Abstract
During human walking, a sudden trip may elicit a Ia afferent fibre mediated short latency stretch reflex. The aim of this study was to investigate soleus (SOL) muscle mechanical behaviour in response to dorsiflexion perturbations, and to relate this behaviour to short latency stretch reflex responses. Twelve healthy subjects walked on a treadmill with the left leg attached to an actuator capable of rapidly dorsiflexing the ankle joint. Ultrasound was used to measure fascicle lengths in SOL during walking, and surface electromyography (EMG) was used to record muscle activation. Dorsiflexion perturbations of 6 deg were applied during mid-stance at walking speeds of 3, 4 and 5 km h−1. At each walking speed, perturbations were delivered at three different velocities (slow: ∼170 deg s−1, mid: ∼230 deg s−1, fast: ∼280 deg s−1). At 5 km h−1, fascicle stretch amplitude was 34–40% smaller and fascicle stretch velocity 22–28% slower than at 3 km h−1 in response to a constant amplitude perturbation, whilst stretch reflex amplitudes were unchanged. Changes in fascicle stretch parameters can be attributed to an increase in muscle stiffness at faster walking speeds. As stretch velocity is a potent stimulus to muscle spindles, a decrease in the velocity of fascicle stretch at faster walking speeds would be expected to decrease spindle afferent feedback and thus stretch reflex amplitudes, which did not occur. It is therefore postulated that other mechanisms, such as altered fusimotor drive, reduced pre-synaptic inhibition and/or increased descending excitatory input, acted to maintain motoneurone output as walking speed increased, preventing a decrease in short latency reflex amplitudes.
When a muscle is rapidly stretched, a short latency stretch reflex is elicited due to the excitation of Ia afferent fibres within the muscle spindles. In some human movements (e.g. hopping), this occurs naturally due to the high rate of stretch encountered by the muscles (Nicol & Komi, 1998; Voigt et al. 1998). In slower movements such as walking, the stretch reflex may not be activated during the normal step cycle, but may be triggered in response to a sudden trip or balance disturbance (e.g. Nielsen & Sinkjaer, 2002; Grey et al. 2004). The precise role and contribution of the stretch reflex remains controversial. The excitability of the electrically evoked Hoffmann reflex (H-reflex) is reported to be smaller during walking than during standing (Capaday & Stein, 1986), and smaller still during running (Capaday & Stein, 1987; Edamura et al. 1991). However, when the H-reflex is normalized to the maximally evoked M-wave adjusted to different phases of the step-cycle, statistically significant differences during walking and running are not observed (Simonsen & Dyhre-Poulsen, 1999).
Although stretch responses to applied perturbations cannot be used to explain background locomotor EMG in normal unperturbed walking (Sinkjaer et al. 2000; Grey et al. 2004, 2007), this information is nonetheless useful in the examination of responses to sudden balance disturbances. Sinkjaer et al. (1996) found that in response to a rapid dorsiflexion stretch of the ankle joint in the mid stance phase, short latency stretch reflex (SLR) amplitudes increased with increasing walking speeds. This study provided valuable information about electrophysiological events in the human soleus muscle, but very little is known about the mechanical behaviour that occurs in a muscle in response to a rapid perturbation that elicits a stretch reflex.
Recent evidence suggests that during isometric contractions, the velocity at which the fascicles of the soleus muscle are stretched progressively decreases with increasing force levels in response to a constant external stretch (Cronin et al. 2008). This is likely to be due to force-dependent alterations in the stiffness ratio between muscle and tendinous tissues (Rack & Westbury, 1984). During a more complex activity such as human walking, very little is known about muscle fascicle stretch responses. Although it is not currently possible to measure muscle spindle output directly during human walking, muscle fascicle length may be a better indicator of this parameter than muscle–tendon unit length (Maas et al. 2009; Maas & Lichtwark, 2009). By combining the use of ultrasonography (Ishikawa & Komi, 2007) with a portable stretch device (Andersen & Sinkjaer, 1995; Andersen & Sinkjaer, 2003), it is possible to simultaneously examine muscle fascicle behaviour and the associated stretch reflex responses. The purpose of this study was to investigate the interaction between muscle fascicle stretch behaviour and short latency stretch reflex responses to artificially induced stretch of the soleus muscle during human walking at different speeds. As muscle activation is known to increase at faster walking speeds (e.g. Edamura et al. 1991), and this increases muscle stiffness relative to the stiffness of the tendinous tissues (Rack & Westbury, 1984), it was hypothesized that muscle fascicle stretch velocity would decrease as walking speed increased, and that this could influence the amplitudes of the resulting stretch reflex responses.
