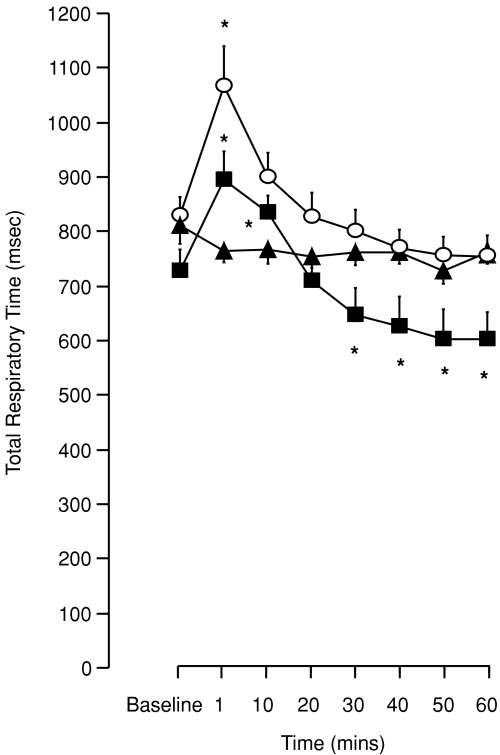
A biphasic change in expiratory and total respiratory times was recorded in animals exposed to eight episodes of hypoxia. In particular, an immediate but transient prolongation of expiratory time (TE) occurred at 1 and 10 min (P < 0.01, Fig. 8B) before returning to baseline levels. At times 40, 50 and 60 min, however, TE was found to be significantly reduced compared to baseline and time-control (P < 0.001, Fig. 8B). A similar pattern of change was found in total respiratory cycle time (TTOT); an initial prolongation of TTOT (1 and 10 min, P < 0.017) followed by a significant reduction in TTOT at times 30, 40, 50 and 60 min (P < 0.03, Fig. 9). After three hypoxic episodes, both TE and TTOT showed only an initial prolongation at 1 min (P < 0.038, Figs 8B and 9) with there being no significant difference from baseline in either variable over the next hour.
Figure 9. Effect of hypoxic number on ventilatory long-term facilitation (LTF) in anaesthetized spontaneously breathing rats.
Total respiratory time following exposure to eight (squares) and three (circles) episodes of intermittent hypoxia or normoxia alone (triangles). *Significant difference from baseline (P < 0.05).
Finally, there was no change in any variable measured (peak, TI, TE, TTOT or frequency) in those animals exposed only to normoxia (Figs 8 and 9).
Discussion
This is the first study to describe LTF of UA dilator (GG) and retractor (HG) tongue muscles and inspiratory pump muscle (Dia) activity in anaesthetized spontaneously breathing adult rats. The principal findings arising from this study include that (i) LTF is dependent on the number of hypoxic exposures in that it is induced following eight but not three episodes of hypoxia, (ii) repetitive hypoxia also results in short-term potentiation of UA muscle activity, (iii) LTF is manifest as an increase in peak GG and Dia inspiratory muscle activity, but as a persistent increase in tonic HG activity, and (iv) repeated exposure to episodic hypoxia induced LTF of respiratory frequency reflected by a reduction in inspiratory and expiratory time. These findings support our initial hypothesis that the number of exposures to hypoxia is a key determinant of LTF at least in the anaesthetized, spontaneously breathing rat.
A major objective of the present study was to demonstrate LTF of UA and respiratory pump muscle activity in an anaesthetized, spontaneously breathing rat given the known benefit of this animal model for studies of the reflex response to UANP (Ryan et al. 2001) and hence its potential utility for investigation of whether LTF of UA muscle activity may also be expressed in response to repeated exposure to UANP stimuli. Our experiments clearly show that there are two separate components to the GG muscle response following exposure to intermittent hypoxia. The number of hypoxic episodes influences expression of these two components. The early response is short-term potentiation (STP) of peak GG activity, with activity maintained at a higher level immediately after cessation of hypoxia and slowly declining towards baseline over a period of 5–10 min (Eldridge, 1980; Mateika & Fregosi, 1997; Golder et al. 2005). This is clearly seen in those animals exposed to three hypoxic challenges. The mechanism for STP remains unknown but the number of hypoxic exposures, duration and severity of hypoxia (Menendez et al. 1999; Dahan et al. 1995), post-hypoxic inspired oxygen concentration (Dahan et al. 1995) and genetic factors (Golder et al. 2005) have all been implicated as important modifiers of its expression.
