Abstract
It is well known that angiogenesis plays a critical role in the pathobiology of tumors. Recent clinical trials have shown that inhibition of angiogenesis can be an effective therapeutic strategy for patients with cancer. However, one of the outstanding issues in anti-angiogenic treatment for cancer is the development of toxicities related to off-target effects of drugs. Transcriptional targeting of tumor endothelial cells involves the use of specific promoters for selective expression of therapeutic genes in the endothelial cells lining the blood vessels of tumors. Recently, several genes that are expressed specifically in tumor-associated endothelial cells have been identified and characterized. These discoveries have enhanced the prospectus of transcriptionaly targeting tumor endothelial cells for cancer gene therapy. In this manuscript, we review the promoters, vectors, and therapeutic genes that have been used for transcriptional targeting of tumor endothelial cells, and discuss the prospects of such approaches for cancer gene therapy.
Keywords: Cancer, angiogenesis, promoter, tumor microenvironment, review
1. Introduction
1.1. Tumor growth and metastasis are angiogenesis dependent
Angiogenesis, the growth of new blood vessels from pre-existing capillaries, is a tightly regulated process that depends on the balance between angiogenesis-stimulating factors and angiogenesis inhibitors. As a physiologic process, angiogenesis is restricted mainly to embryonic development, wound healing, and ovulation. Notably, several diseases such as diabetic blindness, age-related macular degeneration, rheumatoid arthritis, psoriasis, and cancer are considered to be angiogenesis-dependent [1-4]. Dr. Judah Folkman proposed the conceptual framework for anti-angiogenesis therapies for cancer treatment in a seminal paper published in 1971 [5]. He hypothesized that tumors are unable to grow beyond 1 mm3 without sustained recruitment of new capillary blood vessels [5]. Therefore, the prediction was that one could treat cancers by targeting the microvascular endothelial cells in the tumor bed instead of the tumor cells themselves [5,6]. Today, this hypothesis is supported by a large body of evidence and is widely accepted [2,7-9]. Inhibition of tumor angiogenesis has been shown to be an effective anti-cancer treatment [10-14]. The recent approval of anti-angiogenesis (antiangiogenic) drugs, such as Avastin, Sorafenib, and Sunitinib, by the Food and Drug Administration (FDA) as first or second line cancer therapy clearly demonstrates that the therapeutic targeting of blood vessels can be a viable strategy for cancer therapy [15].
1.2. Role of activated endothelial cells in tumor angiogenesis
The process of angiogenesis involves the activation, proliferation, migration and sprouting of endothelial cells [1,16]. The first step to initiate angiogenesis is endothelial cell activation through the angiogenic switch. Once angiogenesis-stimulating factors from the tumor microenvironment bind to their receptors on the endothelial cells of nearby preexisting blood vessels, quiescent endothelial cells become activated. The activated endothelial cells produce molecules including proteolytic enzymes, which open spaces in the surrounding basement membrane, allowing for their migration and proliferation towards the tumor cells [3, 4, 16]. With the involvement of adhesion molecules, enzymes and other molecules of the extracellular matrix, activated endothelial cells organize into tube-like sprouts connected with preexisting vessels, recruit supporting pericytes, and form a new blood vessel that can bridge the pre-existing vessel to the tumor [3,4,16]. Therefore, activated endothelial cells composed a critical element of tumor angiogenesis.
Tumor vessels are abnormal with increased permeability, tortuosity, excessive uneven branches, and surrounded by loosely attached pericytes [4,17]. Notably, tumor associated endothelial cells differ from endothelial cells of normal tissue also at molecular level [18-22]. St. Croix and colleagues [18] compared gene expression patterns of endothelial cells derived from blood vessels of normal and malignant colorectal tissues. They reported that among over 170 transcripts predominantly expressed in endothelial cells, 79 were differently expressed in normal versus malignant tissues, including 46 transcript that were specifically elevated in tumor associated endothelial cells. Buckanovich and colleagues isolated ovarian tumor endothelial cells by laser-capture microdissection, validated 12 novel tumor vascular markers through genome-wide transcriptional profiling [20]. Ghilardi and colleagues investigated by microarray analysis the gene expression profiles of tumor (ovarian carcinoma) derived endothelial cells and normal endothelial cells, and identified 158 transcripts highly expressed by tumor endothelial cells. Among them, ADAM23, GPNMB and PRSS3 were in vivo localized on tumor blood vessels by in situ hybridization [22]. Most likely, the list of genes and proteins that are exclusively expressed in tumor endothelial cells will increase with time. The observed molecular differences between tumor-associated endothelial cells and physiological endothelial cells provide the scientific rationale for transcriptional targeting strategies for cancer therapy.
Targeting tumor endothelial cells for cancer treatment may also benefit from the fact that tumor endothelial cells are easily accessible and tend to be more genetically stable [2]. The high ratio of tumor cells to endothelial cells may lead to an amplification of the effect of angiogenesis-targeted tumor treatment. Together, these features make the transcriptional targeting of endothelial cells an attractive strategy for cancer treatment.
1.3. Targeting tumor endothelial cells
The understanding of the molecular biology of angiogenesis has led to several different approaches that target endothelial cells for inhibition of tumor angiogenesis. To date, over 20 angiogenesis-stimulating factors have been found in the tumor microvascular environment. Among them, vascular endothelial growth factor (VEGF), acidic and basic fibroblast growth factors (aFGF and bFGF), platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) have been extensively studied in vitro and in vivo. VEGF is widely considered the most potent mediator of angiogenesis [13,23]. The binding of VEGF to its receptors on endothelial cells, triggers signaling pathways that result in the activation, proliferation and migration of endothelial cells, as well as in the promotion of endothelial cell survival and vascular permeability. This knowledge led to the development of monoclonal antibodies (e.g. Avastin) and small molecule inhibitors (e.g. Sorafenib and Sunitinib) designed to block VEGF-mediated signaling pathways. Soluble recombinant proteins (e.g. sVEGFR-1) have also been shown to effectively inhibit this pathway [24-26]. In addition, the observation that tumor endothelial cells express αvβ3 and αvβ5 integrins necessary for endothelial cell adhesion, migration, proliferation, and survival led to the development of therapies aimed at the blockade of this pathway [27,28]. Notably, monoclonal antibodies against these integrins have shown inhibitory effects on tumor angiogenesis [29,30].
In addition to the approaches described above, there is an increasing interest in genetically targeting tumor endothelial cells to inhibit angiogenesis and disrupt already established tumor microvascular networks. Gene therapy refers to transfer of exogenous genes into selective somatic cells of a patient to obtain a therapeutic effect [31, 32]. In regards to tumor treatment, it requires that the target cells are easily accessible and critical for the pathophysiology of tumor. The distinct characteristics of tumor endothelial cells make them an excellent target for gene therapy. There are four genetic approaches for genetically targeting tumor endothelial cells, i.e. transductional targeting, transcriptional targeting, posttranscriptional targeting, and functional targeting. The purpose of this manuscript is to review the existing literature on transcriptional targeting of tumor endothelial cell for cancer gene therapy.
2. Strategies for transcriptionaly targeting tumor endothelial cells for cancer therapy
2.1. Scientific basis for transcriptional targeting
Transcriptional targeting involves the use of approaches in which expression of a therapeutic gene in a specific cell population is regulated by placing the gene downstream of a target cell specific promoter. It is different from, but could be combined with, transductional targeting approaches that involve the chemical or genetic modification of vectors to achieve selective delivery of transgenes to desired target cell populations. This can be achieved by enhancing the tropism of a vector to the target cell population [33].
It is well known that eukaryotic genes consist of sequences that encode mRNAs, and non-coding (regulatory) sequences that include promoters and enhancers [34,35]. Promoter is the regulatory sequence just preceding a coding sequence, and is frequently used in transcriptionaly targeted gene therapy strategies. Response elements are also regulatory sequences that function by allowing the binding of transcription factors. A few response elements are located within the promoter region, but most are outside the promoter and often they are clustered to form an enhancer or silencer region. It is believed that the timely and temporal transcription of specific genes is determined by the availability of specific promoters associated with specific combination of transcription factors in any given cell [34-36] (Fig. 1). As indicated earlier, numerous genes have specificity for endothelial cells in general or are uniquely expressed in tumor endothelial cells. Such regulatory elements are critical for successful transcriptional targeting of tumor endothelial cells in cancer gene therapy.
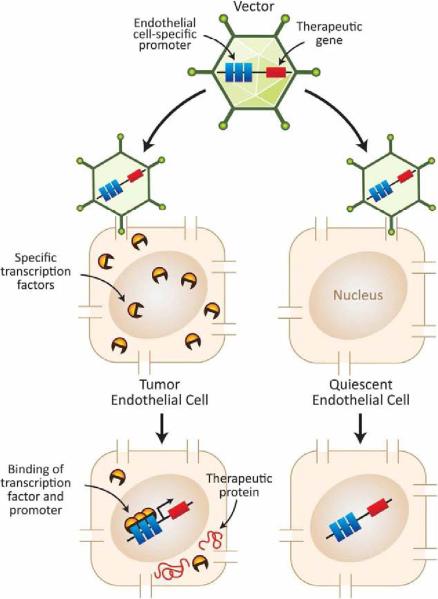
Fig. 1.
Schematic diagram depicting a general strategy for transcriptionaly targeted cancer gene therapy. The specificity of treatment is due to the use of a tumor endothelial cell specific promoter to drive expression of the therapeutic gene. The theory behind this approach is that only tumor endothelial cells, but not normal endothelial cells from physiologic tissues and organs, have the required active set of transcriptional factors to drive expression of the therapeutic gene.
2.2. Endothelial cell-specific promoters
As mentioned above, transcriptional targeting gene therapy strategies aim high specificity and high transcription of transgenes in defined cell populations or tissues through the use of cell/tissue-specific promoters or regulatory elements. Therefore, isolation and characterization of cell/tissue-specific promoters is of considerable interest. Over last 10 years, several endothelial cell-specific promoters were discovered and explored for the transcriptional targeting of activated endothelial cells of tumor blood vessels. They include VEGFR-1 (flt-1) [37], VEGFR-2 (KDR/flk-1) [38], von Willebrand Factor (vWF) [39], tyrosine kinase with immunoglobulin and epidermal growth factor homology domains (Tie1 and Tie2/TEK) [40,41], vascular endothelial (VE)-cadherin [42], intercellular adhesion molecule (ICAM-2) [43], endothelial selectin (E-selectin, ELAM-1, CD62E or LECAM-2) [44], endothelial nitric oxide synthase (eNOS) [45], and preproendothelin-1 (PPE-1) [46] (Table 1). The following is a discussion on the characterization and potential use of such promoters for gene therapy.
Table 1.
