Abstract
Obstruction of the upper airway (UA) is associated with episodes of hypoxia and upper airway negative pressure (UANP). In the companion paper it is shown that episodic hypoxia elicits long-term facilitation (LTF) of tongue protrudor, retractor and respiratory pump muscle activity. However, whether repeated exposure to UANP also induces LTF is unknown. We hypothesized that repetitive exposure to UANP would induce LTF of UA and respiratory pump muscle activity and when coupled with hypoxia, as occurs when the UA obstructs, would lead to an even greater facilitation of muscle activity and the response to UANP. Experiments were performed in 24 anaesthetized, spontaneously breathing rats with intact vagi. To induce LTF, UANP stimuli (−10 cmH2O) of 5 s duration were delivered every 30 s for 3 min (± hypoxia). This was repeated eight times over 1 h, each 3 min episode separated by 5 min of normoxia. Genioglossus (GG), hyoglossus (HG) and diaphragm (Dia) muscle activity was recorded before, during and for 1 h following the last exposure to episodic UANP alone (n= 8), UANP and hypoxia together (n= 8) or normoxia alone (n= 8). During the final hour, single pulses of UANP were applied at 1 min and every 10 min thereafter to determine whether LTF of the response to UANP had been induced. Our results show that LTF of GG muscle activity and its response to UANP was induced following exposure to episodic UANP stimuli alone and UANP applied during hypoxia. However, there was no significant difference between these responses. Episodic UANP alone also induced LTF of HG muscle activity but this effect did not manifest until 40 min following the last episode of repeated UANP stimulation. In the presence of hypoxia, no LTF of HG muscle response to UANP was found. In conclusion, episodic UANP stimulation induces LTF of UA dilator and retractor tongue muscles, but no further facilitation occurs when coupled with hypoxia. This response may serve as an important protective mechanism of respiratory homeostasis during sleep, particularly in patients who suffer from obstructive sleep apnoea.
Respiratory long-term facilitation (LTF) is a long lasting enhancement of respiratory activity following repeated exposure to respiratory stimulation, first described in anaesthetized or decerebrate, paralysed, artificially ventilated cats following repeated electrical stimulation of the carotid sinus nerve (Milhorn et al. 1980). It was subsequently demonstrated in response to repeated exposure to hypoxia and respiratory disinhibition associated with intermittent withdrawal of lung volume feedback and is characterized by increased upper airway (UA) and diaphragm (Dia) activity (Mateika & Fregosi, 1997; McGuire et al. 2002; Fuller, 2005; Harris et al. 2006). LTF is proposed to be an important protective mechanism of respiratory homeostasis during sleep, particularly in patients who suffer from obstructive sleep apnoea (Babcock & Badr, 1998, Aboubakr et al. 2001).
Apnoea during sleep may develop as a result of obstructive airway events, or central sleep apnoeas. During an obstructive apnoea respiratory efforts persist although airflow is absent at the nose and mouth. Central apnoea occurs when both airflow and respiratory efforts are absent. In some patients both patterns occur together, referred to as mixed apnoeas. Patients who suffer from obstructive sleep apnoea (OSA) may experience airway obstruction hundreds of times per night. These events result in repeated episodes of hypoxaemia and interruption of phasic vagal lung-volume feedback, stimuli known to induce LTF (Mateika & Fregosi, 1997; McGuire et al. 2002; Fuller, 2005; Harris et al. 2006; Ryan & Nolan, 2009). LTF, by increasing UA muscle activity and stabilizing respiratory drive could help ameliorate obstructive and mixed apnoeas. Apnoeic episodes also lead to negative transmural pressure generation below the site of obstruction. UANP has been shown to induce a reflex response characterized by activation of motor drive to UA dilator and retractor muscle groups (Ryan et al. 2001) and a reduction in diaphragm activity, responses dedicated to help restore and maintain airway patency. However, whether repeated exposure to episodic UANP also induces LTF of UA muscle activity is unknown.
We have developed a rat model optimized to study hypoxic LTF and the reflex response to UANP. UANP responses can only be demonstrated in the spontaneously breathing rat as it is abolished by neuromuscular blockade (Ryan et al. 2003). We have shown in our anaesthetized spontaneously breathing rat model that LTF of UA dilator and retractor tongue muscles (genioglossus (GG) and hyoglossus (HG), respectively) can be expressed following repeated exposure to episodic hypoxia (Ryan & Nolan, 2009) provided animals are subjected to sufficient hypoxic exposures.
We performed the present study, therefore, to examine whether repeated exposure to UANP stimuli could also induce LTF of UA tongue and respiratory pump muscle activity. Second, we also investigated what effect simultaneous exposure to episodic hypoxia would have on any LTF response in these muscle groups. Given that UANP co-activates UA dilator and retractor muscle groups, we hypothesized that repetitive exposure to UANP stimuli would induce LTF in these muscle groups and that when coupled with hypoxia an even greater long-term facilitation of the response to UANP would occur.
