Abstract
Background
Anesthetics depress both ventilatory and upper airway dilator muscle activity and thus put the upper airway at risk for collapse. However, these effects are agent-dependent and may involve upper airway and diaphragm muscles to varying degrees. We assessed the effects of pentobarbital on upper airway dilator and respiratory pump muscle function in rats, and compared these results with the effects of normal sleep.
Methods
Tracheostomized rats were given increasing doses of pentobarbital to produce deep sedation, then light and deep anesthesia, and negative pressure airway stimuli were applied (n=11). To compare the effects of pentobarbital with those of natural sleep, we chronically instrumented rats (n=10) with genioglossus and neck electromyogram and electroencephalogram electrodes and compared genioglossus activity during wakefulness, sleep (rapid-eye-movement and non- rapid-eye-movement), and pentobarbital anesthesia.
Results
Pentobarbital caused a dose-dependent decrease in ventilation and in phasic diaphragmatic electromyogram by 11±0.1%, but increased phasic genioglossus electromyogram by 23±0.2%. Natural non-rapid-eye-movement sleep and pentobarbital anesthesia (10 mg/kg intraperitoneally) decreased respiratory genioglossus electromyogram by 61±29%, and 45±35% respectively, and natural rapid-eye-movement sleep caused the greatest decrease in phasic genioglossus electromyogram (95±0.3%).
Conclusions
Pentobarbital in rats impairs respiratory genioglossus activity compared to the awake state, but the decrease is no greater than seen during natural sleep. During anesthesia, in the absence of pharyngeal airflow, phasic genioglossus activity is increased in a dose-dependent fashion.
INTRODUCTION
General anesthetic agents1-6, including propofol4,6, isoflurane5, and thiopentone3predispose the upper airway to collapse, at least in part by decreasing upper airway muscle activity1-4. Surprisingly, recent data suggest that certain anesthetics, including pentobarbital, can increase genioglossus phasic activity7,8. We have demonstrated recently that isoflurane dose-dependently increases respiratory genioglossus activity, and that this effect is due to concomitant increases in phasic respiratory drive as assessed by flow rate9. Nonetheless, the overall effect of most anesthetics is to impair upper airway integrity as the transition is made from awake to anesthetized. To our knowledge, direct comparisons of anesthetic effects on airway muscle activity have not been made between anesthesia and various sleep states. Electroencephalographic sleep staging is required for this purpose, since undetected arousal may affect the control of ventilation, which in turn may influence upper airway muscle control. Comparative studies on the effects of sedation, anesthesia and sleep on respiratory muscle function will help understand if anesthesia compared with normal sleep puts a patient at an increased risk to develop an airway obstruction. Furthermore, it might help get a better understanding on whether or not patients with pathological airway anatomy, e.g., patients with obstructive sleep apnea, who recover postoperatively from the effects of anesthetics, are still at an increased risk to develop an airway obstruction. In many institutions, patients with obstructive sleep apnea are closely monitored for apneic events during the first postoperative night even after minor surgical procedures, but it is unclear if this effort-consuming approach to patient care is justified.
Based on the results of Younes and coworkers8, who observed an increased genioglossus activity during sleep in rats given pentobarbital compared placebo, we tested the hypotheses that pentobarbital causes a dose-dependent increase in respiratory genioglossus activity even in the face of declining respiratory activation of the diaphragm. Based on the data of Drummond and coworkers3, who observed a decrease in genioglossus activity during transition from wakefulness to pentobarbital anesthesia, we tested the secondary hypothesis that pentobarbital decreases phasic genioglossus activity when compared with wakefulness. Finally, we tested the hypothesis that pentobarbital's effects on genioglossus activity differ from those observed during natural rapid-eye-movement (REM) and non-REM sleep.
MATERIALS AND METHODS
Sprague-Dawley adult male rats (300-400 g; Harlan Sprague Dawley, Indianapolis, IN) were used in these protocols. All procedures involving animals were approved by the Institutional Animal Care and Use Committees at Harvard Medical School and Beth Israel Deaconess Medical Center.