Methods
Ethical approval
The experiments were approved by the local ethics committee (approval number N2008004), and conducted in accordance with the Declaration of Helsinki. All of the volunteers provided written informed consent prior to the experiments.
Subjects
Twelve healthy subjects (8 males and 4 females; age 27 ± 4 years; height 172 ± 9 cm; weight 69 ± 11 kg) with no history of neurological disorder volunteered to participate in this study.
Apparatus and instrumentation
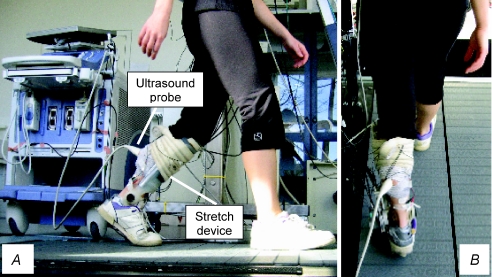
Subjects walked on a treadmill (Woodway; Waukesha, WI, USA) with the left leg attached to a portable robotic actuator capable of eliciting a stretch reflex by rapidly dorsiflexing the ankle joint. The weight of the actuator was approximately 900 g. Full details of the device are presented elsewhere (Andersen & Sinkjaer, 1995; Andersen & Sinkjaer, 2003). Briefly, the device consists of a functional joint that is aligned with the ankle axis of rotation of the subject and attached to the foot and leg with a polypropylene cast. The actuator is connected to an AC servomotor that applies torque to the functional joint through flexible Bowden cables. Ankle angle is measured with an optical encoder incorporated within the functional joint, and ankle velocity is determined by numerical differentiation of the ankle angular record. An ultrasound device (Alpha-10; Aloka, Japan) operating at a scanning frequency of 150 frames s−1 was used to measure fascicle lengths in the soleus (SOL) muscle during walking. The probe was secured over the skin surface with a custom-made polystyrene support device to minimise any probe movement relative to the muscle. The effectiveness of this approach has been confirmed by placing reference markers within view of the ultrasound image to confirm that the probe does not move in relation to the muscle (e.g. Magnusson et al. 2001). The ultrasound settings were individually adjusted to optimise the contrast between muscle fascicles and connective tissues, which greatly aids the analysis process. The weight of the probe was approximately 130 g. The ultrasound apparatus was positioned by the side of the treadmill during the walking experiments. The reliability of the ultrasound method of fascicle length calculation was determined by calculating the coefficient of variation between three different trials at each stretch velocity and at each walking speed. The mean coefficient of variation for the present study was 6 ± 1%, which is similar to values reported previously (Ishikawa et al. 2003; Cronin et al. 2008). The individual coefficients of variation for each experimental condition are reported in the Results section. EMG activity was recorded in the SOL muscle of the left leg using bipolar surface electrodes (Neuroline 720, Ambu, Denmark) with an inter-electrode distance of 2 cm. The EMG signals were band-pass filtered (10–1000 Hz). All EMG and ankle displacement signals were sampled at 2 kHz and stored for later analysis. A heel switch based on a force-sensitive resistor was placed in the insole of the left shoe to trigger the signal acquisition, and to synchronize the EMG, ankle trajectory and ultrasound data. An example of the experimental setup is shown in Fig. 1.
Figure 1. An example of a fully instrumented subject walking on the treadmill with the ankle stretch device and ultrasound probe secured to the left leg.
A, side view; B, rear view.
Walking protocol
Subjects initially walked on the treadmill for 5–10 min at a speed of 3 km h−1 to become accustomed to the robotic actuator. At the end of this period, data were acquired from approximately 30 steps during normal unperturbed walking to generate control profiles of the ankle trajectory and SOL EMG activity. Average stance phase duration was then calculated as the time difference from heel contact to toe-off in the left leg, as defined by the heel switch signals. For the stretch reflex trials, recordings were made at three different walking speeds; 3, 4 and 5 km h−1. The process of generating control profiles was repeated at each speed to ensure that the perturbations were elicited at the same relative time in the stance phase. To elicit stretch reflexes, dorsiflexion perturbations were imposed at approximately the mid-stance phase (between 41–43% of stance phase, see Results) at a rate of one perturbation every 5–10 steps. At each walking speed, perturbations were induced at three different mean velocities (slow: ∼170 deg s−1, mid: ∼230 deg s−1, fast: ∼280 deg s−1) in a random order, until a minimum of 30 trials were obtained at each stretch velocity and in the control condition (unperturbed walking). This resulted in the collection of at least 120 trials per subject at each walking speed. Perturbation velocities were selected to be sufficiently high to elicit a SLR response in all conditions, and three different velocities were chosen to examine the velocity dependence of fascicle stretch responses during walking. For all stretch trials, the perturbation amplitude was 6 ± 0.8 deg. At each walking speed, the subjects walked for l0–15 min without rest and all data were recorded within this time period. To ensure that data were recorded from similar steps, only perturbed steps with a maximal deviation within ±2% of the averaged control stride duration were included in the analysis.