Methods
General procedures
Experiments were conducted in 24 adult male Wistar rats (weight ranging from 280 to 350 g). The work was performed under license from the Department of Health and Children, Ireland, and conformed to national and university guidelines regarding animal experimentation. Surgical anaesthesia was induced with sodium pentobarbitone (60 mg kg−1i.p.; Sagatal, Rhone Merieux, Ireland) and maintained with intravenous infusion of Saffan (alphaxalone/alphadalone, 4–8 mg ml−1 h−1i.v.) to maintain a stable systemic arterial pressure and respiratory rate as well as to suppress reflex withdrawal, arterial pressure and respiratory responses to paw pinch. The rats were placed in the supine position on a thermostatically controlled heating blanket (Harvard homeothermic blanket system, Harvard Apparatus, Edenbridge, UK) to maintain rectal temperature close to 37°C. The right femoral artery and vein were cannulated to record systemic arterial pressure (Statham P23Dd, Heto Rey, PR, USA) and for injection of drugs respectively. The left femoral vein was cannulated to allow infusion of Saffan anaesthetic.
Animals were tracheostomized and the recurrent laryngeal nerves were preserved bilaterally. The animals were allowed to breathe spontaneously through the tracheal cannula, which was attached to a T-piece. A gas-mixing apparatus, comprising a bank of rotameters, one each for O2, N2, and CO2, delivered the inspired gas mix to one arm of this T-piece at a total gas flow of approximately 1 l min−1, and exhaled gases and excess fresh gas flowed out through the other arm of the T-piece. An oxygen-rich mix (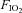 = 0.28) was normally delivered to the T-piece to maintain normal arterial oxygenation. Where required, animals could be exposed to a brief 3 min episode of isocapnic hypoxia by simultaneously changing
= 0.28) was normally delivered to the T-piece to maintain normal arterial oxygenation. Where required, animals could be exposed to a brief 3 min episode of isocapnic hypoxia by simultaneously changing 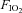 to 0.1 and increasing
to 0.1 and increasing 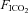 to 0.1, followed by a return to normoxic conditions for a 5 min period.
to 0.1, followed by a return to normoxic conditions for a 5 min period.
The UA preparation was similar to that previously described (Ryan et al. 2001). In brief, the UA was isolated by inserting a second cannula in the trachea, pointing cranially with its tip lying approximately 5 mm below the vocal cords. Two small diameter polyethylene tubes were inserted in the nares and an airtight seal ensured by sealing with glue and Vaseline. The isolated UA was connected to a pressure reservoir via a solenoid valve so that pulses of negative pressure could be applied. Pressure stimuli began at end-expiration and were of 5 s duration. Pressures of −10 cmH2O were used. In all experiments, pressure was applied simultaneously to both the nasal cannula and upper tracheal cannula so that the entire UA was subjected to the transmural pressure change and recorded using a transducer (Validyne DP45, ± 35 cmH2O, Northridge, CA, USA) connected to the upper tracheal cannula.
Electromyography (EMG) recording
All EMG recordings were obtained using disposable concentric EMG needles (diameter 0.6 mm/23 G, length 75 mm, SLE Diagnostics, Surrey, UK). This included recordings of the right genioglossus (GG), hyoglossus (HG) and diaphragm (Dia) muscle activities in all animals (n= 24). Activity was amplified (Neurolog NL100AK pre-amplifier and NL104 amplifier, Digitimer, Welwyn Garden City, UK), filtered (0.3–2 kHz, NL 125, Digitimer), processed by a leaky integrator (time constant 100 ms, NL 703, Digitimer.) and fed to an audio monitor and an oscilloscope. EMG activity, together with systemic arterial pressure and UA pressure were recorded and stored on computer using a CED micro1401 interface and Spike 2 software (CED, Cambridge, UK).
Experimental protocols
After all surgical procedures were complete, a period of at least 30 min was allowed to stabilize the preparation. Three series of experiments were performed (n= 24). Baseline EMG activity and the response to UANP (baseline pressures) were recorded at the beginning of each series.
In the first series of experiments GG, HG and Dia EMG activities were recorded in response to episodic UANP alone stimulation, applied under normoxic normocapnic conditions (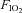 = 0.28, see Table 1 for blood gas data). Eight rats were exposed to eight trains of five UANP stimuli, 5 min apart. UANP stimuli (pulses of −10 cmH2O of 5 s duration) were delivered every 30 s for a 3 min period (5 stimuli in total in each train) followed by a 5 min stabilization period. This cycle was repeated 8 times for 1 h (LTF induction hour). After the final train of UANP stimuli, EMG activities were recorded for a further hour and measured at 1 min and every 10 min thereafter (LTF hour). At each of these time points a single pulse of −10 cmH2O (5 s duration) was applied to examine the reflex response to UANP. We chose −10 cmH2O as our stimulus as we have previously shown in this preparation that the stimulus–response curve to negative pressure saturates at pressures more negative than −20 cmH2O; −10 cmH2O therefore served as a sub-maximal stimulus.
= 0.28, see Table 1 for blood gas data). Eight rats were exposed to eight trains of five UANP stimuli, 5 min apart. UANP stimuli (pulses of −10 cmH2O of 5 s duration) were delivered every 30 s for a 3 min period (5 stimuli in total in each train) followed by a 5 min stabilization period. This cycle was repeated 8 times for 1 h (LTF induction hour). After the final train of UANP stimuli, EMG activities were recorded for a further hour and measured at 1 min and every 10 min thereafter (LTF hour). At each of these time points a single pulse of −10 cmH2O (5 s duration) was applied to examine the reflex response to UANP. We chose −10 cmH2O as our stimulus as we have previously shown in this preparation that the stimulus–response curve to negative pressure saturates at pressures more negative than −20 cmH2O; −10 cmH2O therefore served as a sub-maximal stimulus.
Table 1.