Following eight hypoxic episodes this early potentiation of activity persists as a long-lasting enhancement of peak GG activity, or LTF, with activity remaining elevated for the remainder of the next hour. LTF was not seen, however, after three episodes of hypoxia. Thus, our experiments show that exposure to a critical number of hypoxic events is required to induce LTF of GG muscle activity. These results support other recent findings demonstrating LTF of motor output in the main trunk (Bach & Mitchell, 1996; Fuller et al. 2001a; Zabka et al. 2005) and individual branches of the rat XII nerve to tongue protrudor and retractor muscles (Fuller, 2005) in anaesthetized, vagotomized artificially ventilated animals, as well as GG tongue muscle activity in spontaneously breathing neonatal rats (McKay et al. 2004) and adult cats (Mateika & Fregosi, 1997). The physiological significance of LTF of UA dilator muscle activity may lie in its potential role as an airway protective response particularly in conditions in which intermittent hypoxia is a frequent occurrence such as sleep-disordered breathing. This is supported by studies in sleeping humans (Shkoukani et al. 2002) demonstrating that repetitive hypoxia reduces UA resistance and increases inspiratory minute ventilation, changes believed to indicate LTF of UA dilator muscle activity.
Episodic hypoxia also induced LTF of tonic activity of the hyoglossus tongue retractor muscle. Similar to the GG, LTF was expressed only after exposure to 8 × 3 min episodes of hypoxia. However, unlike the GG, LTF of HG muscle activity manifest as a persistent increase in tonic HG muscle activity with there only being a small transient increase, or STP, in peak HG activity within the first 10 min after the final hypoxic exposure. This is a novel finding and is the first study we are aware of that demonstrates LTF of tonic muscle discharge. The significance of such a response to episodic hypoxia is unclear. We can only speculate that increasing tonic activity may contribute to the stability of the UA.
Ventilatory LTF also developed only after eight hypoxic episodes with a pattern similar to that seen in other studies of LTF: a significant increase in peak Dia muscle activity and respiratory frequency, which developed slowly over the 30 min following the final hypoxic episode and was sustained for 1 h.
While we would conclude that the number of hypoxic episodes is critical to the development of LTF, our data require that we somewhat temper this conclusion. The acute Dia response for animals exposed to eight episodes of hypoxia was greater than that for those exposed to three episodes. This difference was not statistically significant, because of a large variance in the acute Dia response to hypoxia, nor was there any difference in the level of hypoxia between the two groups. Nonetheless, we cannot completely rule out the possibility that the acute response to hypoxia was greater in this group, or that some other factor stimulated respiratory activity, and together with the greater number of episodes contributed to the appearance of LTF in animals exposed to eight episodes of hypoxia. The only other study of LTF in anaesthetized spontaneously breathing rats (Janssen & Fregosi, 2000) did not show any increase in inspiratory intercostal pump muscle activity. Its authors attributed this to the high baseline CO2 at which episodic hypoxic stimulation was initiated. Similar reasons were believed to explain the absence of LTF of diaphragm muscle activity in vagotomized cats (Mateika & Fregosi, 1997). In the present study, the change in peak Dia EMG activity was ∼42% 60 min after the last hypoxic episode and this change in activity is comparable to previous reports of sustained increases in phrenic nerve amplitude in anaesthetized artificially ventilated (Bach & Mitchell, 1996; Kinkead & Mitchell, 1999; Fuller et al. 2001b) and awake rats (Olson et al. 2001). Interestingly, the latter study found that measurements of ventilatory LTF were low unless correction of hypocapnia to baseline levels after hypoxic exposure was performed, emphasising the importance of isocapnic conditions when inducing LTF. Animal and human studies have shown that the inhibitory effects of hypocapnia also counter-influence STP (Badr et al. 1992; Gleeson & Sweer, 1993; Engwall et al. 1994) so that unless CO2 levels remain steady, STP expression may be variable. We found that although peak Dia activity was increased during the immediate post-hypoxic period, this increase in activity did not reach statistical significance. We do not believe, however, that hypocapnia accounts for the absence of significant STP in Dia activity in our study, as CO2 levels were held relatively constant throughout the experimental protocol (see Table 1). Similarly, it is unlikely that there were large discrepancies between systemic and brain stem 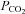 levels which if present may have contributed to the absence of STP in the present study. The discrepancy between UA and respiratory pump muscle STP responses may reflect differences in the pattern of response to hypoxia in the distinct pre-motor neurons controlling these diverse respiratory outflows. In support of this, Golder et al. (2005) found in the rat that genetic differences exist in the time domains of the hypoxic response, and that these are differentially expressed in hypoglossal and phrenic nerves.