Data acquisition and analysis
EMG signals were rectified, low-pass filtered (40 Hz), and ensemble averaged (25–30 trials at each perturbation velocity and each walking speed) to produce EMG profiles. Reflex amplitude and latency were then calculated from the averaged traces. To detect the onset latency of the reflex response, the averaged SOL EMG traces during control and perturbed steps were superimposed, and a window was defined from 20 to 60 ms after the stretch onset, which incorporates the physiological range for the onset of the SLR (e.g. Grey et al. 2004). Within this window, the onset of the SLR was determined by visual inspection. The amplitude of the reflex response at a given perturbation velocity was measured as the peak EMG value within a 30 ms window placed over the SOL EMG record starting at the reflex onset. Reflex amplitude was quantified relative to the mean non-perturbed EMG within the defined window. The latter value was also used to represent background EMG. For the ultrasound analysis, an individual fascicle was identified in each image along its length between the superficial and deep aponeuroses. Fascicles were only chosen for analysis if they were visible throughout the entire stance phase. The selected fascicle was manually tracked frame by frame throughout the step cycle. Pennation angle was defined as the angle between the fascicle and the superficial aponeurosis, and average fascicle velocity was calculated by differentiating fascicle stretch amplitude with respect to time. For each subject and each condition, ultrasound data from three trials were analysed and averaged, and all subjects’ data were then pooled. Three trials were analysed as the coefficients of variation were very small, and previous data from our laboratory have shown that the analysis of five trials has no effect on coefficients of variation compared to analysing just three (CV ranging between 5 and 6% in both cases; Cronin, N.J. unpublished observations). To select the trials to be analysed in a given condition, the three trials where the ankle range of motion was closest to that of the mean ankle range of motion were selected, to ensure that these trials were representative of the median response.
Statistics
Mauchly's test of sphericity was performed on the data, and repeated measures ANOVA was used to examine changes in EMG and fascicle parameters between different walking speeds and between different perturbation velocities. Bonferroni post hoc tests were used to identify the location of the differences, and to correct for multiple comparisons. Pearson's product–moment correlation was used to correlate stretch reflex amplitude and fascicle stretch velocity at different walking speeds. For all statistical tests, significant differences were determined based on a level of significance of P < 0.05. Results are presented as means ±s.d. unless otherwise stated.
Results
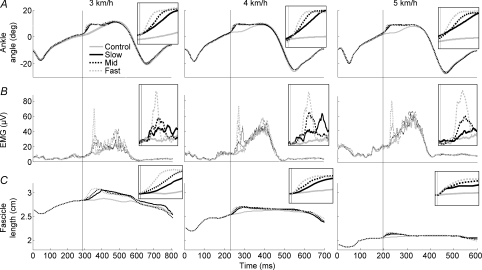
Using the methodology presented here, it was possible to induce perturbations corresponding to 6 ± 0.8 deg amplitude at different velocities to the ankle joint during the stance phase of human walking at different speeds. Data from one subject are presented in Fig. 2. Regardless of the perturbation velocity and walking speed, a short latency stretch reflex response was clearly evident in the SOL EMG patterns, and a clear stretch of the muscle fascicles was observed in the ultrasound data. The mean coefficients of variation between the three selected ultrasound trials for each experimental condition were as follows: 4, 6 and 7% (slow, mid and fast, respectively) at 3 km h−1; 6, 5 and 3% at 4 km h−1; and 7, 7 and 6% at 5 km h−1.
Figure 2. Typical stretch responses from a single subject at each walking speed.