Mean arterial blood gases
| Baseline | LTF induction | LTF hour early | LTF hour middle | LTF hour late | ||
|---|---|---|---|---|---|---|
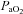 |
Normoxia | 117.6 ± 2.6 | 120.3 ± 3.8 | 125.7 ± 2.7 | 120.7 ± 4.7 | 122.1 ± 2.3 |
| UANP alone | 101 ± 4.3 | 108.8 ± 5.2 | 111.1 ± 3.9 | 116.1 ± 4.2 | 116.9 ± 3.8 | |
| UANP + hypoxia | 112 ± 3.7 | 36.7 ± 0.8 | 110 ± 3.2 | 115.7 ± 2.8 | 116.7 ± 3 | |
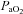 |
Normoxia | 43.2 ± 1.3 | 41.4 ± 1.3 | 43.4 ± 1.8 | 42.2 ± 1.7 | 43.1 ± 1.9 |
| UANP alone | 46.7 ± 1.1 | 46.7 ± 0.7 | 46.3 ± 0.9 | 46.6 ± 0.6 | 45.2 ± 1.1 | |
| UANP + hypoxia | 43.7 ± 0.8 | 46.1 ± 0.7 | 43.9 ± 1.2 | 42 ± 1.0 | 42.5 ± 1 | |
| pH | Normoxia | 7.31 ± 0.01 | 7.32 ± 0.01 | 7.3 ± 0.01 | 7.36 ± 0.01 | 7.3 ± 0.01 |
| UANP alone | 7.3 ± 0.01 | 7.32 ± 0.01 | 7.31 ± 0.01 | 7.3 ± 0.01 | 7.3 ± 0.01 | |
| UANP + hypoxia | 7.32 ± 0.01 | 7.33 ± 0.01 | 7.35 ± 0.01 | 7.34 ± 0.02 | 7.36 ± 0.01 |
Group mean (±s.e.m.) data showing partial pressure of arterial carbon dioxide (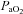 ) partial pressure of arterial oxygen (
) partial pressure of arterial oxygen (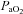 ) and pH during experimental protocols where spontaneously breathing anaesthetized rats were exposed to repeated UANP alone (n= 8), UANP with hypoxia (n= 8) or normoxia alone (n= 8).
) and pH during experimental protocols where spontaneously breathing anaesthetized rats were exposed to repeated UANP alone (n= 8), UANP with hypoxia (n= 8) or normoxia alone (n= 8).
In the second series of experiments, UANP stimuli were applied in a similar way. However, in these eight rats inspired gas mixtures were switched from normoxic normocapnia to isocapnic hypoxia (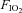 = 0.1,
= 0.1, 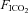 = 0.08 to 0.1) during each 3 min period when UANP stimuli were being applied.
= 0.08 to 0.1) during each 3 min period when UANP stimuli were being applied.
This sequence of UANP and hypoxia together was repeated eight times (as above), each one followed by a 5 min period of normoxia with no UANP stimuli. After the final exposure to pressure and hypoxic stimulation together, inspired gases were returned to normoxic inspired mixtures and EMG activity was recorded for a further hour under these conditions and measured after 1 min and every 10 min thereafter for 1 h. At each of these time points a single pulse of −10 cmH2O was applied to examine if LTF of the response to UANP had been induced.
Blood gases were taken at the commencement of the experimental protocol, immediately before each hypoxic and/or UANP exposure, approximately 150 s into each hypoxic and/or UANP exposure, and at 5, 20, 40 and 60 min into the post-hypoxia or post-UANP hour. Arterial blood samples were withdrawn in 200 μl aliquots and were analysed immediately after sampling for blood gases and pH. The maximum number of samples per animal was 21. When a sample for arterial blood gas analysis was withdrawn, approximately 300 μl of normal saline was administered to maintain intravascular volume.
In the third series of time-control experiments (n= 8), GG, HG and Dia EMG activities were recorded under constant conditions in which normoxia was maintained and UA transmural pressure remained zero throughout the entire protocol, and EMG activity and arterial blood gases were measured at similar time points to that described above for the first two series of experiments.
Data analysis
The activity of all muscles was quantified in arbitrary units. Peak activity was measured as this has been the most commonly chosen measurement in previous studies of LTF and was calculated as the difference between peak activity reached during end-inspiration and zero activity measured at the end of each experiment after the animal died. Peak activities of GG, HG and Dia inspiratory EMG activities were measured under baseline conditions at the start of each experiment. Where pulses of UANP were delivered, peak activity on the first-breath and on the breath immediately preceding each pressure intervention was measured. The first breath activities for each of the five UANP stimuli applied during each 3 min period (LTF induction hour) were averaged. Measurements were made on time-matched breaths in the time-control experiments. Peak activity was then expressed as a percentage change from baseline activity.
The effect of UANP ± hypoxia on respiratory timing was also analysed. Inspiratory time (TI) was recorded as the interval between the onset of diaphragm EMG activity and the beginning of its sharp decline at the end of inspiration. Total respiratory cycle time (TTOT) was measured as the interval between the onset of diaphragm inspiratory activity and the onset of the inspiratory burst of the next respiratory cycle. We calculated expiratory time (TE) by subtracting TI from TTOT.
Analysis of variance (ANOVA) for repeated measures was used to test statistical hypotheses. The Student–Newman–Keuls and Dunnett's tests were used for post hoc multiple comparisons. Data were log transformed to eliminate skewness or to homogenize variance across groups. P < 0.05 was accepted as indicating a statistically significant effect.