levels which if present may have contributed to the absence of STP in the present study. The discrepancy between UA and respiratory pump muscle STP responses may reflect differences in the pattern of response to hypoxia in the distinct pre-motor neurons controlling these diverse respiratory outflows. In support of this, Golder et al. (2005) found in the rat that genetic differences exist in the time domains of the hypoxic response, and that these are differentially expressed in hypoglossal and phrenic nerves.
The time course of changes in respiratory frequency and timing after hypoxia showed a biphasic pattern. The initial effect of episodic isocapnic hypoxia was a reduction in breathing frequency lasting no longer than 10 min in animals exposed to eight hypoxic episodes but was also present after 1 min in the group exposed to three hypoxias. This post-hypoxic frequency decline (PHFD) was reflected by a prolongation of expiratory time over the same time period. PHFD has been reported previously in anaesthetized rats after exposure to hypoxia (Hayashi et al. 1993; Coles et al. 1998; Bach et al. 1999; Fuller, 2005) but not in other species (Cao et al. 1992; Mitchell et al. 2001a) implying that this pattern of ventilatory response may be species dependent. However, following this PHFD, respiratory frequency significantly increased 30 min from the end of the last hypoxic exposure and continued to increase for the remainder of that hour. These changes were again dependent on the number of hypoxic episodes: the phenomenon was detected after eight exposures but not after three. Our findings of an increase in breathing frequency are in agreement with prior studies in anaesthetized artificially ventilated (Bach & Mitchell, 1996) and awake (Olson et al. 2001) rats, goats (Turner & Mitchell, 1997) and ducks (Mitchell et al. 2001a), but not anaesthetized spontaneously breathing (Janssen & Fregosi, 2000) or paralysed artificially ventilated rats (Fuller, 2005).
The results from this study, therefore, show for the first time that under our experimental conditions, LTF can be induced in anaesthetized spontaneously breathing rats with intact vagi. These results are in contrast to the only other previous study in a similar animal model in which no evidence of LTF was found (Janssen & Fregosi, 2000). Such differences may be accounted for in the following ways. First, and most importantly, we have shown that the number and intensity of carotid chemoreceptor stimulations during each hypoxic episode is important in determining the size of ventilatory LTF in this model. In their study, Janssen & Fregosi (2000) used 3 × 5 min episodes of 12–13% hypoxia. This number of hypoxic episodes was chosen based on the number required to evoke LTF in anaesthetized, vagotomized artificially ventilated rats (Bach & Mitchell, 1996). However, McGuire et al. (2002) demonstrated in awake rats that three or five episodes of hypoxia at levels other than 10% were not sufficient to induce LTF but 10, 20 and 72 episodes were. Although we used a shorter duration of exposure to hypoxia (3 versus 5 min), our results are in agreement with those of McGuire et al. (2002) such that a greater number of exposures to episodic hypoxia is more effective in eliciting LTF.