Promoters, vectors and transgenes studied in transcriptional targeting endothehal cell gene therapy.
| Promoter | Vector | Transgene | Animal model/cell lineage | In vitro/In vivo | References |
|---|---|---|---|---|---|
| VEGFR-1 | adenovirus | LacZ | Teratocarcinoma | in vitro | [60] |
| adenovirus | LacZ | Human saphenous vein, mouse liver | in vitro, ex vivo, in vivo | [53] | |
| adenovirus | Luc | Stroke-prone spontaneously hypertension | in vitro, ex vivo | [141] | |
| adenovirus | TRAIL | Prostate cancer | in vitro, in vivo | [160] | |
| adenovirus | Luc, CEA | Mouse endothelial cell | in vitro, in vivo | [54] | |
| baculovirus | GFP | Retinal vasculature | in vitro, in vivo | [164] | |
| plasmid | LacZ | Lewis lung carcinoma, melanoma | in vitro, in vivo | [55] | |
| retrovirus | LacZ | Kaposi's sarcoma | in vitro, in vivo | [96] | |
| VEGFR-2 | adenovirus | HSV-TK | HCC | in vitro, in vivo | [156] |
| adenovirus | iCaspase-9 + LacZ | Tumor xenografts | in vitro, in vivo | [74, 153] | |
| adenovirus | LacZ | HUVEC | in vitro | [136] | |
| retrovirus | LacZ | Kaposi's sarcoma | in vitro, in vivo | [96] | |
| retrovirus | TNFα | Endothelioma cell | in vitro | [101] | |
| lentivirus | GFP/nitroreductase | HUVEC | in vitro | [97] | |
| VEGFR-2-endoglin | adenovirus | LacZ | HUVEC | in vitro | [136] |
| VEGF | adenovirus | sVEGFR-2 | Prostate cancer | in vitro, in vivo | [142] |
| Tie1 | lentivirus | GFP/nitroreductase | HUVEC | in vitro | [97] |
| Tie2 | plasmid | LacZ | Transgenic mice | in vitro, in vivo | [165] |
| plasmid | slΚB alpha | Transgenic mice | in vitro, in vivo | [166] | |
| lentivirus | GFP/nitroreductase | Mammary carcinoma xenograft | in vitro, in vivo | [97] | |
| lentivirus | EGFP | HUVEC | in vitro | [134] | |
| vWF | adenovirus | LacZ | Human saphenous vein, mouse liver | in vitro | [53] |
| plasmid | LacZ | Lewis lung carcinoma, melanoma | in vitro, in vivo | [55] | |
| adenovirus/ retrovirus | HSV-TK/lacZ | HUVEC | in vitro | [116] | |
| plasmid | antisense iNOS RNA | Mouse brain endothelial cell | in vitro | [117] | |
| VE-Cadherin | plasmid | HSV-TK | Transgenic mice | in vitro, in vivo | [93] |
| lentivirus | GFP/nitroreductase | in vitro | [97] | ||
| ICAM-2 | adenovirus | LacZ | Human saphenous vein, mouse liver | in vitro | [53] |
| retrovirus | LacZ | Kaposi's sarcoma | in vitro, in vivo | [96] | |
| lentivirus | GFP/nitroreductase | HUVEC | in vitro | [97] | |
| plasmid | CD59 | Transgenic mice | in vitro, in vivo | [95] | |
| E-selectin | adenovirus | Luc | Mouse endothelial cell | in vitro | [102] |
| retrovirus | TNFα | Mouse endothelial cell | in vitro | [101] | |
| plasmid | Diphtheria toxin | HUVEC | in vitro | [167] | |
| PPET-1 | retrovirus | HSV-TK | Tumor xenograft | in vitro, in vivo | [124] |
| adenovirus | GFP | Lewis lung carcinoma xenograft | in vitro, in vivo | [122] | |
| plasmid | Lipoxygenases | Transgenic mice | in vitro, in vivo | [123] | |
| eNOS | plasmid | LacZ | Transgenic mice | in vitro, in vivo | [112] |
| Beta-actin | rAAV | sVEFGR-2 | Renal tumor | in vitro, in vivo | [146] |
2.2.1. VEGFR-1 and VEGFR-2 promoters
VEGFR-1 and VEGFR-2 are the two primary VEGF receptors in vascular endothelial cells [47-51]. The VEGFR-1 promoter was isolated and functionally characterized by Morishita and collaborators [37]. They demonstrated that a 1-kb DNA fragment of the 5′-flanking region of human VEGFR-1 gene (region from -748 to +284 bp) is involved in endothelial-related gene expression. The authors indicated that VEGFR-1 is expressed primarily in the endothelium and is likely to play a role in tumor angiogenesis and embryonic vascularization. They predicted that the VEGFR-1 promoter could be useful in functional studies on the regulation of endothelial-specific gene expression and also as a tool for the targeting of the expression of exogenously introduced genes to the endothelium [37]. DNA sequencing revealed that one TATA box, four GC boxes, nine E26 transformation specific (ETS) motifs and one cAMP response element (CRE) motif were present in the upstream (489 bp) region of exon 1 [52]. Functional analyses showed that the -229 to +8 region is essential for cell type-specific expression of VEGFR-1. Deletion mutant analysis indicated the possible existence of negative and positive regulatory elements in the region comprised between -911 and -435, +8 and +276, respectively [52]. These results suggested that multiple regulatory factors are involved in the transcriptional regulation of VEGFR-1 gene expression in a cell type-specific manner. Nicklin and collaborators [53] used an adenoviral vector in an ex vivo human gene therapy model and demonstrated that VEGFR-1 promoter induces LacZ gene expression primarily in endothelial cells. Reynolds and colleagues [54] reported that the combination of transductional targeting to pulmonary endothelium with angiotensin-converting enzyme (ACE), and to endothelial cells with the VEGFR-1 promoter, resulted in a synergistic improvement in the selectivity of reporter gene expression in the lung endothelial versus the liver. Minami and collaborators [55] compared the vWF promoter and the VEGFR-1 promoter by transcriptional targeting the hypoxanthine phosphoribosyl transferase gene locus of mice. The authors demonstrated that the vWF promoter allows for reporter gene expression in a subset of endothelial cells in the heart, skeletal muscle, and in the brain [55]. In contrast, the VEGFR-1 promoter directs expression in all vascular beds, except for the liver. Several reports indicate that the VEGFR-1 gene is deregulated in tumors [56-59]. Of note, using an adenoviral vector containing the LacZ reporter gene under the control of the VEGFR-1 promoter, Bauerschmitz and colleagues [60] demonstrated high reporter gene expression in teratocarcinoma lines. Such studies demonstrate that the VEGFR1 promoter may not be specific to tumor endothelial cells and implied that gene therapy using a VEGFR-1 promoter based strategy might also target tumor cells directly, in addition to the tumor blood vessels.
Patterson and collaborators isolated and characterized the human VEGFR-2 promoter [38]. They demonstrated that the transcription start site was localized to a nucleotide 303 base pairs (bp) upstream of the initiation methionine codon. The 5′-flanking sequence is GC rich, contains five Sp1 elements and a single transcription start site, but no TATA consensus sequence. The maximal promoter activity of the 5′-flanking region resides within a fragment from -225 to +127 relative to the transcription initiation site, and that deletions from -95 to -37 result in complete loss of promoter activity, defining this segment as the core promoter for human VEGFR-2 [38]. Wu and colleagues [61] further demonstrated that the VEGFR-2 promoter contains a functional initiator element (Inr) that is bound by TFII-I (transcriptional factor II-1), a multifunctional Inr-binding nuclear protein that regulates transcription activity. Both the functional Inr and the TFII-I activity are essential for initiation of transcription [61]. It was reported that a hypoxia-inducible factor-2 alpha (HIF-2 alpha) and an ETS binding site in tandem within the VEGFR-2 promoter acts as a strong enhancer element for this gene. The interaction between HIF-2 alpha and endothelial ETS factors is required for the full transcriptional activation of VEGFR-2 in endothelial cells [62]. Mounting evidence shows that VEGFR-2 is highly expressed in tumor endothelial cells [63-66]. Millarer and colleagues reported that VEGFR-2 plays an important role in angiogenesis and tumor formation in a C6 glioma xenograft model using a dominant-negative strategy [67]. With the same strategy, they demonstrate that VEGFR-2 is involved in the growth of a wide range of solid tumors, including mammary, ovarian, and lung carcinoma, as well as glioblastoma. Notably, survival times in rats bearing intracerebral tumors were prolonged using the same dominant-negative strategy [68]. Several publications also showed that VEGFR-2 is expressed by tumor cells [69-72]. In respect of transcriptional targeting tumor endothelial cell with VEGFR-2 promoter, Heidenreich and collaborators investigated the activity of cis-acting sequences of the murine VEGFR-2 (Flk1) gene in the tumor endothelium of tumor models [73]. Tumors were grown in transgenic mice that express the LacZ reporter gene under the control of a 939-bp Flk-1 promoter fragment and an enhancer element located within a 2.3-kb fragment of the first intron. In the experimental tumor models examined, strong endothelium-specific reporter gene expression was observed while being absent from most blood vessels in normal adult tissue [73]. The expression patterns of the LacZ reporter gene correlated well between established tumors grown in Flk1-LacZ transgenic mice and tumors grown in Flk-1 +/LacZ knock-in mice that express the LacZ reporter gene from the endogenous Flk-1 locus. The endothelium-specific activity of the Flk-1 promoter/enhancer sequences in three different experimental tumor models demonstrates that the regulatory sequences that mediate the up-regulation of Flk-1 in the tumor endothelium are contained in the Flk-1 promoter/enhancer sequences used, and that these elements function relatively independently of the tumor type [73]. Of note, our research group demonstrated that a 494 bp DNA fragment containing sequence from -226 to +268 of human VEGFR2 promoter allows for potent and specific expression of transgenes in proliferating human endothelial cells in vitro and in vivo [74] (Fig. 2).
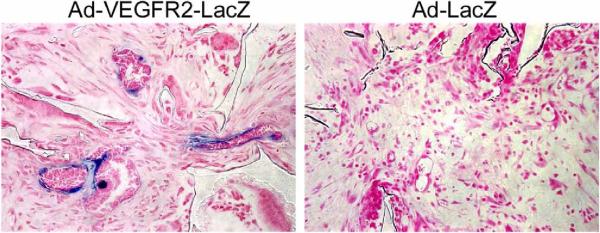
Fig. 2.
Example of transcriptionally targeted expression of a reporter gene in angiogenic endothelial cells. Adenoviruses expressing LacZ under the transcriptional regulation of the human VEGFR-2 promoter mediated expression of reporter gene primarily in neovascular endothelial cells in vivo. Representative photomicrographs depicting B-gal staining of scaffolds containing human endothelial cells and implanted in immunodeficient mice. Fourteen days after implantation, scaffolds were injected with either adenoviruses expressing LacZ under human VEGFR2 promoter regulation (Ad-hVEGFR2-LacZ), or with promoterless control adenoviruses (Ad-LacZ).
2.2.2. Tie1 and Tie2 promoters
Tie1 and Tie2 are receptor tyrosine kinases expressed by endothelial cells [75-81]. They are uniformly expressed in blood vessels during embryonic development. However, the expression of Tie2 mRNA in extra-embryonic tissues begins on day 7.5 pc, half a day earlier than the expression of Tie1 [40,82]. The Alitalo group reported that the mRNA expression of Tie1 and Tie2 is enhanced during angiogenesis associated with ovulation and wound healing, as well as in human glioblastomas and metastatic melanomas [81,83,84]. It has been reported that Tie1 is indispensable for maintaining vascular integrity, and its absence results in vessel rupture and hemorrhage [85,86]. Tie2 regulates the survival of endothelial cells, controls vascular permeability, and regulates angiogenic capillary sprouting [85,87]. It has been demonstrated that the 0.8 kb mouse Tie1 promoter fragment is sufficient to confer the endothelial cell-specific expression [40]. The Tie1 promoter lacks the typical TATA and CAAT boxes, contains a GC sequences, a GT repeat and AP-2, Ets-1 and PEA3 binding sites. An octamer (ATGCAAAT) site at -312 bp appears to confer endothelial cell specific gene expression [40, 88]. Schlaeger and collaborators isolated and characterized a 1.2-kb 5′ flanking region of the Tie2 promoter, and demonstrated that this promoter is capable of directing reporter gene expression specifically in a subset of endothelial cells in transgenic mouse embryos. However, transgene activity was restricted to early embryonic stages and not detectable in adult mice. They also identified and characterized an autonomous endothelial-specific enhancer in the first intron of the mouse Tie2 gene. Combination of the 1.2-kb Tie2 promoter with a fragment containing this enhancer allows it to target reporter gene expression specifically and uniformly to virtually all-vascular endothelial cells throughout embryogenesis and adulthood [41,89]. In the context of transcriptional targeting the tumor endothelium, De Palma and collaborators transduced bone marrow progenitors with lentiviral vectors expressing genes from transcription-regulatory elements of Tie2 promoter/enhancer [90]. When tumors were grown in the transplanted mice, the transduced hematopoietic population 'homed' to the tumor and closely interacted with the vascular endothelial cells. These Tie2-expressing cells had a distinguishable phenotype and were present selectively at angiogenic sites. By delivering a 'suicide' gene, they selectively eliminated the TEM cells and achieved significant inhibition of angiogenesis and tumor growth without systemic toxicity [90].