Results
The group mean (±s.e.m., n= 24) 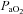 ,
, 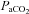 and pH values are shown in Table 1 for each of the three series of experiments performed. Representative recordings of Dia, GG and HG responses following repeated exposure to trains of UANP alone and UANP together with hypoxia are shown in Figs 1 and 2 respectively. Repeated exposure to UANP alone and UANP together with hypoxia induced LTF of Dia activity. This manifested as a long lasting increase in peak activity and breathing frequency. In addition, an early transient post-hypoxic reduction in breathing frequency also developed, attributed to a significant prolongation of expiratory time, changes that were not seen following repeated UANP-alone stimulation.
and pH values are shown in Table 1 for each of the three series of experiments performed. Representative recordings of Dia, GG and HG responses following repeated exposure to trains of UANP alone and UANP together with hypoxia are shown in Figs 1 and 2 respectively. Repeated exposure to UANP alone and UANP together with hypoxia induced LTF of Dia activity. This manifested as a long lasting increase in peak activity and breathing frequency. In addition, an early transient post-hypoxic reduction in breathing frequency also developed, attributed to a significant prolongation of expiratory time, changes that were not seen following repeated UANP-alone stimulation.
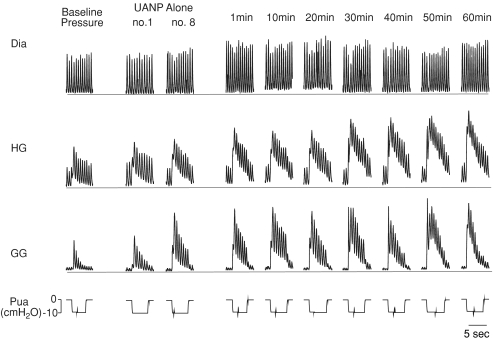
Figure 1. Effect of episodic upper airway negative pressure (UANP) on upper airway and respiratory pump muscle activities in anaesthetized spontaneously breathing rat.
Original record shows genioglossus (GG), hyoglossus (HG), and diaphragm (Dia) muscle activities and their responses to UANP under baseline conditions, during the first (no. 1) and last (no. 8) of eight 3 min repeated episodes of UANP stimulation alone and at individual time points over the subsequent hour. The horizontal line in each muscle recording represents zero activity; amplification settings were similar for all EMG recordings. Pua, upper airway pressure.
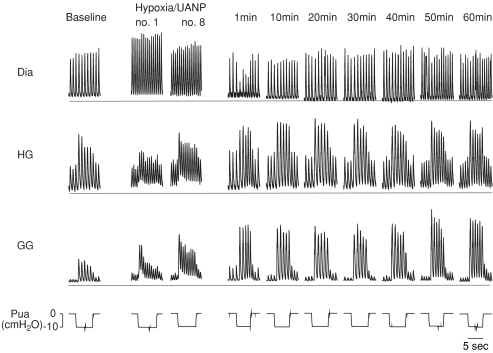
Figure 2. Effect of episodic upper airway negative pressure (UANP) and hypoxia together on upper airway and respiratory pump muscle activities in anaesthetized spontaneously breathing rat.
Original record shows genioglossus (GG), hyoglossus (HG), and diaphragm (Dia) muscle responses to UANP alone under baseline conditions, on the first (no. 1) and last (no. 8) of eight repeated episodes of UANP and hypoxia stimulation together, and in response to UANP alone at individual time points over the subsequent hour following repeated exposure to UANP and hypoxia. The horizontal line in each muscle recording represents zero activity; amplification settings were similar for all EMG recordings. Pua, upper airway pressure.
GG and HG muscle activity both exhibited phasic inspiratory activity and were co-activated in response to UANP stimulation with increases in phasic activity and recruitment of tonic activity. Following repeated exposure to UANP alone (Fig. 1) and UANP together with hypoxia (Fig. 2) LTF of GG activity was induced. The magnitude of this response was similar in both circumstances. GG and HG muscle responses to repeat UANP were significantly greater when compared to the response to UANP stimuli delivered under baseline conditions at the beginning of the experiment, indicating LTF of UANP responses. Repeated exposure to UANP and hypoxia together (Fig. 2) induced LTF of GG (similar in magnitude to that obtained following episodic UANP alone) but not HG muscle responses to UANP indicating that expression of LTF in response to UANP, at least in the HG, is attenuated by hypoxia.
LTF of genioglossus muscle activity
Baseline inspiratory GG EMG activity was similar in all three protocols performed. In those animals exposed to eight 3 min episodes of 5 trains of UANP stimulation alone (n= 8), peak inspiratory GG activity significantly increased during the first recorded breath of each pressure intervention (P < 0.00001, Figs 1 and 3). When compared with the baseline pressure responses delivered at the beginning of each experiment, the GG response to UANP increased significantly during repeated trains of stimuli so that by the 6th episode and thereafter the response to UANP was significantly greater than baseline. This increase persisted during the subsequent hour such that the LTF responses were twice as big as baseline by the end of that hour. However, while UANP alone resulted in LTF of GG UANP responses there was no effect on ongoing GG activity as measured on the breath before each UANP.
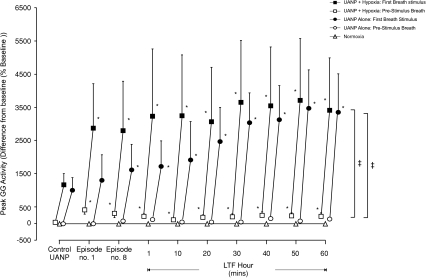
Figure 3. Group mean (±s.e.m., n= 8) effect of repeated exposure to upper airway negative pressure (UANP) and/or hypoxia on peak genioglossus muscle activity in anaesthetized spontaneously breathing rats.