Protocol 1 (acute experiments)
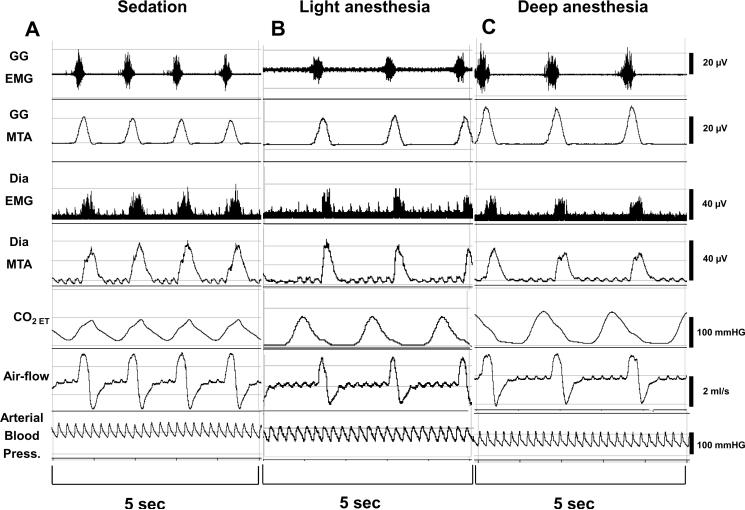
In 11 rats, after induction of anesthesia with pentobarbital (35±2.8mg, ip, (NEMBUTAL, Ovation Pharmaceuticals Inc., Deerfield, IL), and surgical instrumentation, we performed intermittent tail-clamping maneuvers until the rat regained a withdrawal response (`deep sedation'). Deep sedation was said to occur when the rat displayed gross and purposeful movement10 that resolved following the end of tail clamping. After measurements of respiratory muscle function and breathing, we then started a pentobarbital infusion at a rate of 75mg/kg/h11 and performed the second series of measurements of respiratory function (`light anesthesia') when the rats' withdrawal response to tail-clamping was again abolished. Another pentobarbital infusion was then given to apply an additional 50% of the pentobarbital dose that had been required to turn `deep sedation' into `light anesthesia' (`deep anesthesia'). A representative recording is given in figure 1.
Figure 1.
Original recording from an acute experiment. Typical response to increasing the pentobarbital dose. Genioglossus and diaphragmatic electromyograms, respiratory flow, end-tidal carbon dioxide (CO2) concentration, and arterial blood-pressure during increasing doses of pentobarbital. With increasing pentobarbital concentrations, phasic genioglossus activity and CO2 increased, whereas diaphragmatic activity, respiratory rate, and respiratory flow decreased. MTA=moving time average, Dia=diaphragm.
We cannulated the femoral artery and vein with PE50 tubing, transected and cannulated the trachea with PE240 tubing through which the rat spontaneously breathed, and then inserted by open surgery two insulated stainless steel wires (California Fine Wire Co., Grover Beach, CA) into the genioglossus muscle and the diaphragm, one on each side of the midline. For the purpose of measuring the genioglossus negative pressure reflex, the rats' nares were occluded by a plastic cap placed over the muzzle and sealed with glue. Sub-atmospheric pressure could be applied through a fitting on this cap by gating a vacuum source with a solenoid valve. For measurement of the magnitude of pharyngeal pressure, we inserted a pressure-sensitive catheter (Millar Instruments, Houston, TX) through the tracheostomy so that the tip was located in the rostral trachea just below the level of the thyroid cartilage9. Measurements of pharyngeal pressure were made under zero flow conditions.
We measured breathing using pneumotachography for a period of two minutes at each measurement point. This was followed by a series of three 4 sec negative pressure maneuvers applied to the pharynx every 60s. The genioglossus negative pressure reflex was taken as the percent change in genioglossus amplitude during negative pressure (the average of the second, third and fourth breaths after the onset of negative pressure application) compared to the average during the three breaths just prior to negative pressure application. For each rat we applied a stimulus that produced a consistent increase in genioglossus activity under baseline conditions (on average -25 cm H2O) 9. The negative pressure stimulus was kept constant throughout the remainder of each experiment.
Protocol 2: Chronically instrumented rats
In order to assess the effects of pentobarbital on genioglossus activity during wakefulness and sleep, we chronically instrumented 10 rats with electrodes for genioglossus and neck electromyography, as well as cortical electroencephalogram12. Animals were anesthetized with chloral hydrate (350mg/kg) and the skull was exposed. Two flexible electromyography wire electrodes (Plastics One Inc., Roanoke, VA) were placed into the genioglossus muscle through a ventral incision, and the wires were subcutaneously tunneled to the neck. Another two electromyography wires were surgically implanted in the neck muscles. For measurement of electroencephalographic signals two screw electrodes (Plastics One Inc.) were inserted into holes drilled into the skull, one approximately 0.2 centimeter anterior and one approximately 0.5 centimeter posterior to the coronal line and approximately 0.3 centimeter lateral to the midline. The free ends of the leads were connected to a socket (Plastics One Inc.) that was attached to the skull with dental cement. The incisions were then closed with wound clips and suture.
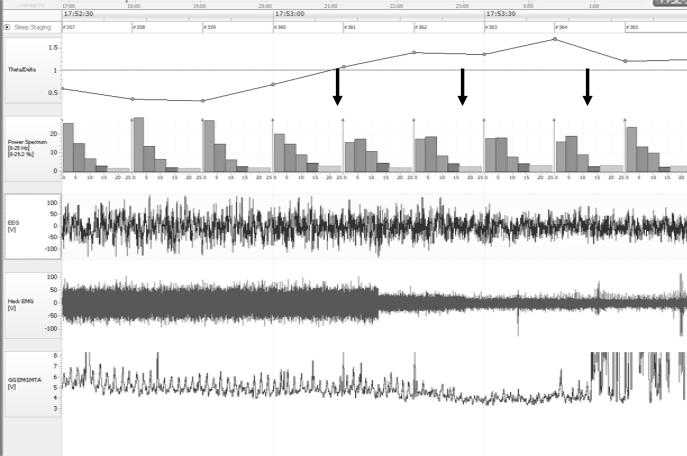
Rats were allowed to recovery 7 days from chronic instrumentation. During the subsequent night we performed measurements in various sleep stages in the absence of pentobarbital administration (see figure 2). With a time delay of 24 h or more, rats were then given pentobarbital (10 mg or 20 mg i.p., n=10) and we measured time-course of genioglossus and neck muscle function during the transition from wakefulness to anesthesia (figure 3). Pentobarbital 10 mg/kg and 20 mg/kg were given to the same rats on different days. 72 h or more elapsed between the measurements.