2.2.3. VE-cadherin and ICAM-2 promoter
VE-cadherin is one of eight members of the cadherin family of cell adhesion molecules [91]. VE-cadherin appears to be specifically expressed at the earliest stages of vascular development in embryonic tissue [42]. A 2.5-kb (-2486 to +24) promoter of VE-cadherin was isolated and characterized as enable of directing endothelial cell-specific expression in transgenic mice [92]. VE-cadherin promoter contains a GT box (-48 to -40) and two Ets binding sites (-93 to -90 and -109 to -106). Therefore, transcription factors of the Ets family and Sp family appear to regulate this promoter's activity [92]. The Huber group produced transgenic mice expressing the HSV-thymidine kinase gene under regulation of the VE-cadherin promoter [93]. Lewis lung carcinoma cells were injected subcutaneously to establish tumors and to test the effect of ganciclovir on tumor growth. In two independent transgenic lines, ganciclovir treatment resulted in a 66-71% reduction in tumor volume as compared to controls. Tumor growth inhibition was accompanied by a marked reduction in tumor microvascular density and an increase in tumor cell death, suggesting that tumor growth inhibition was caused by a reduction in tumor angiogenesis [93].
ICAM-2, a cell surface glycoprotein, is a receptor for lymphocyte function-associated Ag-1 (LFA-1). ICAM-2 can be expressed in resting lymphocytes and monocytes, but in general its expression in tissues is observed primarily in the vascular endothelium [94]. The ICAM-2 promoter has been identified as a 0.33 kb, TATA-less, containing a Sp1 site, two GAGA sites and a 8-bp palindromic sequence [43]. Using the ICAM-2 promoter for driving the expression of human CD59 in transgenic mice, a strong and uniform expression of CD59 on the endothelial cells of blood vessels in the heart, kidney, lung, liver, and pancreas was observed [95]. The possibility of using ICAM-2 promoter for transcriptional targeting gene therapy was explored with varying degrees of success in several manuscripts in vitro and in vivo [53,96,97].
2.2.4. E-selectin promoter
E-selectin is a heavily glycosylated transmembrane protein of endothelial cells. However, it's not constitutively expressed in endothelial cells, instead, its expression is transcriptionally restricted in the endothelial cells activated by TNF-α and IL-1 [98]. Following stimulation with these cytokines, peak expression of E-selectin occurs within 4 hours and then declines by 24 hours [98]. E-selectin expression was high in proliferative phase hemangiomas specimens and was co-localized with dividing microvascular endothelial cells, but not detected in quiescent endothelium [99]. These two key characteristics of E-selectin expression, i.e. cytokine inducibility and tissue specificity, have prompted the interest in the regulatory elements of this gene. The E-selectin promoter contains a consensus TATAA element located upstream of the transcriptional start site, an inverted CCAAT box and consensus NF-κB and AP-1 binding sites [44]. A recent study involving analyses of progressive deletion together with site-specific mutagenesis of the E-selectin promoter indicated that the Abd-B-like HOX DNA-binding motif, CAATTTTATTAA, located in the proximal region spanning bp -210 to -221 upstream of the transcription start site was necessary for promoter induction by HOXA9 [100]. They also showed that HOXA9 binds temporally, in a TNFα-dependent manner, to the region containing this Abd-B-like element in vivo [100]. Jaggar and collaborators employed the E-selectin promoter for transcriptional targeting of stimulated endothelial cells and demonstrated a 10- to 11-fold increase in transgene expression in endothelial cells as compared to control fibroblasts [101]. This promoter was also investigated in regards to angiogenesis-targeted gene therapy. Exposure of endothelial cells transduced by a reporter gene driven by e-selectin promoter to TNFα, or tumor conditional medium, also induced high expression of the reporter gene [102]
2.2.5. eNOS promoter
eNOS is a well-characterized endothelial cell gene. It plays a critical role in the control of blood vessel tone and remodeling, homeostasis, angiogenesis, and the mobilization of endothelial progenitor cells [103-107]. eNOS is responsible for the production of the majority of the nitric oxide (NO) in endothelial cells. eNOS promoter does not contain a TATA box, but has Sp1, Ets, GATA, NF-1, AP-1, shear-stress response elements, and sterol regulatory elements [45]. Therefore, a variety of transcription factors known to regulate endothelial gene expression are capable of regulating eNOS promoter activation [108]. In gene therapy, eNOS is primarily used as a therapeutic gene for cardiovascular diseases [109-111]. Transgenic mice were generated with a construct containing the 1,600-bp 5′ flanking region of the eNOS promoter coupled to the coding sequence of LacZ. In multiple independent lines of transgenic mice, reporter gene expression was restricted to the endothelium. However, β-galactosidase activity was limited to a subpopulation of endothelial cells within the heart, skeletal muscle, brain, and aorta, but notably absent in other vascular beds that otherwise express the endogenous gene [112]. Notably, the eNOS promoter was selectively upregulated by conditioned media from cardiac myocytes, skeletal myocytes, and brain astrocytes. The induction of the eNOS promoter by cardiac myocytes was mediated by both platelet derived growth factor (PDGF)-dependent and PDGF-independent signaling pathways, whereas induction by skeletal myocytes and brain astrocytes was mediated by a PDGF-independent pathway [112]. These findings suggest that the eNOS gene is regulated by vascular bed-specific signaling pathways.
2.2.6. Von Willebrand Factor promoter
vWF is a complex multimeric glycoprotein synthesized by vascular endothelial cells and megakaryocytes and is a commonly used immunohistochemical marker for these cell lineages. It is required for the adhesion of platelets to sites of vascular damage, linking specific platelet membrane receptors to constituents of sub-endothelial connective tissue. It also binds to and stabilizes blood coagulation factor VIII in the circulation [113]. The vWF gene spans around 178 kb and contains 52 exons. The 5′ flanking region contains an AT-rich region resembling a TATA element and a (GT)19 repetitive sequence. The 5′ non-coding region is encoded entirely by the first exon, and the second exon begins with the first nucleotide of the initiator codon. Most of the signal peptide is encoded by the second exon [39]. A 733-bp vWF promoter that included 487 bp of 5′flanking sequence and the first exon (246 bp) was employed to generate transgenic mice. In situ histochemical analyses of the reporter activity demonstrated that the vWF promoter targeted expression of reporter gene to a subpopulation of endothelial cells in the yolk sac and adult brain. While the activity was absent in the vascular beds of the spleen, lung, liver, kidney, testes, heart, and aorta, as well as in megakaryocytes [114]. The same strategy with a vWF promoter containing 2,182 bp of 5′ flanking sequence, the first exon and first intron driving a LacZ to generated transgenic mice, beta-galactosidase expression was detected within endothelial cells in the brain, heart, and skeletal muscle [115].
In vitro reporter gene assays showed that the vWF putative promoter, which encompasses most of the first noncoding exon, had stronger activity in endothelial cells. Although the promoter activity was low when employed as an internal promoter for retroviral and adenoviral vectors, when used to drive the herpes simplex virus thymidine kinase (HSV-TK) gene the vWF promoter preferentially directed the suppression of endothelial cell growth in the presence of prodrug ganciclovir [116]. A 750-bp vWF promoter was evaluated to drive antisense RNA against iNOS in cerebral endothelial cells. A significant reduction in NO synthesis by antisense RNA against iNOS gene demonstrated the protection of transfected endothelial cells against cytokine-mediated injuries [117].
2.2.7. PPET-1 promoter
Endothelin-1 (ET-1) is a 21-amino acid peptide that was originally isolated and characterized as a vasoconstrictor synthesized by endothelial cells [118]. More recently, ET-1 has been recognized as the most potent and predominant endothelin that plays a pivotal role in the regulation of vascular tone and in the etiology of the atherosclerotic vascular disease [119]. Human ET-1 is derived from a 212-amino acid precursor, preproendothelin (PPET-1) [46]. The gene for human PPET-1 contains 5 exons. A 119-bp promoter (-86 to -204) was identified and characterized as capable of inducing potent and specific transcription in endothelial cells [120]. Transgenic mice were generated to evaluate the activity of the PPET-1 promoter containing the 5.9-kb 5′ flanking region. High-level expression of the reporter gene in endothelial cells of both large and small arteries and lower levels of expression in veins and capillaries were observed [121]. A tissue-specific gene therapy to angiogenic blood vessels of tumor metastasis was investigated using an adenoviral vector containing the PPET-1 promoter. Genes activated by the PPET-1 promoter were highly expressed in bovine aortic endothelial cells in vitro [122]. Systemic injection of the recombinant adenoviral vectors expressing AdPPET-1-luciferase to C57BL/6 mice resulted in higher activity of PPET-1 promoter in the aorta and vascularized tissues such as heart, kidney, lung and pancreas as compared with adenoviruses driving expression of the reporter gene with the non-specific CMV promoter [122]. Systemic administration of the adenoviral vector in mice bearing Lewis lung carcinoma resulted in high and specific activity of PPET-1 in the new vasculature of primary tumors and lung metastasis. Cellular distribution of the gene revealed highest expression in angiogenic endothelial cells at the metastatic sites [122]. Using transgenic mice that overexpress 15-LO-1 (15-lipoxygenase) in endothelial cells under the regulation of the PPET-1 promoter, Harats and colleagues found that 15-LO-1 inhibited tumor and metastasis growth in the transgenic mice in two different models of cancer (mammary gland and Lewis lung carcinoma). This inhibition was correlated with a higher number of apoptotic cells in metastases of transgenic mice [123]. Mavria and collaborators employed a hybrid PPET-1 promoter transcriptionally targeting HSV-TK gene to the endothelial cells of xenograft tumors. They observed that tumor growth was reduced and survival was increased in response to ganciclovir treatment. Treatment resulted in widespread vascular disruption and tumor cell apoptosis [124].
2.3. Vectors for transcriptional targeting of tumor endothelial cell
To achieve the aim of transcriptionally targeted gene therapy, it is necessary to have a delivery system that is biocompatible, nontoxic, and able to efficiently deliver and express transgenes in the targeted cell population. A variety of vectors for transcriptional targeting endothelial cells have been explored, including viral and non-viral vectors (Table 1). Viruses have efficient mechanisms to introduce their genetic materials into host cells. Therefore, viral vectors are typically designed by keeping their transduction ability, but removing the genes that regulate replication and pathogenicity, as well as the genes that regulate immunogenic viral antigens. Viral vectors have been a common starting point in many experimental gene therapy protocols. Currently, viral vectors offer the best choices for efficient gene delivery. In addition, they can be easily produced and scaled to high titers in a convenient and reproducible production scheme [32, 125]. According to their ability to integrate the genetic material into the host genome, viruses can be classified into two major groups: A) Integrating viruses, such as retrovirus and lentivirus, which induce stable expression of transgene in targeted cell; and B) non-integrating viruses, such as adenovirus and herpes virus, which mediate transient expression of foreign genes [126].
2.3.1. Retroviral vectors
Retroviruses are single-stranded RNA (ssRNA) viruses in which tRNA serves as a primer for the mRNA synthesis [127]. The mRNA is then reverse transcribed into DNA, which is then integrated into the host genome at multiple sites, particularly those that are transcriptionally active. Retroviral vector-mediated gene transfer has been central to the development of gene therapy, especially when permanent gene transfer is the desired outcome [127]. In respect of targeting tumor endothelial cells for gene therapy, retroviral vectors have the positive aspect of depending on cell division for integration and expression, providing one level of specificity [128]. Millauer and collaborators investigated the biological relevance of the VEGFR-2/ligand system for angiogenesis using a retrovirus encoding a dominant-negative mutant of the VEGFR-2 to infect endothelial target cells in vivo, and observed that tumor growth is prevented in nude mice [67]. Another research group developed hybrid long terminal repeat (LTR) retroviruses for transcriptional targeting of tumor endothelial cells for gene therapy [96,129,130]. By using these hybrid LTR retroviruses, sequences from each of the human promoters for VEGFR-1, ICAM-2, and VEGFR-2 were investigated. The chosen fragments were used to replace the enhancer or combined enhancer and proximal promoter regions of the viral LTR. All showed activity in primary human breast microvascular endothelial cells [96]. Another publication from this group reported the use of regulatory elements of the endothelial-specific PPET-1 promoter to express HSV-TK gene in endothelial cells of subcutaneous tumor xenografts. Administration of ganciclovir (GCV) reduced tumor growth and resulted in extensive hemorrhagic necrosis of the xenograft [128]. Notably, their experiments also showed that vascular targeting combined with appropriate chemotherapy is more effective than either therapy alone [128].