Second, Janssen & Fregosi believed that eucapnic levels of 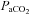 at baseline (∼7–9 Torr above apnoeic threshold of 32.2 ± 2.4 Torr) in spontaneously breathing animals masked LTF expression in their study. In support of this Fregosi & Mitchell (1994) suggested that the level to which
at baseline (∼7–9 Torr above apnoeic threshold of 32.2 ± 2.4 Torr) in spontaneously breathing animals masked LTF expression in their study. In support of this Fregosi & Mitchell (1994) suggested that the level to which 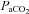 is raised above the apnoeic threshold might be critical for the manifestation of LTF with saturation of the response to sensory stimulation occurring if CO2 is too high (Eldridge et al. 1981). We found, however, that LTF of UA and respiratory pump muscle activity is expressed, under isocapnic conditions, at mean
is raised above the apnoeic threshold might be critical for the manifestation of LTF with saturation of the response to sensory stimulation occurring if CO2 is too high (Eldridge et al. 1981). We found, however, that LTF of UA and respiratory pump muscle activity is expressed, under isocapnic conditions, at mean 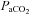 values (45 ± 1.5 mmHg) in excess of those postulated to mask LTF (Janssen & Fregosi, 2000). This is in agreement with a recent study demonstrating LTF of ventilation and genioglossus muscle activity in the presence of elevated levels of carbon dioxide in awake humans (Harris et al. 2006). Of equal importance, maintaining isocapnic conditions throughout the experiment is also crucial as it eliminates hyperventilation induced hypocapnia during hypoxic stimulation and post-hypoxic hypocapnia due to LTF of ventilation, which in turn may have profound inhibitory effects on the ability to express LTF (Olson et al. 2001; McGuire et al. 2002) and STP (Badr et al. 1992; Gleeson & Sweer, 1993; Engwall et al. 1994).
values (45 ± 1.5 mmHg) in excess of those postulated to mask LTF (Janssen & Fregosi, 2000). This is in agreement with a recent study demonstrating LTF of ventilation and genioglossus muscle activity in the presence of elevated levels of carbon dioxide in awake humans (Harris et al. 2006). Of equal importance, maintaining isocapnic conditions throughout the experiment is also crucial as it eliminates hyperventilation induced hypocapnia during hypoxic stimulation and post-hypoxic hypocapnia due to LTF of ventilation, which in turn may have profound inhibitory effects on the ability to express LTF (Olson et al. 2001; McGuire et al. 2002) and STP (Badr et al. 1992; Gleeson & Sweer, 1993; Engwall et al. 1994).
Finally, LTF has most commonly been demonstrated in vagotomized animals, in the belief that the degree of LTF may be attenuated by a long-lasting inhibitory memory elicited by repeated stimulation of lung stretch receptors (Xi et al. 1993). This was demonstrated to be the case in anaesthetized cats (Mateika & Fregosi, 1997) but LTF was not seen in anaesthetized spontaneously breathing rats even with vagi cut (Janssen & Fregosi, 2000). In the present study, we found that vagally intact anaesthetized spontaneously breathing rats exhibit LTF, supporting previous results in unanaesthetized rats (Olson et al. 2001). This suggests perhaps that in the rat at least, vagal inhibitory feedback is not as important a determinant of LTF expression as in other species.
In conclusion, it is clear from our findings that it is possible to induce LTF in anaesthetized spontaneously breathing rats with intact vagi, and that the number of exposures to hypoxia is a key determinant of the response. Our results demonstrating LTF of GG and HG muscle activity lend further support to the proposal that this response may serve as a crucial defence mechanism to help maintain airway patency during sleep, in agreement with studies demonstrating LTF in patients with obstructive sleep apnoea (Babcock & Badr, 1998; Aboubakr et al. 2001). The ability to induce LTF in this animal model allows us to further investigate whether LTF of these UA muscles may also be induced following exposure to repetitive UANP stimulation.