A, ankle trajectory (n= 25–30); B, SOL EMG activity (n= 25–30); C, fascicle length (n= 3). Vertical lines denote perturbation onset. Note that the time scale differs between walking speeds. The enlarged insets are not shown to scale, but are intended to highlight the individual traces more clearly.
The mean perturbation velocities induced at the ankle joint in the slow, mid and fast conditions were 172 ± 17, 231 ± 21 and 281 ± 23 deg s−1, respectively. In order to ensure that these velocities were consistent between walking speeds, the slope and amplitude of the ankle angular displacement records were analysed in response to each perturbation condition. No significant differences in the perturbation parameters were observed between walking speeds at any of the perturbation velocities (P values between 0.218 and 0.713). These data confirm that a constant stretch was induced at the ankle joint between walking speeds. As variations in the timing of a perturbation within the stance phase can cause modulation of stretch reflex responses (e.g. Sinkjaer et al. 1996), the onset of the perturbation was determined relative to the total stance phase duration at each walking speed. This analysis revealed that the perturbation timing did not significantly differ between walking speeds (43 ± 3% at 3 km h−1, 41 ± 2% at 4 km h−1 and 41 ± 2% at 5 km h−1; P values between 0.592 and 0.881).
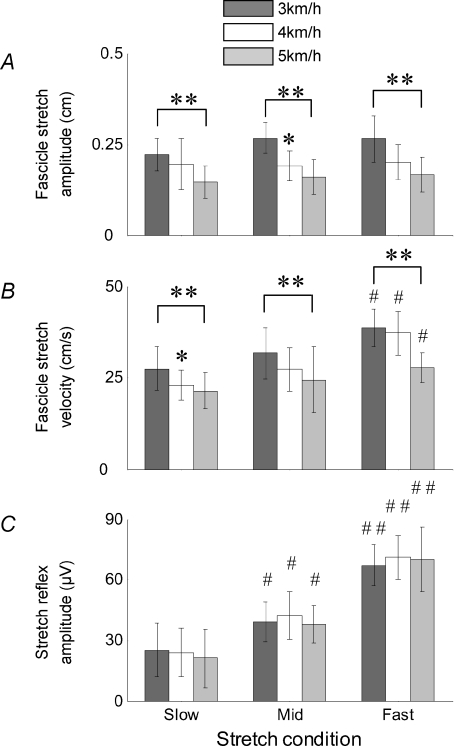
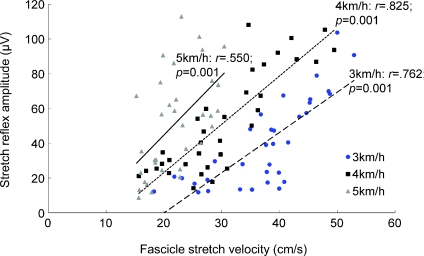
Within a given walking speed, there was a general trend of a concurrent increase in stretch reflex amplitude and fascicle stretch velocity with increasing perturbation velocity (Fig. 3). Reflex amplitude and fascicle stretch velocity were well correlated at all walking speeds, although the strength of the correlation was greatest at 4 km h−1 (Fig. 4). As shown in Fig. 4, when plotting reflex amplitude against fascicle stretch velocity, the slope of the relationship increased with faster walking speeds (from 2.32 at 3 km h−1 to 3.42 at 5 km h−1).
Figure 3. Pooled data for the group (n= 12).
A, fascicle stretch amplitude; B, fascicle stretch velocity; C, stretch reflex amplitude. Stretch reflex data were averaged from 25–30 trials per subject, and then pooled to produce the group average. For each subject, fascicle stretch responses are based on the average of 3 trials at each perturbation velocity and each walking speed. *, ** and *** denote a significant difference from the previous walking speed at the P < 0.05, P < 0.01 and P < 0.001 levels, respectively. #, ## and ### denote a significant difference from the previous stretch condition at the same significance levels.
Figure 4. Scatter plots of stretch reflex amplitudes against fascicle stretch velocities at each walking speed.
Correlation coefficients and significance values are displayed for each walking speed. The linear regression equations were as follows: 2.32x– 46.4 at 3 km h−1, 2.78x– 32.4 at 4 km h−1, 3.42x– 23.6 at 5 km h−1.