2.3.2. Lentiviral vectors
Lentiviral vectors have all the characteristics of retroviruses for gene therapy, such as large cloning capacity (close to 10 kb); integrating transgenes into the chromosomes of target cells, a prerequisite for long-term expression; and not transferring virus-derived coding sequences, avoiding the recognition and destruction of transduced cells by vector-specific cytotoxic T lymphocytes. Most importantly, because their preintegration complex (virus “shell”) can get through the intact membrane of the nucleus of the target cell, lentiviral vectors can be used to delivery transgenes to both dividing and non-dividing cells [131]. In the context of transcriptional targeting gene therapy, Lotti and colleagues developed a lentiviral vector by replacing the viral long terminal repeat (LTR) enhancer with cell lineage-specific, genomic control elements [132]. Naldini and collaborators [90,97,133] generated a panel of vesicular stomatitis virus-pseudotyped lentiviral vectors engineered for endothelial cell-specific expression. They cloned a wide repertoire of transcription regulatory sequences from genes preferentially expressed in endothelial cells (Tie1, Tie2, Flk-1, VE-Cad, and ICAM-2) into self-inactivating lentivirus to drive expression of the marker gene encoding green fluorescent protein (GFP). Alternatively, they expressed the conditionally toxic nitroreductase, and compared these viruses with viruses ubiquitously expressing phosphoglycerate kinase (PGK) and non-specific cytomegalovirus (CMV) promoters. They evaluated the efficiency and specificity of vector expression in a panel of human primary cultures, including endothelial cells, fibroblasts, neurons, lymphocytes, and hematopoietic progenitors, and in tumor cell lines in vitro. They observed that vectors containing promoter and enhancer sequences from the Tie2 gene achieved remarkable specificity of expression in endothelial cells in vitro and in vivo. Another research team developed a lentiviral vector using a modified Sindbis virus envelope with enhanced tropism to endothelial cells, and with endothelial cell-specific promoters [134].
2.3.3. Adenoviral vectors
Adenoviral vectors have been used for gene therapy because of their stability, high infection efficiency, high transgene expression in vivo, and relative low risk for secondary mutagenesis. Notably, adenoviruses mediate transient expression of transgenes [31,135]. Savontaus and colleagues reported that conditionally replicating adenovirus (CRAD) can be transcriptionally targeted to dividing endothelial cells [136]. Curiel's research group published several manuscripts using adenoviral vectors in transcriptional targeting strategies for cancer therapy [53,54,60,137-140]. They investigated a variety of tissue/cell-specific promoters for transcriptional targeting of the vascular endothelium of tumors. They also modified the adenoviral vectors to improve their targeting specificity. For instance, in order to selectively target pulmonary vascular endothelial cells, in addition to using VEGFR-1 promoter, they replaced the native fiber in the adenoviral capsid by a chimeric fibritin trimerization motif fused to the CD40 ligand. This resulted in permanent ablation of native viral receptor tropism and simultaneously offered flexibility in the generation of novel vector tropism to specific cell populations. In this case, in vivo administration of modified adenovirus (AdflthCD40) to hCAR mice resulted in hCD40 expression in the pulmonary vasculature, which was successfully targeted with systematically administered Ad5Luc.FF/CD40L [140]. A transductional targeting approach was used by Work and collaborators to improve vascular cell infectivity through RGD peptide insertion into adenovirus fibers, combined with transcriptional targeting to endothelial cells using a fragment of VEGFR-1 promoter. They observed that Adenoviral-mediated transduction can be beneficially modified in vitro and in vivo by combining fiber modification and a cell-selective promoter within a single-component vector system [141]. Kaliberov and collaborators investigated the effects of VEGFR-2 (KDR) gene transfer using a recombinant adenoviral vector under control of the human VEGF promoter element on the growth of human prostate cancer cells [142]. Their data suggested that selective transcriptional targeting of soluble VEGFR-2 employing a radiation inducible promoter can effectively inhibit tumor growth.
2.3.4. Adeno-associated viral vectors
Adeno-associated virus (AAV) is nonpathogenic human parvovirus with a 4.7 kb ssDNA genome. AAV infection requires helper functions that can be supplied by coinfection with helper viruses. AAV have several major advantages such as stable integration, low immunogenicity, long-term expression, and the ability to infect both dividing and nondividing cells. Among the multiple serotypes that have been isolated, AAV serotype 2 (AAV2) is the best characterized and has been the most frequently employed recombinant AAV vector [143]. Zacchigna and colleagues constructed AAV vector containing the matrix metalloproteinases (MMP) inhibitor (Timp1) under control of CMV promoter. Following in vivo administration to a Kaposi's sarcoma (KS) xenograft model, it significantly impaired tumor progression, limited the development of vascular structures, and resulted in extensive areas of cell death [144]. White and collaborators employed human venous endothelial cell-targeting peptide-modified AAV transductionally targeted LacZ to venous endothelial cells [145]. The Davidoff group [146] reported that using AAV transductional delivery of soluble, truncated VEGFR-2 inhibited endothelial cell activation in vitro and Matrigel plug neovascularization in vivo. They also observed significant inhibition of local angiogenesis and tumor growth in an orthotropic tumor model of renal cancer. Similar strategy was used by Streck and collaborators [147] to investigate tumor metastasis. They observed a decrease in tumor weight in the liver that correlated with lower intratumoral microvessel density and higher levels of tumor cell apoptosis. Notably, by the time of preparation of this manuscript no strategy with AAV for transcriptional targeting tumor endothelial cells has been published. This might be related to the fact that AAV has relatively poor tropism to endothelial cells [53, 148].
2.3.5. Non-viral vectors
Non-viral vectors have characteristics such as simplicity of use, ease of large-scale production, and lack of adverse immune response that could be potentially beneficial for gene therapy [149]. Non-viral vectors are categorized into two general groups: (A) naked plasmid DNA delivered by a physical method, such as electroporation and gene gun; and (B) chemical carriers such as cationic polymer and lipid, metallic nanoparticles, and carbon nanotubes [149,150]. However, the feasibility of gene delivery to tumor endothelial cells by non-viral vectors has still not been clearly demonstrated.
2.4. Therapeutic genes that have been transcriptionaly targeted to tumor endothelial cells
The therapeutic gene used in the vectors discussed above should ideally promote tumor vasculature occlusion or disruption, reduce tumor growth, and eliminate metastasis. Theoretically, genes that directly or indirectly block the activation, proliferation, migration and reorganization of endothelial cell are typically the best candidates. They include (A) genes that encode proteins that directly kill transduced target cells (endothelial cells and pericytes), activate prodrugs, or sensitize cells to the cytotoxic effects of chemotherapeutic agents or ionizing radiation; (B) antisense oligonucleotides; (C) receptors of angiogenic factors that can function in a dominant negative manner; or (D) ribozymes that are capable of cleavage of other angiogenesis-associated RNA molecules (Table 1).
2.4.1. Soluble VEGF receptors
Inhibition of the signaling activity of VEGF receptors has shown promise in the treatment of cancer. This is supported by the recent FDA approval of two small molecule inhibitors (i.e. Sorafenib and Sunitinib) for the treatment of selected malignancies. Another alternative for blockade of VEGF signaling is the use of soluble VEGF receptors, which can function as inhibitors by directly binding to VEGF in the extracellular matrix. Alternative splicing of VEGFR-1 generates two distinct products: a full-length membrane-spanning receptor which contains an extracellular region with seven immunoglobulin-like loops, a single transmembrane region and a split tyrosine kinase domain; and a truncated soluble form which lack of the tyrosine kinase domain. This native soluble receptor is able to bind VEGF at higher affinity than full-length VEGFR-1 but is unable to signal, thus acts as VEGF antagonist [1]. Soluble VEGFR-1 has been extensively used in gene therapy strategies. It was reported that a recombinant adenovirus vector expressing sVEGFR-1 could successfully inhibit the development of corneal angiogenesis [2]. Mahendra and colleagues evaluated the potential of a recombinant adeno-associated virus-2 (rAAV) encoding the human soluble VEGFR-1 in vitro and in vivo. Results indicated significant growth inhibitory activity of the soluble VEGFR-1 in human umbilical vein endothelial cell proliferation in vitro and protection against the growth of an angiogenesis-dependent human ovarian cancer xenograft in vivo with increased disease-free survival [3]. Increased therapeutic effects on both the growth index of the implanted tumor cells and tumor-free survival also correlated with an increasing dose of the vector used [3]. Zhang and collaborators inserted soluble VEGFR-1 gene (sflt-1) into an E1B-55-kDa-deleted oncolytic adenovirus (ZD55) to construct ZD55-sflt-1. They demonstrated that the secretion of sFlt-1 from ZD55-sflt-1 led to potent inhibition of VEGF-induced proliferation and tube formation by human endothelial cells. Moreover, marked reduction of tumor growth and increased long-term survival were observed in ZD55-sflt-1-treated nude mice with subcutaneous SW620 tumor [4]. The efficacy of this virus correlated with a decrease in microvessel density and an increase in apoptotic tumor cells. In addition, ZD55-sflt-1 showed a synergistic effect with the chemotherapeutic agent 5-FU [4]. Their results suggested that ZD55-sflt-1, which uses the features of oncolytic adenovirus for antiangiogenic gene therapy with a soluble VEGF receptor, could be an efficacious strategy for cancer treatment.
Soluble VEGFR-2 was also investigated for its anti-angiogenesis and anti-tumor activity. Davidoff and colleagues constructed an AAV vector in which the expression of soluble VEGFR-2 (sKDR) was driven by a composite ß-actin-based promoter. After intraportal injection of this vector, high-level and stable transgene expression was achieved in mice. This strategy led to a systemic state of angiogenesis inhibition. Indeed, sera from these mice inhibited endothelial cell activation in vitro and Matrigel plug neovascularization in vivo. Significant antitumor efficacy was observed in murine models of pediatric kidney tumors. Tumor development was prevented in 10 of 15 (67%) mice, with significant growth restriction of tumors in the remaining mice [146]. Kaliberov and colleagues used an adenovirus to transcriptionally express soluble VEGFR-2 under control of the human VEGF promoter. In vitro analysis showed significant inhibition of the proliferation and migration of human vascular endothelial cells and prostate cancer cells. In vivo tumor therapy studies demonstrated significant inhibition of DU145 tumor growth in mice that received combined AdVEGF-sKDR infection and ionizing radiation versus AdVEGF-sKDR alone or radiation therapy alone [142].
2.4.2. Suicide genes
A suicide gene causes target cells to kill themselves through apoptosis. A variety of suicidal genes targeted to tumor cells have been characterized and employed in cancer gene therapy. Several suicidal gene systems have been also studied in combination with transcriptional targeting to tumor endothelial cells. They include the artificial death switches, herpes simplex type 1 thymidine kinase/ganciclovir (HSV-TK/GCV), Escherichia coli cytosine deaminase/5-fluorocytosine (CD/5-FC), and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL or Apo2L).