Glossary
Abbreviations
- Dia
diaphragm
- GG
genioglossus
- HG
hyoglossus
- LTF
long-term facilitation
- OSA
obstructive sleep apnoea
- PHFD
post-hypoxic frequency decline
- SAP
systemic arterial pressure
- STP
short-term potentiation
- TI
inspiratory time
- TTOT
total respiratory cycle time
- TE
expiratory time
- UA
upper airway
- UANP
upper airway negative pressure
Author contributions
S.R. performed all experiments in the research study; P.N. was the principal investigator/supervisor for research study.
References
- Aboubakr SE, Taylor A, Ford R, Siddiq S, Badr MS. Long-term facilitation in obstructive sleep apnoea patients during NREM sleep. J Appl Physiol. 2001;91:2751–2757. doi: 10.1152/jappl.2001.91.6.2751. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep. 1998;21:709–716. [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia induced long-term facilitation of respiratory activity is serotonin dependent. Resp Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Bach KB, Kinkead R, Mitchell GS. Post-hypoxia frequency decline in rats: sensitivity to repeated hypoxia and α2-adrenoreceptor antagonism. Brain Res. 1999;817:25–33. doi: 10.1016/s0006-8993(98)01181-0. [DOI] [PubMed] [Google Scholar]
- Badr MS, Skatrud JB, Dempsey JA. Determinants of post-stimulus potentiation in humans during NREM sleep. J Appl Physiol. 1992;73:1958–1971. doi: 10.1152/jappl.1992.73.5.1958. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol. 2004;96:440–449. doi: 10.1152/japplphysiol.00733.2003. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529:215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, Mitchell GS. Intermittent hypoxia induces phrenic long-term facilitation in carotid-denervated rats. J Appl Physiol. 2003;94:399–409. doi: 10.1152/japplphysiol.00374.2002. [DOI] [PubMed] [Google Scholar]
- Cao KY, Zwillich CW, Berton-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol. 1992;73:2083–2088. doi: 10.1152/jappl.1992.73.5.2083. [DOI] [PubMed] [Google Scholar]
- Coles SK, Ernsberger P, Dick TE. Post-hypoxic frequency decline does not depend in α2-adrenergic receptors in the adult rat. Brain Res. 1998;794:267–273. doi: 10.1016/s0006-8993(98)00234-0. [DOI] [PubMed] [Google Scholar]
- Dahan A, Berkenbosch A, DeGoede J, Van Den Elsen M, Olievier I, van Kleef J. Influence of hypoxic duration and post-hypoxic inspired O2 concentration on short-term potentiation of breathing in humans. J Physiol. 1995;488:803–813. doi: 10.1113/jphysiol.1995.sp021012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Gill-Kumar P, Millhorn DE. Input- output relationships of central neural circuits involved in respiration in cats. J Physiol. 1981;311:81–95. doi: 10.1113/jphysiol.1981.sp013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL. Subthreshold central neural respiratory activity and afterdischarge. Resp Physiol. 1980;39:327–343. doi: 10.1016/0034-5687(80)90064-x. [DOI] [PubMed] [Google Scholar]
- Engwall MJA, Smith CA, Dempsey JA, Bisgard GE. Ventilatory after-discharge and central respiratory drive interaction in the awake goat. J Appl Physiol. 1994;76:416–423. doi: 10.1152/jappl.1994.76.1.416. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol. 1994;477:469–479. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD. Episodic hypoxia induces long-term facilitation of neural drive to tongue protruder and retractor muscles. J Appl Physiol. 2005;98:1761–1767. doi: 10.1152/japplphysiol.01142.2004. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long-term facilitation of phrenic motor output. Resp Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genomics. 2001a;4:175–181. doi: 10.1152/physiolgenomics.2001.4.3.175. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Mateika JH, Fregosi JH. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol. 1998;507:265–276. doi: 10.1111/j.1469-7793.1998.265bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol. 1999;519:601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001b;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Gleeson K, Sweer LW. Ventilatory pattern after hypoxic stimulation during wakefulness and NREM sleep. J Appl Physiol. 1993;75:397–404. doi: 10.1152/jappl.1993.75.1.397. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Zabka AG, Bavis RW, Baker-Herman T, Fuller DD, Mitchell GS. Differences in time-dependent hypoxic phrenic responses among inbred rat strains. J Appl Physiol. 2005;98:838–844. doi: 10.1152/japplphysiol.00984.2004. [DOI] [PubMed] [Google Scholar]