When examining the differences between walking speeds, it is clear that as walking speed increased, background EMG also increased, while stride duration, pre-stretch fascicle length and fascicle length at the point of ground contact all decreased (Table 1 and Fig. 2). There was also a moderate, non-significant decrease in stretch reflex onset latency between 3 and 4 km h−1 (P= 0.262), which may have been due to estimation errors when identifying the latency. When pooling the data for the entire group of subjects, it is evident that the amplitude and velocity of the stretch to the muscle fascicles both decreased with increasing walking speeds (Fig. 3A and B). This was true regardless of the perturbation velocity, although the patterns of modulation were somewhat different between velocities. Between the slowest and fastest walking speeds, fascicle stretch amplitude decreased by 34 ± 15%, 40 ± 17% and 38 ± 19% in the slow, mid and fast conditions, respectively. During the same intervals, fascicle stretch velocity decreased by 22 ± 20%, 23 ± 14% and 28 ± 16%, respectively. Concurrently, no changes were observed in stretch reflex amplitudes between walking speeds (Fig. 3C).
Table 1.
Changes in neural and mechanical gait parameters at different walking speeds
| Walking speed |
|||
|---|---|---|---|
| 3 km h−1 | 4 km h−1 | 5 km h−1 | |
| Background EMG (μV) | 40 ± 18 | 51 ± 11 | 59 ± 18*## |
| Stretch reflex latency (ms) | 40.7 ± 3.0 | 39.5 ± 3.3 | 39.4 ± 3.1 |
| Stride duration (ms) | 791 ± 36 | 678 ± 32*** | 589 ± 24***### |
| Lfa at ground contact (cm) | 4.29 ± 1.17 | 4.06 ± 1.16** | 3.83 ± 1.14**### |
| Pre-stretch Lfa (cm) | 4.44 ± 1.19 | 4.18 ± 1.16*** | 3.96 ± 1.19**### |
*, ** and *** denote a significant difference from the previous walking speed at the P < 0.05, P < 0.01 and P < 0.001 levels, respectively. #, ## and ### denote a significant difference from 3 km h−1. Lfa: fascicle length. For all variables, n= 12.
Discussion
The main findings of this study were that in response to a constant dorsiflexion perturbation at the ankle joint, the amplitude and velocity of stretch to the soleus muscle fascicles decreased at faster walking speeds. Concurrently, no changes in stretch reflex amplitudes were observed between walking speeds. As stretch velocity is known to be a potent stimulus to muscle spindles (Stein & Kearney, 1995), a decrease in the velocity of fascicle stretch at faster walking speeds would decrease spindle afferent feedback (Rack et al. 1983). Accordingly, it may be anticipated that stretch reflex amplitudes would decrease at faster walking speeds, which was not observed in this study. Although muscle spindle output was not directly measured, the data suggest that other mechanisms may have acted to maintain motoneurone output as walking speed increased, effectively preventing a decrease in the amplitude of the short latency stretch reflex during movement, at least within the range of walking speeds examined here.
Before discussing the findings of this study, it is important to acknowledge some methodological issues. The weight of the portable stretch device used in this study was approximately 900 g, and the additional weight due to the ultrasound probe and support device was approximately 130 g. Although the addition of weight to both legs has been found to increase metabolic rate during walking (Soule & Goldman, 1969), lower extremity kinematics and net muscle moments have been reported to remain unchanged (Browning et al. 2007). Furthermore, previous reports have confirmed that the natural gait pattern is not influenced while walking with this device attached (Andersen & Sinkjaer, 1995), and the subjects in the present study reported no perceptible differences between the weight of each leg after the initial adaptation period. Nonetheless, as weight was only added to one leg in this study, the possibility cannot be excluded that weight imbalance affected our data. To ensure that the stretch applied by the functional joint was transmitted to the ankle joint of the subject, a very tight fitting was made between the calf and the foot of the subject and the functional joint. However, due to the high torque generation of the ankle extensors during walking (Winter, 1987), it is likely that part of the stretch was absorbed by the tissues and fixtures surrounding the ankle joint, and the compliance of the functional joint itself. Therefore, the stretch of the ankle extensors may have been less than that of the functional joint, as previously hypothesized (Sinkjaer et al. 1996). Nonetheless, the stretch device has been designed with the aim of minimizing this error, and visual inspection of the ultrasound images during the experiments confirmed that a perturbation of the functional joint was transmitted to the ankle joint and soleus muscle fascicles at all walking speeds and stretch velocities. It should be noted that the use of imposed perturbations with fixed parameters is not necessarily representative of a trip occurring during human walking. Furthermore, in contrast to a previous study based on similar methodology (Grey et al. 2007), it was not possible to measure muscle forces in this study due to space limitations imposed by the combination of the perturbation and ultrasound devices. Notwithstanding, the methodology presented does facilitate the study of mechanical stretch responses under controlled conditions. One further consideration is whether the stretch responses observed in one region of the muscle are representative of the stretch responses throughout the muscle. As this is the first study to examine fascicle responses to rapid perturbations during human walking, the effects of stretch inhomogeneity are unknown. Numerous studies in animals and humans have reported uniform fascicle behaviour within a muscle during active contractions (Maganaris & Baltzopoulos, 1999; Kawakami et al. 2000; Muramatsu et al. 2002; Soman et al. 2005) and during unperturbed human walking (Lichtwark et al. 2007). However, recent data have been presented that contradict this notion in guinea fowl (Higham et al. 2008) and frogs (Ahn et al. 2003). Further investigation is required in human soleus muscle fascicles to resolve whether stretch homogeneity is a valid assumption in response to mechanical perturbations.