2.4.3. Artificial death switch
Inducible Caspase-9 (iCaspase-9) is a fusion protein that consists of two mutated FKBP (FK506-binding protein)-12 domains (referred as Fv2) fused to caspase-9. iCaspase molecule can be dimerized with AP20187, a nontoxic FK506 analogue with high affinity and specificity for the V36 (Fv) variant of FKBP12 [151,152]. To transcriptionally target tumor endothelial cells, our group subcloned iCaspase-9 into recombinant adenoviruses under VEGFR-2 promoter regulation. VEGFR-2 promoter drives expression of reporter genes primarily in neovascular endothelial cells (Figure 2). Activation of adenovirally delivered iCaspase-9 with the dimerizer compound AP20187 activated endogenous caspase-3 and induced endothelial cell apoptosis in vitro [74,153]. Furthermore, we showed that activation of iCaspase-9 with AP20187 was sufficient to induce apoptosis of neovascular endothelial cells, and to cause a marked decrease in tissue microvessel density in vivo [74,153]. When we infected proliferating human endothelial cells and human tumor cells of different linages with the same recombinant adenovirus, we observed that the vector includes the VRGFR-2 promoter induced apoptosis of proliferating human endothelial cells, but not human tumor cells in vitro [153]. Notably, intra-tumor delivery of Ad-hVEGFR2-iCaspase-9 followed by intraperitoneal injection of AP20187 showed the ablation of tumor microvessels and inhibition of xenografted tumor growth in all tumor models evaluated [153].
2.4.4. HSV-TK/Ganciclovir system
HSV-TK is a well-characterized suicidal gene. Ganciclovir is a nucleoside analogue that can be phosphorylated by HSV-TK and inhibit cellular DNA replication by substitution for normal nucleosides in the DNA chain leading to premature interruption of replication and cell death. HSV-TK/Ganciclovir has been shown to be a successful system for gene therapy [154]. Transcriptional targeting HSV-TK to tumor endothelial cells under the control of vWF promoter led to suppression of endothelial cell growth in the presence of ganciclovir [116]. Pramudji and colleagues developed an adenovirus harboring HSV-TK gene downstream of Caveolin-1 (cav-1) promoter [155]. Caveolin-1 is a structural component of caveolae that is upregulated in metastatic and androgen-resistant prostate cancer and is highly expressed in tumor-associated endothelial cells. To evaluate the activity of Ad-cav-1TK in vivo, orthotropic mouse prostate cancer tumors were generated with RM-9 cells and injected in situ with Adcav-1TK. They observed that the treatment with ganciclovir produced a significant reduction in tumor weight, increased apoptosis, and decreased tumor microvessel density as compared with control vectors [155]. As mentioned above, delivery of HSV-TK gene by recombinant retrovirus to endothelial cells of xenograft tumor under the control of PPET-1 promoter followed by administration of ganciclovir resulted in widespread vascular disruption and tumor cell apoptosis [124]. It was reported that in situ administration of recombinant adenovirus expressing the HSV-TK gene under VEGFR-2 promoter regulation inhibited tumor vascularization, and reduced tumor volume in a xenograft tumor model of hepatocellular carcinoma [156].
2.4.5. CD/5-FC suicide system
Escherichia coli cytosine deaminase (CD) is a microbial enzyme that converts the antifungal agent 5-fluorocytosine (5-FC) into the anti-tumor agent 5-fluorouracil (5-FU). As a pyrimidine antagonist, 5-FU is inserted into DNA or RNA and inhibits DNA synthesis [157]. Therefore, DNA damage occurs and cells are arrested or undergo apoptosis. 5-FU is basically a pro-drug that can be converted into an active drug. Fluorodeoxyuridylate (5-FdUMP), which is the main metabolite of 5-FU, inhibits DNA synthesis through the binding with thymidylate synthase. Another metabolite of 5-FU, 5-fluorouridine monophosphate (5-FUMP) is inserted into RNA and inhibits processing and function of RNA. In addition, 5-FU is able to diffuse across the cell membrane into adjacent cells without going through the gap junctions, resulting in a powerful bystander effect [5]. Szary and colleagues showed that VEGFR-2 promoter-driven cytosine deaminase is efficiently expressed in murine L1 sarcoma and human ovarian carcinoma cell lines leading to sensitization to 5-fluorocytosine in vitro and in vivo.
Unexpectedly, their results indicated that VEGFR-2 promoter activity is not endothelial cell-exclusive and that this promoter can also be used to obtain specific expression of therapeutic genes in certain cancer cells [7]. To evaluate the killing effect of double suicide gene on human endothelial cells, Huang and collaborators constructed an adenovirus containing both the cytosine deaminase (CD) and the TK genes (CDglyTK) under the regulation of the VEGFR-2 promoter. They observed a marked decrease in endothelial cell survival when ganciclovir and 5-FC were administered [8].
2.4.6. TRAIL suicidal gene
TRAIL is a member of the tumor necrosis factor family. The ability to selectively induce apoptosis in tumor cells makes TRAIL a promising anticancer agent. TRAIL induces apoptosis via activation of its death receptors, DR4 (TRAIL-R1) and DR5 (TRAIL-R2) [158]. TRAIL binding initiates receptor conformational changes and formation of a death-inducing signaling complex that results in the autoactivation of caspase-8/10 and apoptosis [159]. TRAIL has also been targeted to the tumor endothelium for gene therapy. Kaliberov and colleagues generated a recombinant adenovirus encoding TRAIL under the control of the VEGFR-1 promoter. Local administration of the adenovirus to prostate cancer xenografts resulted in inhibition of tumor growth [160]. They further confirmed that the microvessel density of the tumor was significantly reduced by the targeted delivery of TRAIL to endothelial cells [160].
3. Summary and prospects for potential clinical applications
The data reviewed here indicates that transcriptionaly targeting tumor endothelial cells is a promising approach for cancer gene therapy. However, there are still some barriers and important issues should be clear addressed considering the clinical application of the transcriptional tumor endothelial targeting systems. Firstly, most endothelial cell-specific promoters used for transcriptional targeting have been characterized in proliferating endothelial cells from normal tissues. This makes the efficacy and safety of this therapeutic strategy for cancer gene therapy still questionable. Another critical issue is related to the safety of viral vectors for cancer gene therapy. One has to take into account that viral vectors have been associated with the development of a leukemic-like disorder in at least two patients with human severe combined immunodeficiency (SCID)-X1 disease [161,162]. However, viral vectors are still more efficient for transgene expression than non-viral vectors. Therefore, developing safe and effective vectors for gene delivery remains a critical challenge. To address these problems, combination of transductional and transcriptional targeting strategies to tumor endothelial cells has been employed and has demonstrated improvements in the specificity of transgene expression and therapeutic efficacy in vivo [9-13]. In addition, studies have suggested the existence of proteins that are exclusively expressed in tumor endothelial cells and that could be exploited for transcriptional targeting of tumor blood vessels [18-22,163]. Characterization and validation of these tumor endothelial cell-specific proteins is currently being done in many laboratories around the world. The knowledge coming out of these studies will be critical for the future application of transcriptional targeting of tumor endothelial cells for cancer gene therapy in the clinic.
Acknowledgments
The authors thank Chris Yung for his work with the illustration model, and Wendy Song for technical assistance with in vivo experiments. This work was supported by grant P50-CA97248 (University of Michigan Head & Neck SPORE) from the NIH/NCI; and grants R01-DE14601, R01-DE15948, R01-DE16586, and R21-DE19279 from the NIH/NIDCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Folkman J. Toward an understanding of angiogenesis: search and discovery. Perspect. Biol. Med. 1985;29:10–36. doi: 10.1353/pbm.1985.0049. [DOI] [PubMed] [Google Scholar]
- [2].Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays. 1991;13:31–36. doi: 10.1002/bies.950130106. [DOI] [PubMed] [Google Scholar]
- [3].Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- [4].Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- [5].Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- [6].Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann. Surg. 1972;175:409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Folkman J. What is the evidence that tumors are angiogenesis dependent? J. Natl. Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- [8].Grunstein J, Roberts WG, Mathieu-Costello O, Hanahan D, Johnson RS. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 1999;59:1592–1598. [PubMed] [Google Scholar]
- [9].Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- [10].Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990;348:555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- [11].Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- [12].O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- [13].Brekken RA, Overholser JP, Stastny VA, Waltenberger J, Minna JD, Thorpe PE. Selective inhibition of vascular endothelial growth factor (VEGF) receptor 2 (KDR/Flk-1) activity by a monoclonal anti-VEGF antibody blocks tumor growth in mice. Cancer Res. 2000;60:5117–5124. [PubMed] [Google Scholar]
- [14].Ishida T, Kundu RK, Yang E, Hirata K, Ho YD, Quertermous T. Targeted disruption of endothelial cell-selective adhesion molecule inhibits angiogenic processes in vitro and in vivo. J. Biol. Chem. 2003;278:34598–34604. doi: 10.1074/jbc.M304890200. [DOI] [PubMed] [Google Scholar]
- [15].Folkman J. Is angiogenesis an organizing principle in biology and medicine? J. Pediatr. Surg. 2007;42:1–11. doi: 10.1016/j.jpedsurg.2006.09.048. [DOI] [PubMed] [Google Scholar]
- [16].Folkman J. What is the role of endothelial cells in angiogenesis? Lab. Invest. 1984;51:601–604. [PubMed] [Google Scholar]
- [17].Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc. Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Croix B, St, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- [19].Christian S, Ahorn H, Novatchkova M, Garin-Chesa P, Park JE, Weber G, Eisenhaber F, Rettig WJ, Lenter MC. Molecular cloning and characterization of EndoGlyx-1, an EMILIN-like multisubunit glycoprotein of vascular endothelium. J. Biol. Chem. 2001;276:48588–48595. doi: 10.1074/jbc.M106152200. [DOI] [PubMed] [Google Scholar]
- [20].Buckanovich RJ, Sasaroli D, O'Brien-Jenkins A, Botbyl J, Hammond R, Katsaros D, Sandaltzopoulos R, Liotta LA, Gimotty PA, Coukos G. Tumor vascular proteins as biomarkers in ovarian cancer. J. Clin. Oncol. 2007;25:852–861. doi: 10.1200/JCO.2006.08.8583. [DOI] [PubMed] [Google Scholar]