- Harris DP, Balasubramanian A, Badr MS, Mateika JH. Long term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1111–1119. doi: 10.1152/ajpregu.00896.2005. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol Regul Integr Comp Physiol. 1993;265:R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Janssen PL, Fregosi RF. No evidence of long term facilitation after episodic hypoxia in spontaneously breathing, anaesthetized rats. J Appl Physiol. 2000;89:1345–1351. doi: 10.1152/jappl.2000.89.4.1345. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxyamidotryptamine. Am J Physiol Regul Integr Comp Physiol. 1999;277:R658–R666. doi: 10.1152/ajpregu.1999.277.3.R658. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol. 1997;82:419–425. doi: 10.1152/jappl.1997.82.2.419. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Effect of episodic number and severity on ventilatory long-term facilitation in awake rats. J Appl Physiol. 2002;93:2155–2161. doi: 10.1152/japplphysiol.00405.2002. [DOI] [PubMed] [Google Scholar]
- McKay L, Janczewski WA, Feldman JL. Episodic hypoxia induces long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol. 2004;557:13–18. doi: 10.1113/jphysiol.2004.064006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez AA, Nuckton TJ, Torres JE, Gozal D. Short-term potentiation of ventilation after different levels of hypoxia. J Appl Physiol. 1999;86:1478–1482. doi: 10.1152/jappl.1999.86.5.1478. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Resp Physiol. 1980;42:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker T, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001b;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Powell FL, Hopkins SR, Milsom WK. Time domains of the hypoxic ventilatory response in awake ducks: episodic and continuous hypoxia. Respir Physiol. 2001a;124:117–128. doi: 10.1016/s0034-5687(00)00197-3. [DOI] [PubMed] [Google Scholar]
- Olson EB, Jr, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchell GS. Ventilatory long-term facilitation in unanaesthetized rats. J Appl Physiol. 2001;91:709–716. doi: 10.1152/jappl.2001.91.2.709. [DOI] [PubMed] [Google Scholar]
- Ryan S, McNicholas WT, O’Regan RG, Nolan PG. Reflex respiratory response to changes in upper airway pressure in the anaesthetized rat. J Physiol. 2001;537:251–265. doi: 10.1111/j.1469-7793.2001.0251k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S, McNicholas WT, O’Regan RG, Nolan P. Upper airway muscle paralysis reduces reflex upper airway motor response to negative transmural pressure in rat. J Appl Physiol. 2003;94:1307–1316. doi: 10.1152/japplphysiol.00052.2002. [DOI] [PubMed] [Google Scholar]
- Shkoukani M, Babcock MA, Badr MS. Effect of episodic hypoxia on upper airway mechanics during NREM sleep. J Appl Physiol. 2002;92:2565–2570. doi: 10.1152/japplphysiol.00938.2001. [DOI] [PubMed] [Google Scholar]
- Suratt P, McTier RF, Wilhoit SC. Upper airway muscle activation is augmented in patients with obstructive sleep apnoea compared with that in normal subjects. Am Rev Respir Dis. 1988;137:889–894. doi: 10.1164/ajrccm/137.4.889. [DOI] [PubMed] [Google Scholar]
- Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol. 1997;499:543–550. doi: 10.1113/jphysiol.1997.sp021947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi L, Smith CA, Saupe KW, Dempsey JA. Effects of memory from vagal feedback on short-term potentiation of ventilation in conscious dogs. J Physiol. 1993;462:547–561. doi: 10.1113/jphysiol.1993.sp019568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Behan M. Ageing and gonadectomy have similar effects on hypoglossal long-term facilitation in male Fischer rats. J Physiol. 2005;563:557–568. doi: 10.1113/jphysiol.2004.077511. [DOI] [PMC free article] [PubMed] [Google Scholar]