With increasing force production, the stiffness ratio of the muscle–tendon unit is progressively altered in favour of the muscle fibres. As the distribution of a stretch between muscle and tendinous tissues depends on their relative stiffnesses (Rack & Westbury, 1984), this alteration decreases the stretch that is distributed to the muscle fascicles, as observed in the present study in the human soleus as walking speed (and presumably also force level) increased. Muscle spindles only respond to the movement of the muscle fibres in which they are situated, so their view of a stretch is partly dependent on the distribution of the movement between muscle fibres and tendon (Rack & Westbury, 1984). Therefore, one might expect that a decrease in the amplitude, and particularly the velocity of the stimulus to the muscle fascicles, would decrease the firing rate of group Ia afferents. However, no changes were observed in short latency stretch reflex amplitude between the walking speeds that were examined in this study. This result differs from a similar previous study where SLR amplitude generally increased with walking speed (Sinkjaer et al. 1996). The reason for this discrepancy is unclear, although it should be noted that data from the previous study were only obtained from six subjects, and no changes in SLR amplitude were observed between walking speeds in two of the subjects. Therefore, it may be difficult to draw reliable conclusions based on such a small sample. In any case, both studies suggest that SLR amplitude is at least maintained with increasing walking speed in response to a constant perturbation.
In the present study, a number of mechanisms may have acted to compensate for changes in spindle afferent feedback at faster walking speeds. For example, pre-synaptic inhibition may have decreased with increasing walking speed. A decrease in afferent feedback from Ia fibres may also have been offset by an increase in descending drive to the motoneurones (see for example Nielsen & Sinkjaer, 2002). Alternatively, Sinkjaer et al. (1996) suggested that during walking, muscle spindle tension may be maintained by a combination of the eccentric contraction that the ankle extensors undergo in the stance phase, and an increased fusimotor drive to the muscle spindles concurrent with the increased α-motoneurone activity. This would remove slack from both extrafusal and intrafusal muscle fibres, thus preventing unloading of the muscle spindles when the extrafusal muscle fibres shorten (Proske et al. 2000), and elevating muscle spindle sensitivity (Taylor et al. 2006). In addition to a general increase in gamma drive during locomotion, previous authors have suggested more specifically that dynamic fusimotor activity may increase as movement speed increases (Prochazka & Hulliger, 1983; Sinkjaer et al. 1996), thus contributing to the monosynaptic excitation of α-motoneurones (Taylor et al. 1985; Greer & Stein, 1990; Bennett et al. 1996). It should be noted that the fusimotor system has been suggested to be fully activated by 20–25% of maximum muscle force (e.g. Proske, 2006). Therefore, at higher force levels or faster speeds of locomotion (such as running), the influence of this mechanism may be less significant. It is of course possible that some combination of the above mechanisms was operative during the conditions studied here.
In conclusion, the results of this study demonstrate a decline in the amplitude and velocity of stretch of the soleus muscle fascicles in response to a constant external ankle joint rotation with increasing walking speed. As the decreased stretch amplitude and velocity would be expected to decrease the afferent feedback coming from muscle spindles (Rack et al. 1983), and since the stretch reflex amplitudes were unchanged according to our data, it is likely that contributions from other mechanisms served to compensate for decreases in spindle afferent feedback, thus preventing a decline in stretch reflex amplitudes in response to perturbations at faster walking speeds.
Acknowledgments
The authors gratefully acknowledge the financial support of The Obel Family Foundation and The Spar Nord Foundation. This work was also supported by grant KAKENHI (20800061).