- [21].Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, Croix B., St Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11:539–554. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ghilardi C, Chiorino G, Dossi R, Nagy Z, Giavazzi R, Bani M. Identification of novel vascular markers through gene expression profiling of tumor-derived endothelium. BMC Genomics. 2008;9:201–219. doi: 10.1186/1471-2164-9-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- [24].Wu Y, Li ZY, Zhao X, Kan B, Wei YQ. Inhibition of ovarian tumor growth by gene therapy with recombinant soluble vascular endothelial growth factor receptor 2. Hum. Gene Ther. 2006;17:941–948. doi: 10.1089/hum.2006.17.941. [DOI] [PubMed] [Google Scholar]
- [25].Kuhnert F, Tam BY, Sennino B, Gray JT, Yuan J, Jocson A, Nayak NR, Mulligan RC, McDonald DM, Kuo CJ. Soluble receptor-mediated selective inhibition of VEGFR and PDGFRbeta signaling during physiologic and tumor angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10185–10190. doi: 10.1073/pnas.0803194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bandyopadhyay A, Lopez-Casillas F, Malik SN, Montiel JL, Mendoza V, Yang J, Sun LZ. Antitumor activity of a recombinant soluble betaglycan in human breast cancer xenograft. Cancer Res. 2002;62:4690–4695. [PubMed] [Google Scholar]
- [27].Silva R, D'Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: the keys to unlocking angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2008;28:1703–1713. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- [28].Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Delbaldo C, Raymond E, Vera K, Hammershaimb L, Kaucic K, Lozahic S, Marty M, Faivre S. Phase I and pharmacokinetic study of etaracizumab (Abegrin), a humanized monoclonal antibody against alphavbeta3 integrin receptor, in patients with advanced solid tumors. Invest. New Drugs. 2008;26:35–43. doi: 10.1007/s10637-007-9077-0. [DOI] [PubMed] [Google Scholar]
- [30].Beekman KW, Colevas AD, Cooney K, Dipaola R, Dunn RL, Gross M, Keller ET, Pienta KJ, Ryan CJ, Smith D, Hussain M. Phase II evaluations of cilengitide in asymptomatic patients with androgen-independent prostate cancer: scientific rationale and study design. Clin. Genitourin. Cancer. 2006;4:299–302. doi: 10.3816/CGC.2006.n.012. [DOI] [PubMed] [Google Scholar]
- [31].George JA., St Gene therapy progress and prospects: adenoviral vectors. Gene Ther. 2003;10:1135–1141. doi: 10.1038/sj.gt.3302071. [DOI] [PubMed] [Google Scholar]
- [32].Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu. Rev. Biochem. 2005;74:711–738. doi: 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- [33].Barker SD, Dmitriev IP, Nettelbeck DM, Liu B, Rivera AA, Alvarez RD, Curiel DT, Hemminki A. Combined transcriptional and transductional targeting improves the specificity and efficacy of adenoviral gene delivery to ovarian carcinoma. Gene Ther. 2003;10:1198–1204. doi: 10.1038/sj.gt.3301974. [DOI] [PubMed] [Google Scholar]
- [34].Orphanides G, Reinberg D. A unified theory of gene expression. Cell. 2002;108:439–451. doi: 10.1016/s0092-8674(02)00655-4. [DOI] [PubMed] [Google Scholar]
- [35].Nettelbeck DM, Jerome V, Muller R. Gene therapy: designer promoters for tumour targeting. Trends Genet. 2000;16:174–181. doi: 10.1016/s0168-9525(99)01950-2. [DOI] [PubMed] [Google Scholar]
- [36].Papadakis ED, Nicklin SA, Baker AH, White SJ. Promoters and control elements: designing expression cassettes for gene therapy. Curr. Gene Ther. 2004;4:89–113. doi: 10.2174/1566523044578077. [DOI] [PubMed] [Google Scholar]
- [37].Morishita K, Johnson DE, Williams LT. A novel promoter for vascular endothelial growth factor receptor (flt-1) that confers endothelial-specific gene expression. J. Biol. Chem. 1995;270:27948–27953. doi: 10.1074/jbc.270.46.27948. [DOI] [PubMed] [Google Scholar]
- [38].Patterson C, Perrella MA, Hsieh CM, Yoshizumi M, Lee ME, Haber E. Cloning and functional analysis of the promoter for KDR/flk-1, a receptor for vascular endothelial growth factor. J. Biol. Chem. 1995;270:23111–23118. doi: 10.1074/jbc.270.39.23111. [DOI] [PubMed] [Google Scholar]
- [39].Mancuso DJ, Tuley EA, Westfield LA, Worrall NK, Shelton-Inloes BB, Sorace JM, Alevy YG, Sadler JE. Structure of the gene for human von Willebrand factor. J. Biol. Chem. 1989;264:19514–19527. [PubMed] [Google Scholar]
- [40].Korhonen J, Lahtinen I, Halmekyto M, Alhonen L, Janne J, Dumont D, Alitalo K. Endothelial-specific gene expression directed by the tie gene promoter in vivo. Blood. 1995;86:1828–1835. [PubMed] [Google Scholar]
- [41].Schlaeger TM, Qin Y, Fujiwara Y, Magram J, Sato TN. Vascular endothelial cell lineage-specific promoter in transgenic mice. Development. 1995;121:1089–1098. doi: 10.1242/dev.121.4.1089. [DOI] [PubMed] [Google Scholar]
- [42].Breier G, Breviario F, Caveda L, Berthier R, Schnurch H, Gotsch U, Vestweber D, Risau W, Dejana E. Molecular cloning and expression of murine vascular endothelialcadherin in early stage development of cardiovascular system. Blood. 1996;87:630–641. [PubMed] [Google Scholar]
- [43].Cowan PJ, Tsang D, Pedic CM, Abbott LR, Shinkel TA, d'Apice AJ, Pearse MJ. The human ICAM-2 promoter is endothelial cell-specific in vitro and in vivo and contains critical Sp1 and GATA binding sites. J. Biol. Chem. 1998;273:11737–11744. doi: 10.1074/jbc.273.19.11737. [DOI] [PubMed] [Google Scholar]
- [44].Collins T, Williams A, Johnston GI, Kim J, Eddy R, Shows T, Gimbrone MA, Jr, Bevilacqua MP. Structure and chromosomal location of the gene for endothelial-leukocyte adhesion molecule 1. J. Biol. Chem. 1991;266:2466–2473. [PubMed] [Google Scholar]
- [45].Marsden PA, Heng HH, Scherer SW, Stewart RJ, Hall AV, Shi XM, Tsui LC, Schappert KT. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J. Biol. Chem. 1993;268:17478–17488. [PubMed] [Google Scholar]
- [46].Bloch KD, Friedrich SP, Lee ME, Eddy RL, Shows TB, Quertermous T. Structural organization and chromosomal assignment of the gene encoding endothelin. J. Biol. Chem. 1989;264:10851–10857. [PubMed] [Google Scholar]
- [47].Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, Sato M. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene. 1990;5:519–524. [PubMed] [Google Scholar]
- [48].de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- [49].Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Bohlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- [50].Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- [51].Quinn TP, Peters KG, De Vries C, Ferrara N, Williams LT. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc. Natl. Acad. Sci. U. S. A. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ikeda T, Wakiya K, Shibuya M. Characterization of the promoter region for flt-1 tyrosine kinase gene, a receptor for vascular endothelial growth factor. Growth Factors. 1996;13:151–162. doi: 10.3109/08977199609003217. [DOI] [PubMed] [Google Scholar]
- [53].Nicklin SA, Reynolds PN, Brosnan MJ, White SJ, Curiel DT, Dominiczak AF, Baker AH. Analysis of cell-specific promoters for viral gene therapy targeted at the vascular endothelium. Hypertension. 2001;38:65–70. doi: 10.1161/01.hyp.38.1.65. [DOI] [PubMed] [Google Scholar]
- [54].Reynolds PN, Nicklin SA, Kaliberova L, Boatman BG, Grizzle WE, Balyasnikova IV, Baker AH, Danilov SM, Curiel DT. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat. Biotechnol. 2001;19:838–842. doi: 10.1038/nbt0901-838. [DOI] [PubMed] [Google Scholar]
- [55].Minami T, Donovan DJ, Tsai JC, Rosenberg RD, Aird WC. Differential regulation of the von Willebrand factor and Flt-1 promoters in the endothelium of hypoxanthine phosphoribosyltransferase-targeted mice. Blood. 2002;100:4019–4025. doi: 10.1182/blood-2002-03-0955. [DOI] [PubMed] [Google Scholar]
- [56].Bellamy WT. Expression of vascular endothelial growth factor and its receptors in multiple myeloma and other hematopoietic malignancies. Semin. Oncol. 2001;28:551–559. doi: 10.1016/s0093-7754(01)90023-5. [DOI] [PubMed] [Google Scholar]
- [57].Bellamy WT, Richter L, Sirjani D, Roxas C, Glinsmann-Gibson B, Frutiger Y, Grogan TM, List AF. Vascular endothelial cell growth factor is an autocrine promoter of abnormal localized immature myeloid precursors and leukemia progenitor formation in myelodysplastic syndromes. Blood. 2001;97:1427–1434. doi: 10.1182/blood.v97.5.1427. [DOI] [PubMed] [Google Scholar]
- [58].von Marschall Z, Cramer T, Hocker M, Burde R, Plath T, Schirner M, Heidenreich R, Breier G, Riecken EO, Wiedenmann B, Rosewicz S. De novo expression of vascular endothelial growth factor in human pancreatic cancer: evidence for an autocrine mitogenic loop. Gastroenterology. 2000;119:1358–1372. doi: 10.1053/gast.2000.19578. [DOI] [PubMed] [Google Scholar]
- [59].von Marschall Z, Cramer T, Hocker M, Finkenzeller G, Wiedenmann B, Rosewicz S. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut. 2001;48:87–96. doi: 10.1136/gut.48.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bauerschmitz GJ, Nettelbeck DM, Kanerva A, Baker AH, Hemminki A, Reynolds PN, Curiel DT. The flt-1 promoter for transcriptional targeting of teratocarcinoma. Cancer Res. 2002;62:1271–1274. [PubMed] [Google Scholar]
- [61].Wu Y, Patterson C. The human KDR/flk-1 gene contains a functional initiator element that is bound and transactivated by TFII-I. J. Biol. Chem. 1999;274:3207–3214. doi: 10.1074/jbc.274.5.3207. [DOI] [PubMed] [Google Scholar]
- [62].Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T, Rauter M, Plate K, Sieweke M, Breier G, Flamme I. Cooperative interaction of hypoxia-inducible factor-2alpha (HIF-2alpha) and Ets-1 in the transcriptional activation of vascular endothelial growth factor receptor-2 (Flk-1) J. Biol. Chem. 2003;278:7520–7530. doi: 10.1074/jbc.M211298200. [DOI] [PubMed] [Google Scholar]
- [63].Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- [64].Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Dvorak HF, Senger DR. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am. J. Pathol. 1993;143:1255–1262. [PMC free article] [PubMed] [Google Scholar]
- [65].Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, Dvorak HF. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–4735. [PubMed] [Google Scholar]
- [66].Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- [67].Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367:576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- [68].Millauer B, Longhi MP, Plate KH, Shawver LK, Risau W, Ullrich A, Strawn LM. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 1996;56:1615–1620. [PubMed] [Google Scholar]
- [69].Dias S, Hattori K, Heissig B, Zhu Z, Wu Y, Witte L, Hicklin DJ, Tateno M, Bohlen P, Moore MA, Rafii S. Inhibition of both paracrine and autocrine VEGF/ VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10857–10862. doi: 10.1073/pnas.191117498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ryden L, Linderholm B, Nielsen NH, Emdin S, Jonsson PE, Landberg G. Tumor specific VEGF-A and VEGFR2/KDR protein are co-expressed in breast cancer. Breast Cancer Res. Treat. 2003;82:147–154. doi: 10.1023/B:BREA.0000004357.92232.cb. [DOI] [PubMed] [Google Scholar]