The mean change in pre-stimulus (open symbols) and first-breath (filled symbols) peak genioglossus (GG) muscle activity expressed as a percentage of baseline in response to UANP stimulation alone under control conditions (average of 5 UANP stimuli), during the first (no. 1) and last (no. 8) of eight repeated episodes of UANP alone (circles), UANP together with hypoxia (squares) and normoxia alone (triangles, no pressure applied), and to single pulses of UANP alone delivered at separate time points over the subsequent hour. *Significant change in peak GG activity from control pressure responses (P < 0.05). ‡Significant difference from pre-stimulus breath activity. LTF, long-term facilitation.
In contrast, hypoxia alone produced a significant increase in GG activity as measured on the breath immediately before UANP (P < 0.001, Figs 2 and 3) and this increase persisted for at least 60 min following exposure to the last episode of pressure/hypoxia (Figs 2 and 3). UANP stimuli applied during hypoxia significantly increased GG activity on the first-breath of each pressure intervention (P < 0.0001, Figs 2 and 3). The change in GG activity in response to UANP and hypoxia together was not significantly different from that measured during repeated episodes of UANP alone (except on the first 3 min exposure; Fig. 3, P= 0.02). Following repeated exposure to episodic UANP and hypoxia, LTF of the GG muscle response to UANP was produced (P < 0.01, Figs 2 and 3). Although it appears that LTF of GG activity was greater in those animals following repeated exposure to UANP and hypoxia compared to that following repeated trains of UANP alone, the magnitude of this increase was not statistically different (P > 0.05, Fig. 3).
No change in peak GG muscle activity occurred in animals exposed only to normoxic normocapnic conditions (P > 0.05, Fig. 3).
LTF of hyoglossus muscle activity
Baseline HG EMG activity was similar in all three protocols performed. All UANP stimuli significantly increased peak HG muscle activity (P < 0.00001, Figs 1, 2 and 4). However, the response to repeated episodes of UANP stimulation alone was not significantly different from control pressure responses (P > 0.05, Figs 1 and 4). Furthermore, after 1 h of episodic UANP stimulation, LTF of the HG muscle responses to UANP developed only after 40 min had elapsed (P < 0.05, Fig. 4).
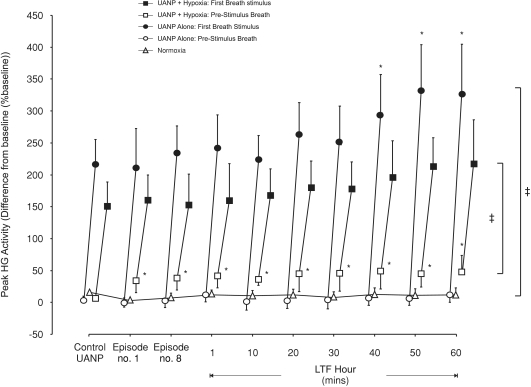
Figure 4. Group mean (±s.e.m., n= 8) effect of repeated exposure to upper airway negative pressure (UANP) and/or hypoxia on peak hyoglossus muscle activity in anaesthetized spontaneously breathing rats.
The mean change in pre-stimulus (open symbols) and first-breath (filled symbols) peak hyoglossus (HG) muscle activity expressed as a percentage of baseline in response to UANP stimulation alone under control conditions (average of 5 UANP stimuli), during the first (no. 1) and last (no. 8) of eight repeated episodes of UANP alone (circles), UANP together with hypoxia (squares) and normoxia alone (triangles, no pressure applied), and to single pulses of UANP alone delivered at separate time points over the subsequent hour. *Significant change in peak HG activity from control pressure responses (P < 0.05). ‡Significant difference from pre-stimulus breath activity. LTF, long-term facilitation.
The change in peak HG activity in response to UANP and hypoxia together was similar to that obtained in response to episodic UANP alone (P= 0.73, Figs 2 and 4). However, LTF of HG muscle responses to UANP did not develop (Fig. 4). Pre-stimulus peak HG activity was significantly increased compared to baseline and this increase, similar to that for the GG, persisted as LTF of baseline peak HG activity for at least 1 h (P < 0.05, Figs 2 and 4).
No change in HG EMG activity occurred in those animals exposed only to normoxia (n= 8, Fig. 4).
LTF of diaphragm muscle activity
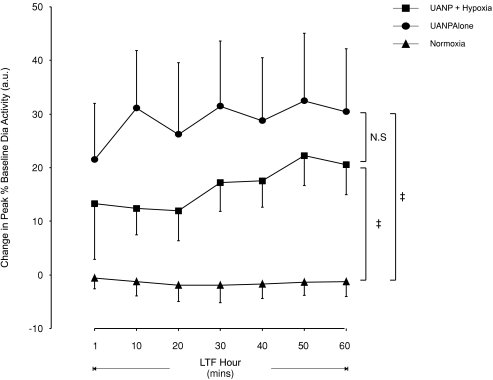
Peak Dia activity significantly decreased only in response to UANP applied 1 min after the last episode of repeated UANP and hypoxic stimulation (Fig. 2) but no significant change in first breath responses to UANP occurred at any other time either following episodic UANP alone or UANP together with hypoxia (data not shown). LTF of pre-stimulus breath activity was induced, however, following repeated exposure to episodic UANP stimulation and UANP together with hypoxia. This prolonged increase of baseline peak Dia activity was maintained for 1 h (P < 0.001, Figs 1, 2 and 5). Normoxia alone (n= 8) did not significantly affect peak diaphragm activity (Fig. 5).