Glossary
Abbreviations
- EMG
electromyography
- Lfa
fascicle length
- SLR
short latency stretch reflex
- SOL
soleus muscle
Author contributions
All of the listed authors have made a significant contribution to this study in terms of the study design and/or data analysis, writing and revision of the manuscript, and providing final approval of the submitted material. All of the experiments described in this manuscript were performed at Aalborg University in Denmark.
References
- Ahn AN, Monti RJ, Biewener AA. In vivo and in vitro heterogeneity of segment length changes in the semimembranosus muscle of the toad. J Physiol. 2003;549:877–888. doi: 10.1113/jphysiol.2002.038018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JB, Sinkjaer T. An actuator system for investigating electrophysiological and biomechanical features around the human ankle joint during gait. Trans Rehabil Eng. 1995;3:299–306. [Google Scholar]
- Andersen JB, Sinkjaer T. Mobile ankle and knee perturbator. IEEE Trans Biomed Eng. 2003;50:1208–1211. doi: 10.1109/TBME.2003.816073. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, De Serres SJ, Stein RB. Regulation of soleus muscle spindle sensitivity in decerebrate and spinal cats during postural and locomotor activities. J Physiol. 1996;495:835–850. doi: 10.1113/jphysiol.1996.sp021636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning RC, Modica JR, Kram R, Goswami A. The effects of adding mass to the legs on the energetics and biomechanics of walking. Med Sci Sports Exerc. 2007;39:515–525. doi: 10.1249/mss.0b013e31802b3562. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Difference in the amplitude of the human soleus H reflex during walking and running. J Physiol. 1987;392:513–522. doi: 10.1113/jphysiol.1987.sp016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin NJ, Peltonen J, Ishikawa M, Komi PV, Avela J, Sinkjaer T, et al. Effects of contraction intensity on muscle fascicle and stretch reflex behavior in the human triceps surae. J Appl Physiol. 2008;105:226–232. doi: 10.1152/japplphysiol.90432.2008. [DOI] [PubMed] [Google Scholar]
- Edamura M, Yang JF, Stein RB. Factors that determine the magnitude and time course of human H-reflexes in locomotion. J Neurosci. 1991;11:420–427. doi: 10.1523/JNEUROSCI.11-02-00420.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Stein RB. Fusimotor control of muscle spindle sensitivity during respiration in the cat. J Physiol. 1990;422:245–264. doi: 10.1113/jphysiol.1990.sp017982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey MJ, Mazzaro N, Nielsen JB, Sinkjaer T. Ankle extensor proprioceptors contribute to the enhancement of the soleus EMG during the stance phase of human walking. Can J Physiol Pharmacol. 2004;82:610–616. doi: 10.1139/y04-077. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Nielsen JB, Mazzaro N, Sinkjaer T. Positive force feedback in human walking. J Physiol. 2007;581:99–105. doi: 10.1113/jphysiol.2007.130088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham TE, Biewener AA, Wakeling JM. Functional diversification within and between muscle synergists during locomotion. Biol Lett. 2008;4:41–44. doi: 10.1098/rsbl.2007.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Finni T, Komi PV. Behaviour of vastus lateralis muscle-tendon during high intensity SSC exercises in vivo. Acta Physiol Scand. 2003;178:205–213. doi: 10.1046/j.1365-201X.2003.01149.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Komi PV. The role of the stretch reflex in the gastrocnemius muscle during human locomotion at various speeds. J Appl Physiol. 2007;103:1030–1036. doi: 10.1152/japplphysiol.00277.2007. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Ichinose Y, Kubo K, Ito M, Imai M, Fukunaga T. Architecture of contracting human muscles and its functional significance. J Appl Biomech. 2000;16:88–98. [Google Scholar]