- [71].Straume O, Akslen LA. Increased expression of VEGF-receptors (FLT-1, KDR, NRP-1) and thrombospondin-1 is associated with glomeruloid microvascular proliferation, an aggressive angiogenic phenotype, in malignant melanoma. Angiogenesis. 2003;6:295–301. doi: 10.1023/B:AGEN.0000029408.08638.aa. [DOI] [PubMed] [Google Scholar]
- [72].Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Potential autocrine function of vascular endothelial growth factor in head and neck cancer via vascular endothelial growth factor receptor-2. Mod. Pathol. 2005;18:485–494. doi: 10.1038/modpathol.3800295. [DOI] [PubMed] [Google Scholar]
- [73].Heidenreich R, Kappel A, Breier G. Tumor endothelium-specific transgene expression directed by vascular endothelial growth factor receptor-2 (Flk-1) promoter/enhancer sequences. Cancer Res. 2000;60:6142–6147. [PubMed] [Google Scholar]
- [74].Song W, Sun Q, Dong Z, Spencer DM, Nunez G, Nor JE. Antiangiogenic gene therapy: disruption of neovascular networks mediated by inducible caspase-9 delivered with a transcriptionally targeted adenoviral vector. Gene Ther. 2005;12:320–329. doi: 10.1038/sj.gt.3302306. [DOI] [PubMed] [Google Scholar]
- [75].Partanen J, Armstrong E, Makela TP, Korhonen J, Sandberg M, Renkonen R, Knuutila S, Huebner K, Alitalo K. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol. Cell. Biol. 1992;12:1698–1707. doi: 10.1128/mcb.12.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Runting AS, Stacker SA, Wilks AF. Tie2, a Putative Protein Tyrosine Kinase from a New Class of Cell Surface Receptor. Growth Factors. 1993;9:99–105. [PubMed] [Google Scholar]
- [77].Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML. Tek, a Novel Tyrosine Kinase Gene Located on Mouse Chromosome 4, is Expressed in Endothelial Cells and their Presumptive Precursors. Oncogene. 1992;7:1471–1480. [PubMed] [Google Scholar]
- [78].Korhonen J, Partanen J, Armstrong E, Vaahtokari A, Elenius K, Jalkanen M, Alitalo K. Enhanced expression of the tie receptor tyrosine kinase in endothelial cells during neovascularization. Blood. 1992;80:2548–2555. [PubMed] [Google Scholar]
- [79].Iwama A, Hamaguchi I, Hashiyama M, Murayama Y, Yasunaga K, Suda T. Molecular cloning and characterization of mouse TIE and TEK receptor tyrosine kinase genes and their expression in hematopoietic stem cells. Biochem. Biophys. Res. Commun. 1993;195:301–309. doi: 10.1006/bbrc.1993.2045. [DOI] [PubMed] [Google Scholar]
- [80].Ziegler SF, Bird TA, Schneringer JA, Schooley KA, Baum PR. Molecular cloning and characterization of a novel receptor protein tyrosine kinase from human placenta. Oncogene. 1993;8:663–670. [PubMed] [Google Scholar]
- [81].Korhonen J, Polvi A, Partanen J, Alitalo K. The mouse tie receptor tyrosine kinase gene: expression during embryonic angiogenesis. Oncogene. 1994;9:395–403. [PubMed] [Google Scholar]
- [82].Dumont DJ, Fong GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev. Dyn. 1995;203:80–92. doi: 10.1002/aja.1002030109. [DOI] [PubMed] [Google Scholar]
- [83].Hatva E, Kaipainen A, Mentula P, Jaaskelainen J, Paetau A, Haltia M, Alitalo K. Expression of endothelial cell-specific receptor tyrosine kinases and growth factors in human brain tumors. Am. J. Pathol. 1995;146:368–378. [PMC free article] [PubMed] [Google Scholar]
- [84].Kaipainen A, Vlaykova T, Hatva E, Bohling T, Jekunen A, Pyrhonen S, Alitalo K. Enhanced expression of the tie receptor tyrosine kinase mesenger RNA in the vascular endothelium of metastatic melanomas. Cancer Res. 1994;54:6571–6577. [PubMed] [Google Scholar]
- [85].Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- [86].Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14:5884–5891. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Martin V, Liu D, Fueyo J, Gomez-Manzano C. Tie2: a journey from normal angiogenesis to cancer and beyond. Histol. Histopathol. 2008;23:773–780. doi: 10.14670/HH-23.773. [DOI] [PubMed] [Google Scholar]
- [88].Iljin K, Dube A, Kontusaari S, Korhonen J, Lahtinen I, Oettgen P, Alitalo K. Role of ets factors in the activity and endothelial cell specificity of the mouse Tie gene promoter. FASEB J. 1999;13:377–386. doi: 10.1096/fasebj.13.2.377. [DOI] [PubMed] [Google Scholar]
- [89].Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3058–3063. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat. Med. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- [91].Suzuki S, Sano K, Tanihara H. Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue. Cell Regul. 1991;2:261–270. doi: 10.1091/mbc.2.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gory S, Vernet M, Laurent M, Dejana E, Dalmon J, Huber P. The vascular endothelial-cadherin promoter directs endothelial-specific expression in transgenic mice. Blood. 1999;93:184–192. [PubMed] [Google Scholar]
- [93].Dancer A, Julien S, Bouillot S, Pointu H, Vernet M, Huber P. Expression of thymidine kinase driven by an endothelial-specific promoter inhibits tumor growth of Lewis lung carcinoma cells in transgenic mice. Gene Ther. 2003;10:1170–1178. doi: 10.1038/sj.gt.3301981. [DOI] [PubMed] [Google Scholar]
- [94].Staunton DE, Dustin ML, Springer TA. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989;339:61–64. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- [95].Cowan PJ, Shinkel TA, Witort EJ, Barlow H, Pearse MJ, d'Apice AJ. Targeting gene expression to endothelial cells in transgenic mice using the human intercellular adhesion molecule 2 promoter. Transplantation. 1996;62:155–160. doi: 10.1097/00007890-199607270-00002. [DOI] [PubMed] [Google Scholar]
- [96].Richardson TB, Kaspers J, Porter CD. Retroviral hybrid LTR vector strategy: functional analysis of LTR elements and generation of endothelial cell specificity. Gene Ther. 2004;11:775–783. doi: 10.1038/sj.gt.3302220. [DOI] [PubMed] [Google Scholar]
- [97].De Palma M, Venneri MA, Naldini L. In vivo targeting of tumor endothelial cells by systemic delivery of lentiviral vectors. Hum. Gene Ther. 2003;14:1193–1206. doi: 10.1089/104303403322168028. [DOI] [PubMed] [Google Scholar]
- [98].Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu. Rev. Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- [99].Kraling BM, Razon MJ, Boon LM, Zurakowski D, Seachord C, Darveau RP, Mulliken JB, Corless CL, Bischoff J. E-selectin is present in proliferating endothelial cells in human hemangiomas. Am. J. Pathol. 1996;148:1181–1191. [PMC free article] [PubMed] [Google Scholar]
- [100].Bandyopadhyay S, Ashraf MZ, Daher P, Howe PH, DiCorleto PE. HOXA9 participates in the transcriptional activation of E-selectin in endothelial cells. Mol. Cell. Biol. 2007;27:4207–4216. doi: 10.1128/MCB.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jaggar RT, Chan HY, Harris AL, Bicknell R. Endothelial cell-specific expression of tumor necrosis factor-alpha from the KDR or E-selectin promoters following retroviral delivery. Hum. Gene Ther. 1997;8:2239–2247. doi: 10.1089/hum.1997.8.18-2239. [DOI] [PubMed] [Google Scholar]
- [102].Walton T, Wang JL, Ribas A, Barsky SH, Economou J, Nguyen M. Endothelium-specific expression of an E-selectin promoter recombinant adenoviral vector. Anticancer Res. 1998;18:1357–1360. [PubMed] [Google Scholar]
- [103].Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- [104].Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J. Clin. Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Freedman JE, Sauter R, Battinelli EM, Ault K, Knowles C, Huang PL, Loscalzo J. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circ. Res. 1999;84:1416–1421. doi: 10.1161/01.res.84.12.1416. [DOI] [PubMed] [Google Scholar]
- [106].Lee PC, Salyapongse AN, Bragdon GA, Shears LL, 2nd, Watkins SC, Edington HD, Billiar TR. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am. J. Physiol. 1999;277:H1600–8. doi: 10.1152/ajpheart.1999.277.4.H1600. [DOI] [PubMed] [Google Scholar]
- [107].Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat. Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- [108].Fish JE, Marsden PA. Endothelial nitric oxide synthase: insight into cell-specific gene regulation in the vascular endothelium. Cell Mol. Life Sci. 2006;63:144–162. doi: 10.1007/s00018-005-5421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Chen AF, Jiang SW, Crotty TB, Tsutsui M, Smith LA, O'Brien T, Katusic ZS. Effects of in vivo adventitial expression of recombinant endothelial nitric oxide synthase gene in cerebral arteries. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12568–12573. doi: 10.1073/pnas.94.23.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Deng W, Bivalacqua TJ, Chattergoon NN, Hyman AL, Jeter JR, Jr, Kadowitz PJ. Adenoviral gene transfer of eNOS: high-level expression in ex vivo expanded marrow stromal cells. Am. J. Physiol. Cell. Physiol. 2003;285:C1322–C1329. doi: 10.1152/ajpcell.00141.2003. [DOI] [PubMed] [Google Scholar]
- [111].Smith RS, Jr, Agata J, Xia CF, Chao L, Chao J. Human endothelial nitric oxide synthase gene delivery protects against cardiac remodeling and reduces oxidative stress after myocardial infarction. Life Sci. 2005;76:2457–2471. doi: 10.1016/j.lfs.2004.11.028. [DOI] [PubMed] [Google Scholar]
- [112].Guillot PV, Guan J, Liu L, Kuivenhoven JA, Rosenberg RD, Sessa WC, Aird WC. A vascular bed-specific pathway. J. Clin. Invest. 1999;103:799–805. doi: 10.1172/JCI6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sadler JE. von Willebrand factor. J. Biol. Chem. 1991;266:22777–22780. [PubMed] [Google Scholar]
- [114].Aird WC, Jahroudi N, Weiler-Guettler H, Rayburn HB, Rosenberg RD. Human von Willebrand factor gene sequences target expression to a subpopulation of endothelial cells in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1995;92:4567–4571. doi: 10.1073/pnas.92.10.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Aird WC, Edelberg JM, Weiler-Guettler H, Simmons WW, Smith TW, Rosenberg RD. Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J. Cell Biol. 1997;138:1117–1124. doi: 10.1083/jcb.138.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ozaki K, Yoshida T, Ide H, Saito I, Ikeda Y, Sugimura T, Terada M. Use of von Willebrand factor promoter to transduce suicidal gene to human endothelial cells, HUVEC. Hum. Gene Ther. 1996;7:1483–1490. doi: 10.1089/hum.1996.7.13-1483. [DOI] [PubMed] [Google Scholar]
- [117].Yang DI, Chen S, Ezekiel UR, Xu J, Wu Y, Hsu CY. Antisense RNA to inducible nitric oxide synthase reduces cytokine-mediated brain endothelial cell death. Ann. N. Y. Acad. Sci. 2005;1042:439–447. doi: 10.1196/annals.1338.037. [DOI] [PubMed] [Google Scholar]
- [118].Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- [119].Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annu. Rev. Physiol. 1999;61:391–415. doi: 10.1146/annurev.physiol.61.1.391. [DOI] [PubMed] [Google Scholar]
- [120].Lee ME, Bloch KD, Clifford JA, Quertermous T. Functional analysis of the endothelin-1 gene promoter. Evidence for an endothelial cell-specific cis-acting sequence. J. Biol. Chem. 1990;265:10446–10450. [PubMed] [Google Scholar]