Figure 5. Long-term facilitation of peak diaphragm muscle activity following exposure to repeated episodes of upper airway negative pressure (UANP) and/or hypoxia in anaesthetized spontaneously breathing rats.
Group mean (±s.e.m., n= 8) change in pre-stimulus breath peak diaphragm (Dia) muscle activity expressed as a percentage of baseline following exposure to episodic UANP alone (circles), UANP together with hypoxia (squares) and normoxia alone (triangles). ‡Significant change in peak Dia activity from corresponding time points during normoxia alone (P < 0.05). N.S., no significant difference at any time point measured across the hour.
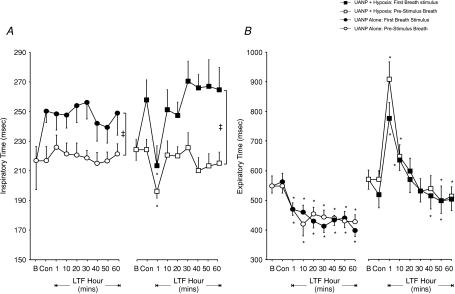
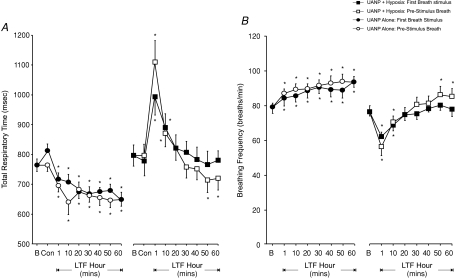
A significant increase in inspiratory time (TI) in response to UANP was obtained whether pressure was applied in the presence or absence of hypoxia (P < 0.01, Fig. 6A), but there was no evidence for LTF of TI after completion of each stimulus protocol. Expiratory times (TE) measured on the breath immediately preceding and on the first breath during UANP stimulation were similar. A biphasic pattern of change in TE was obtained following exposure to repeated episodes of UANP and hypoxia together (Fig. 6B). This involved an initial prolongation of TE measured after 1 and 10 min, followed by a gradual but significant reduction in TE after 40 min (P < 0.05, Fig. 6B). A similar biphasic response to episodic UANP was not found following exposure to repeated UANP stimuli alone (Fig. 6B). Instead, TE was significantly reduced at all time points measured over the subsequent hour (P < 0.01). These changes in TE were reflected in the mean changes in total respiratory time (TTOT, Fig. 7A) and respiratory breathing frequency (Fig. 7B). In particular, an early transient prolongation of TTOT (P < 0.01, Fig. 7A) and a concomitant reduction in breathing frequency (P < 0.01, Fig. 7B) was obtained at 1 and 10 min following episodic UANP stimulation in the presence of hypoxia. These changes were subsequently reversed so that after 50 min TTOT and frequency were significantly reduced and increased respectively. Following episodic UANP alone, a prolonged reduction in TTOT was obtained with TTOT being significantly reduced at all time points measured over 60 min (Fig. 7A). This response was mirrored by a significant increase in respiratory breathing frequency over the same time period (Fig. 7B).
Figure 6. Long-term facilitation (LTF) of inspiratory and expiratory diaphragm (Dia) muscle activity (group mean ±s.e.m.) in anaesthetized spontaneously breathing rats.
A, inspiratory time (TI) measured during control pressure stimuli (Con, average 5 stimuli) and at different time points following repeated exposure to upper airway negative pressure (UANP) stimulation alone (circles) and UANP together with hypoxia (squares). Open symbols represent TI on breaths immediately preceding and filled symbols TI on the first breath of UANP stimulation. B, expiratory time (TE) measured during control pressure stimuli (Con, average 5 stimuli) and at different time points following repeated exposure to upper airway negative pressure (UANP) stimulation alone (circles) and UANP together with hypoxia (squares). Open symbols represent TE on breaths immediately preceding and filled symbols TE on the first breath during UANP stimulation. *Significant difference from control (P < 0.05). ‡Significant difference at all corresponding time points. B, baseline activity.
Figure 7. Long-term facilitation (LTF) of diaphragm (Dia) muscle total respiratory time and breathing frequency (group mean ±s.e.m.) in anaesthetized spontaneously breathing rats.
Breathing frequency measured at different time points following exposure to upper airway negative pressure (UANP) stimulation alone (circles) and UANP together with hypoxia (squares). Open symbols represent breathing frequency on breaths immediately preceding and filled symbols, frequency on the first breath during UANP stimulation. *Significant difference from baseline (P < 0.05). B, baseline activity.
Discussion
This is the first study to examine whether LTF of UA dilator and retractor muscle and respiratory pump muscle activity and their responses to UANP develops following repeated exposure to episodic UANP stimulation. The major new findings arising from this study include that (i) a powerful LTF of UANP response is induced by repetitive UANP exposure in both tongue protrudor (GG) and retractor (HG) muscle groups, characterized by an early short-term potentiation (STP) and later LTF of UANP response, (ii) UANP together with hypoxia also induced LTF but in the presence of hypoxia there was no additional facilitation of the UANP response compared to that following repetitive UANP alone, (iii) hypoxia induced LTF of ongoing GG and HG muscle activity, and (iv) ventilatory LTF manifests as a prolonged increase in peak Dia activity, a reduction in TE and TTOT, and an increase in breathing frequency following exposure to episodic UANP alone. In addition, an initial transient post-hypoxic frequency decline was induced following exposure to episodic UANP and hypoxia together.