- Lichtwark G, Bougoulias K, Wilson AM. Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J Biomech. 2007;40:157–164. doi: 10.1016/j.jbiomech.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Maas H, Gregor RJ, Hodson-Tole EF, Farrell BJ, Prilutsky BI. Distinct muscle fascicle length changes in feline medial gastrocnemius and soleus during slope walking. J Appl Physiol. 2009;106:1169–1180. doi: 10.1152/japplphysiol.01306.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas H, Lichtwark G. Is muscle–tendon unit length a valid indicator for muscle spindle output? J Physiol. 2009;587:13–14. doi: 10.1113/jphysiol.2008.165555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V. Predictability of in vivo changes in pennation angle of human tibialis anterior muscle from rest to maximum isometric dorsiflexion. Eur J Appl Physiol Occup Physiol. 1999;79:294–297. doi: 10.1007/s004210050510. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Aagaard P, Dyhre-Poulsen P, Kjaer M. Load-displacement properties of the human triceps surae aponeurosis in vivo. J Physiol. 2001;531:277–288. doi: 10.1111/j.1469-7793.2001.0277j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T, Muraoka T, Kawakami Y, Fukunaga T. Superficial aponeurosis of human gastrocnemius is elongated during contraction: implications for modeling muscle-tendon unit. J Biomech. 2002;35:217–223. doi: 10.1016/s0021-9290(01)00196-8. [DOI] [PubMed] [Google Scholar]
- Nicol C, Komi PV. Significance of passively induced stretch reflexes on achilles tendon force enhancement. Muscle Nerve. 1998;21:1546–1548. doi: 10.1002/(sici)1097-4598(199811)21:11<1546::aid-mus29>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Sinkjaer T. Afferent feedback in the control of human gait. J Electromyogr Kinesiol. 2002;12:213–217. doi: 10.1016/s1050-6411(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Hulliger M. Muscle afferent function and its significance for motor control mechanisms during voluntary movements in cat, monkey, and man. Adv Neurol. 1983;39:93–132. [PubMed] [Google Scholar]
- Proske U. Kinesthesia: The role of muscle receptors. Muscle Nerve. 2006;34:545–558. doi: 10.1002/mus.20627. [DOI] [PubMed] [Google Scholar]
- Proske U, Wise AK, Gregory JE. The role of muscle receptors in the detection of movements. Prog Neurobiol. 2000;60:85–96. doi: 10.1016/s0301-0082(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Rack PM, Ross HF, Thilmann AF, Walters DK. Reflex responses at the human ankle: The importance of tendon compliance. J Physiol. 1983;344:503–524. doi: 10.1113/jphysiol.1983.sp014954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack PM, Westbury DR. Elastic properties of the cat soleus tendon and their functional importance. J Physiol. 1984;347:479–495. doi: 10.1113/jphysiol.1984.sp015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen EB, Dyhre-Poulsen P. Amplitude of the human soleus H reflex during walking and running. J Physiol. 1999;515:929–939. doi: 10.1111/j.1469-7793.1999.929ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Ladouceur M, Christensen LO, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Larsen B. Soleus stretch reflex modulation during gait in humans. J Neurophysiol. 1996;76:1112–1120. doi: 10.1152/jn.1996.76.2.1112. [DOI] [PubMed] [Google Scholar]
- Soman A, Hedrick TL, Biewener AA. Regional patterns of pectoralis fascicle strain in the pigeon Columba livia during level flight. J Exp Biol. 2005;208:771–786. doi: 10.1242/jeb.01432. [DOI] [PubMed] [Google Scholar]
- Soule RG, Goldman RF. Energy cost of loads carried on the head, hands, or feet. J Appl Physiol. 1969;27:687–690. doi: 10.1152/jappl.1969.27.5.687. [DOI] [PubMed] [Google Scholar]
- Stein RB, Kearney RE. Nonlinear behavior of muscle reflexes at the human ankle joint. J Neurophysiol. 1995;73:65–72. doi: 10.1152/jn.1995.73.1.65. [DOI] [PubMed] [Google Scholar]
- Taylor A, Durbaba R, Ellaway PH, Rawlinson S. Static and dynamicγ-motor output to ankle flexor muscles during locomotion in the decerebrate cat. J Physiol. 2006;571:711–723. doi: 10.1113/jphysiol.2005.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Stein RB, Murphy PR. Impulse rates and sensitivity to stretch of soleus muscle spindle afferent fibers during locomotion in premammillary cats. J Neurophysiol. 1985;53:341–360. doi: 10.1152/jn.1985.53.2.341. [DOI] [PubMed] [Google Scholar]
- Voigt M, Dyhre-Poulsen P, Simonsen EB. Modulation of short latency stretch reflexes during human hopping. Acta Physiol Scand. 1998;163:181–194. doi: 10.1046/j.1365-201X.1998.00351.x. [DOI] [PubMed] [Google Scholar]
- Winter D. The Biomechanics and Motor Control of Human Gait. Waterloo, Canada: University of Waterloo Press; 1987. [Google Scholar]