- [121].Harats D, Kurihara H, Belloni P, Oakley H, Ziober A, Ackley D, Cain G, Kurihara Y, Lawn R, Sigal E. Targeting gene expression to the vascular wall in transgenic mice using the murine preproendothelin-1 promoter. J. Clin. Invest. 1995;95:1335–1344. doi: 10.1172/JCI117784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Varda-Bloom N, Shaish A, Gonen A, Levanon K, Greenbereger S, Ferber S, Levkovitz H, Castel D, Goldberg I, Afek A, Kopolovitc Y, Harats D. Tissue-specific gene therapy directed to tumor angiogenesis. Gene Ther. 2001;8:819–827. doi: 10.1038/sj.gt.3301472. [DOI] [PubMed] [Google Scholar]
- [123].Harats D, Ben-Shushan D, Cohen H, Gonen A, Barshack I, Goldberg I, Greenberger S, Hodish I, Harari A, Varda-Bloom N, Levanon K, Grossman E, Chaitidis P, Kuhn H, Shaish A. Inhibition of carcinogenesis in transgenic mouse models over-expressing 15-lipoxygenase in the vascular wall under the control of murine preproendothelin-1 promoter. Cancer Lett. 2005;229:127–134. doi: 10.1016/j.canlet.2005.02.017. [DOI] [PubMed] [Google Scholar]
- [124].Mavria G, Harrington KJ, Marshall CJ, Porter CD. In vivo efficacy of HSV-TK transcriptionally targeted to the tumour vasculature is augmented by combination with cytotoxic chemotherapy. J. Gene Med. 2005;7:263–275. doi: 10.1002/jgm.662. [DOI] [PubMed] [Google Scholar]
- [125].Hermens WT, Verhaagen J. Viral vectors, tools for gene transfer in the nervous system. Prog. Neurobiol. 1998;55:399–432. doi: 10.1016/s0301-0082(98)00007-0. [DOI] [PubMed] [Google Scholar]
- [126].Brandwijk RJ, Griffioen AW, Thijssen VL. Targeted gene-delivery strategies for angiostatic cancer treatment. Trends Mol. Med. 2007;13:200–209. doi: 10.1016/j.molmed.2007.03.001. [DOI] [PubMed] [Google Scholar]
- [127].Vile RG, Tuszynski A, Castleden S. Retroviral vectors. From laboratory tools to molecular medicine. Mol. Biotechnol. 1996;5:139–158. doi: 10.1007/BF02789062. [DOI] [PubMed] [Google Scholar]
- [128].Mavria G, Porter CD. Reduced growth in response to ganciclovir treatment of subcutaneous xenografts expressing HSV-tk in the vascular compartment. Gene Ther. 2001;8:913–920. doi: 10.1038/sj.gt.3301483. [DOI] [PubMed] [Google Scholar]
- [129].Jager U, Zhao Y, Porter CD. Endothelial cell-specific transcriptional targeting from a hybrid long terminal repeat retrovirus vector containing human prepro-endothelin-1 promoter sequences. J. Virol. 1999;73:9702–9709. doi: 10.1128/jvi.73.12.9702-9709.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Mavria G, Jager U, Porter CD. Generation of a high titre retroviral vector for endothelial cell-specific gene expression in vivo. Gene Ther. 2000;7:368–376. doi: 10.1038/sj.gt.3301093. [DOI] [PubMed] [Google Scholar]
- [131].Trono D. Lentiviral vectors: turning a deadly foe into a therapeutic agent. Gene Ther. 2000;7:20–23. doi: 10.1038/sj.gt.3301105. [DOI] [PubMed] [Google Scholar]
- [132].Lotti F, Menguzzato E, Rossi C, Naldini L, Ailles L, Mavilio F, Ferrari G. Transcriptional targeting of lentiviral vectors by long terminal repeat enhancer replacement. J. Virol. 2002;76:3996–4007. doi: 10.1128/JVI.76.8.3996-4007.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Shichinohe T, Bochner BH, Mizutani K, Nishida M, Hegerich-Gilliam S, Naldini L, Kasahara N. Development of lentiviral vectors for antiangiogenic gene delivery. Cancer Gene Ther. 2001;8:879–889. doi: 10.1038/sj.cgt.7700388. [DOI] [PubMed] [Google Scholar]
- [134].Pariente N, Mao SH, Morizono K, Chen IS. Efficient targeted transduction of primary human endothelial cells with dual-targeted lentiviral vectors. J. Gene Med. 2008;10:242–248. doi: 10.1002/jgm.1151. [DOI] [PubMed] [Google Scholar]
- [135].Ali M, Lemoine NR, Ring CJ. The use of DNA viruses as vectors for gene therapy. Gene Ther. 1994;1:367–384. [PubMed] [Google Scholar]
- [136].Savontaus MJ, Sauter BV, Huang TG, Woo SL. Transcriptional targeting of conditionally replicating adenovirus to dividing endothelial cells. Gene Ther. 2002;9:972–979. doi: 10.1038/sj.gt.3301747. [DOI] [PubMed] [Google Scholar]
- [137].Stackhouse MA, Buchsbaum DJ, Kancharla SR, Grizzle WE, Grimes C, Laffoon K, Pederson LC, Curiel DT. Specific membrane receptor gene expression targeted with radiolabeled peptide employing the erbB-2 and DF3 promoter elements in adenoviral vectors. Cancer Gene Ther. 1999;6:209–219. doi: 10.1038/sj.cgt.7700049. [DOI] [PubMed] [Google Scholar]
- [138].Mahasreshti PJ, Navarro JG, Kataram M, Wang MH, Carey D, Siegal GP, Barnes MN, Nettelbeck DM, Alvarez RD, Hemminki A, Curiel DT. Adenovirus-mediated soluble FLT-1 gene therapy for ovarian carcinoma. Clin. Cancer Res. 2001;7:2057–2066. [PubMed] [Google Scholar]
- [139].Everts M, Kim-Park SA, Preuss MA, Passineau MJ, Glasgow JN, Pereboev AV, Mahasreshti PJ, Grizzle WE, Reynolds PN, Curiel DT. Selective induction of tumor-associated antigens in murine pulmonary vasculature using double-targeted adenoviral vectors. Gene Ther. 2005;12:1042–1048. doi: 10.1038/sj.gt.3302491. [DOI] [PubMed] [Google Scholar]
- [140].Izumi M, Kawakami Y, Glasgow JN, Belousova N, Everts M, Kim-Park S, Yamamoto S, Wang M, Le LP, Reynolds PN, Curiel DT. In vivo analysis of a genetically modified adenoviral vector targeted to human CD40 using a novel transient transgenic model. J. Gene Med. 2005;7:1517–1525. doi: 10.1002/jgm.806. [DOI] [PubMed] [Google Scholar]
- [141].Work LM, Ritchie N, Nicklin SA, Reynolds PN, Baker AH. Dual targeting of gene delivery by genetic modification of adenovirus serotype 5 fibers and cell-selective transcriptional control. Gene Ther. 2004;11:1296–1300. doi: 10.1038/sj.gt.3302292. [DOI] [PubMed] [Google Scholar]
- [142].Kaliberov SA, Kaliberova LN, Buchsbaum DJ. Combined ionizing radiation and sKDR gene delivery for treatment of prostate carcinomas. Gene Ther. 2005;12:407–417. doi: 10.1038/sj.gt.3302432. [DOI] [PubMed] [Google Scholar]
- [143].Tenenbaum L, Lehtonen E, Monahan PE. Evaluation of risks related to the use of adeno-associated virus-based vectors. Curr. Gene Ther. 2003;3:545–565. doi: 10.2174/1566523034578131. [DOI] [PubMed] [Google Scholar]
- [144].Zacchigna S, Zentilin L, Morini M, Dell'Eva R, Noonan DM, Albini A, Giacca M. AAV-mediated gene transfer of tissue inhibitor of metalloproteinases-1 inhibits vascular tumor growth and angiogenesis in vivo. Cancer Gene Ther. 2004;11:73–80. doi: 10.1038/sj.cgt.7700657. [DOI] [PubMed] [Google Scholar]
- [145].White SJ, Nicklin SA, Buning H, Brosnan MJ, Leike K, Papadakis ED, Hallek M, Baker AH. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation. 2004;109:513–519. doi: 10.1161/01.CIR.0000109697.68832.5D. [DOI] [PubMed] [Google Scholar]
- [146].Davidoff AM, Nathwani AC, Spurbeck WW, Ng CY, Zhou J, Vanin EF. rAAV-mediated long-term liver-generated expression of an angiogenesis inhibitor can restrict renal tumor growth in mice. Cancer Res. 2002;62:3077–3083. [PubMed] [Google Scholar]
- [147].Streck CJ, Zhou J, Ng CY, Zhang Y, Nathwani AC, Davidoff AM. Longterm recombinant adeno-associated, virus-mediated, liver-generated expression of an angiogenesis inhibitor improves survival in mice with disseminated neuroblastoma. J. Am. Coll. Surg. 2004;199:78–86. doi: 10.1016/j.jamcollsurg.2004.02.011. [DOI] [PubMed] [Google Scholar]
- [148].Denby L, Nicklin SA, Baker AH. Adeno-associated virus (AAV)-7 and -8 poorly transduce vascular endothelial cells and are sensitive to proteasomal degradation. Gene Ther. 2005;12:1534–1538. doi: 10.1038/sj.gt.3302564. [DOI] [PubMed] [Google Scholar]
- [149].Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- [150].Ragusa A, Garcia I, Penades S. Nanoparticles as nonviral gene delivery vectors, IEEE Trans. Nanobioscience. 2007;6:319–330. doi: 10.1109/tnb.2007.908996. [DOI] [PubMed] [Google Scholar]
- [151].MacCorkle RA, Freeman KW, Spencer DM. Synthetic activation of caspases: artificial death switches. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3655–3660. doi: 10.1073/pnas.95.7.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Nor JE, Hu Y, Song W, Spencer DM, Nunez G. Ablation of microvessels in vivo upon dimerization of iCaspase-9. Gene Ther. 2002;9:444–451. doi: 10.1038/sj.gt.3301671. [DOI] [PubMed] [Google Scholar]
- [153].Song W, Dong Z, Jin T, Mantellini MG, Nunez G, Nor JE. Cancer gene therapy with iCaspase-9 transcriptionally targeted to tumor endothelial cells. Cancer Gene Ther. 2008;15:667–675. doi: 10.1038/cgt.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Nawa A, Ishida D, ChenHong L. New paradigm of herpes simplex virus type 1 thymidine kinase (HSV-TK)/ganciclovir (GCV) Nippon Rinsho. 2006;64(Suppl 3):345–349. [PubMed] [Google Scholar]
- [155].Pramudji C, Shimura S, Ebara S, Yang G, Wang J, Ren C, Yuan Y, Tahir SA, Timme TL, Thompson TC. In situ prostate cancer gene therapy using a novel adenoviral vector regulated by the caveolin-1 promoter. Clin. Cancer Res. 2001;7:4272–4279. [PubMed] [Google Scholar]
- [156].Li BJ, Zhang C, Yi YX, Hao Y, Liu XP, Ou QJ. Vascular damage and anti-angiogenic effects of tumor vessel-targeted adenovirus-mediated herpes simplex virus thymidine kinase gene. World J. Gastroenterol. 2007;13:4006–4010. doi: 10.3748/wjg.v13.i29.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Smythe WR. Prodrug/drug sensitivity gene therapy: current status. Curr. Oncol. Rep. 2000;2:17–22. doi: 10.1007/s11912-000-0006-z. [DOI] [PubMed] [Google Scholar]
- [158].LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- [159].Huang Y, Sheikh MS. TRAIL death receptors and cancer therapeutics. Toxicol. Appl. Pharmacol. 2007;224:284–289. doi: 10.1016/j.taap.2006.12.007. [DOI] [PubMed] [Google Scholar]
- [160].Kaliberov SA, Kaliberova LN, Stockard CR, Grizzle WE, Buchsbaum DJ. Adenovirus-mediated FLT1-targeted proapoptotic gene therapy of human prostate cancer. Mol. Ther. 2004;10:1059–1070. doi: 10.1016/j.ymthe.2004.08.024. [DOI] [PubMed] [Google Scholar]
- [161].Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, Bousso P, Deist FL, Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- [162].Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, L. Villeval J, Fraser CC, Cavazzana-Calvo M, Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- [163].Zurita AJ, Arap W, Pasqualini R. Mapping tumor vascular diversity by screening phage display libraries. J. Control. Release. 2003;91:183–186. doi: 10.1016/s0168-3659(03)00236-0. [DOI] [PubMed] [Google Scholar]
- [164].Luz-Madrigal A, Clapp C, Aranda J, Vaca L. In vivo transcriptional targeting into the retinal vasculature using recombinant baculovirus carrying the human flt-1 promoter. Virol. J. 2007;4:88–100. doi: 10.1186/1743-422X-4-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Evans V, Hatzopoulos A, Aird WC, Rayburn HB, Rosenberg RD, Kuivenhoven JA. Targeting the Hprt locus in mice reveals differential regulation of Tie2 gene expression in the endothelium. Physiol. Genomics. 2000;2:67–75. doi: 10.1152/physiolgenomics.2000.2.2.67. [DOI] [PubMed] [Google Scholar]
- [166].Kisseleva T, Song L, Vorontchikhina M, Feirt N, Kitajewski J, Schindler C. NF-kappaB regulation of endothelial cell function during LPS-induced toxemia and cancer. J. Clin. Invest. 2006;116:2955–2963. doi: 10.1172/JCI27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Maxwell IH, Kaletta C, Naujoks K, Maxwell F. Targeting diphtheria toxin A-chain transcription to activated endothelial cells using an E-selectin promoter. Angiogenesis. 2003;6:31–38. doi: 10.1023/a:1025894616613. [DOI] [PubMed] [Google Scholar]