We hypothesized that episodic UANP stimulation would induce LTF of UA dilator and retractor muscle responses to UANP and that this response would be increased further in the presence of hypoxia. We found that episodic UANP alone induces marked LTF of GG and HG muscle UANP responses. These are novel findings and demonstrate that LTF following episodic UANP stimulation is not confined to airway dilators and instead provide additional support that these opposing muscle groups work together to help maintain airway patency (Mateika & Fregosi, 1997; Fuller et al. 1998, 1999; Kuna & Vanoye, 1999; McKay et al. 2004; Harris et al. 2006). The present study is the first to define LTF of UA muscle responses to UANP. As UANP is an important and sensitive signal indicating compromise to airway patency, LTF and co-activation of dilator and retractor UA muscles in response to episodic UANP strongly suggests that this response may serve as an important physiological response dedicated to help prevent pharyngeal collapse and stabilize the UA especially in those individuals susceptible to recurrent episodes of airway obstruction during sleep.
These results further support our recent findings demonstrating LTF of both GG and HG muscle activities in anaesthetized spontaneously breathing rats following repetitive exposure to episodic hypoxia (Ryan & Nolan, paper submitted) and compliments that reported by Fuller (2005) in which LTF of medial and lateral hypoglossal motoneurons was induced following exposure to episodic hypoxia in anaesthetized, vagotomized, paralysed artificially ventilated rats. During obstruction of the UA, changes in UA transmural pressure are accompanied by development of hypoxaemia. We investigated whether simultaneous exposure to both stimuli would lead to an even greater LTF of UA muscle activity and the response to UANP. Our results, however, do not support our initial hypothesis. LTF of GG but not HG muscle responses to UANP were induced after exposure to episodic UANP and hypoxia together but the magnitude of this response was not significantly different when compared to that observed following repeated exposure to UANP alone. It would appear, therefore, that in the rat, UANP alone expresses marked LTF of UA muscle activity, similar to hypoxia alone (Ryan & Nolan, paper submitted). However, simultaneous exposure to both stimuli has no greater additional effect than that obtained from either stimulus alone, so that the interaction between chemoreceptor (hypoxia) and laryngeal mechanoreceptor (UANP) inputs in the control of UA muscle activity is occlusive in the rat. Shea et al. (2000) also found no evidence of facilitation of UA muscle responses under conditions of increased respiratory drive in human subjects. They found that hypoxaemia at levels physiologically analogous to those developed during obstructive sleep apnoeas did not affect the reflex response to UANP.
LTF of ongoing Dia activity occurred following exposure to episodic UANP alone or together with hypoxia. This prolonged increase in ongoing activity was a surprising result. UANP is known to inhibit inspiratory drive to diaphragm (Mathew & Farber, 1983; Ryan et al. 2001) and so we would have expected a decrease in Dia muscle activity to occur following repeated exposure to UANP stimuli. Our findings may represent a rebound (post-inhibitory) excitatory response but the significance of this response pattern is unclear.
Baseline TE was significantly shortened following repetitive exposure to UANP. Individual UANP stimuli delivered after the last exposure to trains of UANP alone had no additional shortening effect on TE. The reduction in TE was reflected by a concomitant reduction in TTOT and an increase, or LTF, in breathing frequency. This is the first study to describe LTF ventilatory changes following exposure to repetitive UANP. The ventilatory LTF induced by episodic UANP stimulation in our study has properties similar to that described following repeated episodes of hypoxia alone. For example, Morris & Gozal (2004) reported an increase in respiratory frequency measured as a decrease in TE and TI following carotid chemoreceptor stimulation in humans and anaesthetized vagotomized cats. Similarly, a prolonged increase in respiratory frequency has also been demonstrated in anaesthetized, vagotomized, artificially ventilated (Bach & Mitchell 1996; Baker & Mitchell, 2000) and awake (Olson et al. 2001) rats, goats (Turner & Mitchell 1997) and ducks (Mitchell et al. 2001a) but not anaesthetized spontaneously breathing rats (Janssen & Fregosi 2000). In the presence of hypoxia an initial increase in baseline TE developed leading to a corresponding transient reduction in frequency. We and others have demonstrated this post-hypoxic frequency decline following episodic exposure to hypoxia alone in anaesthetized spontaneously breathing (Ryan & Nolan, 2009; Janssen & Fregosi, 2000) or ventilated rats (Hayashi et al. 1993; Coles et al. 1998; Bach et al. 1999; Fuller, 2005). We did not find any evidence of reduced breathing frequency when exposed only to UANP alone, indicating that this effect is mediated by exposure to hypoxia and not UANP.
In conclusion, we have shown that episodic UANP induces LTF of UA dilator and retractor tongue muscles. As UANP is closely associated with narrowing or closure of the UA, LTF of these muscle activities and their response to episodic UANP provides strong support that it may form part of a vital airway protective response especially in those people who suffer from repeated episodes of UA obstruction during sleep. The coincident increase in baseline muscle activity may also compensate for the increase susceptibility to airway collapse during sleep in these individuals. These results also support our recent findings that LTF can be expressed in anaesthetized spontaneously breathing rats following exposure to episodic hypoxia. However, we did not find any evidence to support an additive effect when both these stimuli were delivered simultaneously, which suggests that the interaction between chemoreceptor (hypoxia) and laryngeal mechanoreceptor (UANP) inputs in the control of UA muscle activity is occlusive, at least in the rat. Nevertheless, these results provide important new information in to our understanding of LTF and control of UA patency.
Glossary
Abbreviations
- Dia
diaphragm
- GG
genioglossus
- HG
hyoglossus
- LTF
long-term facilitation
- OSA
obstructive sleep apnoea
- PHFD
post-hypoxic frequency decline
- SAP
systemic arterial pressure
- STP
short-term potentiation
- TI
inspiratory time
- TTOT
total respiratory cycle time
- TE
expiratory time
- UA
upper airway
- UANP
upper airway negative pressure
Author contributions
S.R. performed all experiments in the research study; P.N. was the principal investigator/supervisor for research study.
References
- Aboubakr SE, Taylor A, Ford R, Siddiq S, Badr MS. Long-term facilitation in obstructive sleep apnoea patients during NREM sleep. J Appl Physiol. 2001;91:2751–2757. doi: 10.1152/jappl.2001.91.6.2751. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep. 1998;21:709–716. [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia induced long-term facilitation of respiratory activity is serotonin dependent. Resp Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Bach KB, Kinkead R, Mitchell GS. Post-hypoxia frequency decline in rats: sensitivity to repeated hypoxia and α2-adrenoreceptor antagonism. Brain Res. 1999;817:25–33. doi: 10.1016/s0006-8993(98)01181-0. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529:215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles SK, Ernsberger P, Dick TE. Post-hypoxic frequency decline does not depend in α2-adrenergic receptors in the adult rat. Brain Res. 1998;794:267–273. doi: 10.1016/s0006-8993(98)00234-0. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol. 1999;519:601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol. 1998;507:265–276. doi: 10.1111/j.1469-7793.1998.265bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD. Episodic hypoxia induces long-term facilitation of neural drive to tongue protruder and retractor muscles. J Appl Physiol. 2005;98:1761–1767. doi: 10.1152/japplphysiol.01142.2004. [DOI] [PubMed] [Google Scholar]
- Harris DP, Balasubramanian A, Badr MS, Mateika JS. Long term facilitation. of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:1111–1119. doi: 10.1152/ajpregu.00896.2005. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol Regul Integr Comp Physiol. 1993;265:R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Janssen PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J Appl Physiol. 2000;89:1345–1351. doi: 10.1152/jappl.2000.89.4.1345. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Vanoye CR. Mechanical effects of pharyngeal constrictor activation on pharyngeal airway function. J Appl Physiol. 1999;86:411–417. doi: 10.1152/jappl.1999.86.1.411. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol. 1997;82:419–425. doi: 10.1152/jappl.1997.82.2.419. [DOI] [PubMed] [Google Scholar]
- Mathew OP, Farber JP. Effect of upper airway negative pressure on respiratory timing. Resp Physiol. 1983;54:259–268. doi: 10.1016/0034-5687(83)90062-2. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Effect of episodic number and severity on ventilatory long-term facilitation in awake rats. J Appl Physiol. 2002;93:2155–2161. doi: 10.1152/japplphysiol.00405.2002. [DOI] [PubMed] [Google Scholar]
- McKay L, Janczewski WA, Feldman JL. Episodic hypoxia induces long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol. 2004;557:13–18. doi: 10.1113/jphysiol.2004.064006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Resp Physiol. 1980;42:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Powell FL. Time domains of the hypoxic ventilatory response in awake ducks: episodic and continuous hypoxia. Respir Physiol. 2001;124(2):117–128. doi: 10.1016/s0034-5687(00)00197-3. [DOI] [PubMed] [Google Scholar]
- Morris KF, Gozal D. Persistent respiratory changes following intermittent hypoxic stimulation in cats and human beings. Respir Physiol Neurobiol. 2004;140(1):1–8. doi: 10.1016/j.resp.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Olson EB, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitche GS. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol. 2001;91:709–716. doi: 10.1152/jappl.2001.91.2.709. [DOI] [PubMed] [Google Scholar]
- Ryan S, Nolan P. Episodic hypoxia induces long-term facilitation of upper airway muscle activity in spontaneously breathing anaesthetized rats. J Physiol. 2009;587:3329–3342. doi: 10.1113/jphysiol.2009.169680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S, McNicholas WT, O’Regan RG, Nolan P. Reflex respiratory response to changes in upper airway pressure in the anaesthetized rat. J Physiol. 2001;537:251–265. doi: 10.1111/j.1469-7793.2001.0251k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S, McNicholas WT, O’Regan RG, Nolan P. Upper airway muscle paralysis reduces reflex upper airway motor response to negative transmural pressure in rat. J Appl Physiol. 2003;94:1307–1316. doi: 10.1152/japplphysiol.00052.2002. [DOI] [PubMed] [Google Scholar]
- Shea SA, Akahoshi T, Edwards JK, White D. Influence of chemoreceptor stimuli on genioglossal response to negative pressure in humans. Am J Resp Crit Care Med. 2000;162:559–565. doi: 10.1164/ajrccm.162.2.9908111. [DOI] [PubMed] [Google Scholar]
- Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol. 1997;499:543–550. doi: 10.1113/jphysiol.1997.sp021947. [DOI] [PMC free article] [PubMed] [Google Scholar]