Figure 2.
Rat polysomnography. Experiment in a chronically instrumented rat (sleep experiment). Typical transition from non-REM to REM sleep. First arrow: point of conversion of theta/delta ratio to greater than 1. Second arrow: Start point of genioglossus activity measurements (low neck-muscle activity). Third arrow: End-point of genioglossus activity measurements (onset of tongue muscle twitches). EEG=electroencephalogram, EMG=electromyogram, REM=rapid-eye-movement.
Figure 3.
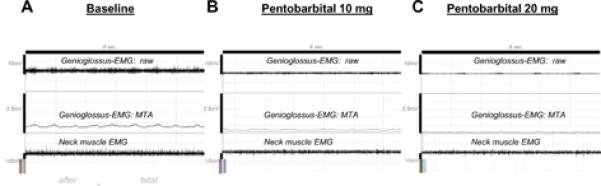
Original recording from an experiment in a chronically instrumented rat (anesthesia experiment). Typical response to pentobarbital.
A. Baseline condition. During wakefulness, the genioglossus muscle showed a strong phasic activity in this rat.
B. 5 minutes after administration of pentobarbital 10 mg. Phasic and tonic muscle activities were markedly lower.
C. 5 minutes after administration of pentobarbital 20 mg (same rat as shown in A.) Genioglossus and neck muscle activity are markedly decreased; note that the activity of the latter is relatively preserved compared with the genioglossus.
EMG=electromyogram, MTA=moving time average.
The sockets were connected via flexible recording cables and a commutator to the recording equipment. The animal stayed in its cage during the whole measurement to minimize stress, and it was allowed to habituate to the new situation for a minimum of 5 hours before the recordings were performed. The electromyograms and electroencephalograms were recorded after the rats had been immobile for at least 5 minutes.
Genioglossus activity was measured during the non-anesthetized state, 10 minutes after pentobarbital injection, and after euthanasia. The latter measurement was performed to discriminate between electrical noise and tonic genioglossus activity. After euthanasia the two wires connected to the genioglossus muscle were stimulated electrically and movements of the tongue were observed to ensure the correct position of the wires. A representative recording of genioglossus activity in a chronically instrumented rat is depicted in figure 3.
Sleep stages were determined by analyzing the electroencephalogram and electromyograms recorded during the whole experiment. Genioglossus activity was measured for 10 seconds during 5 consecutive episodes of REM and non-REM sleep, and the averages for these two stages were compared to values measured during quiet wakefulness (no movements, eyes open, neck muscle activity higher compared with sleep). REM sleep was defined by two criteria: theta/delta ratio of greater than 1, and decrease in neck muscle activity (by 25% compared with non-REM, see figure 2). Episodes of tongue muscle twitches typically occurring during REM were not analyzed. NREM sleep was characterized by beta-rhythm (14 to 30 Hz) or delta-activity (0.5 to 4 Hz) in the electroencephalographic, as well as closed eyes. Electroencephalographic signals were analyzed on a desktop computer using Sleep Sign for Animals (Kissei Comtec Co., LTD., Nagano, Japan).
Data acquisition and analysis
The primary outcome variable was genioglossus activity. Genioglossus electromyography signals were amplified (Grass Instruments, West Warwick, RI), filtered (100-1000 Hz), moving time averaged (100 ms), and digitized by a computer. Signals were analyzed with Clampfit (Molecular Devices, Sunnyvale, CA) and Igor Pro (WaveMetrics, Inc., Lake Oswego, OR). Phasic (respiratory) moving time average was used for analysis. Tonic genioglossus activity was defined as nadir genioglossus activity during expiration minus genioglossus activity measured in the dead rat following euthanasia (reflecting the degree of electrical noise) 13. End-tidal carbon dioxide was measured by CAPSTAR-100 Carbon Dioxide Analyzer, (CWE Inc., Ardmore, PA).
Statistical analysis
We tested the hypotheses that pentobarbital increases the respiratory genioglossus activity and decreases respiratory diaphragmatic activity. Statistical comparisons were made by using a general linear model for repeated measures (RMAOVA). We used data from all rats studied under protocol 1 and tested for an effect of the independent variable `pentobarbital dose' on the dependent variables `phasic genioglossus activity' and `phasic diaphragmatic activity'. Based on the data of Drummond and coworkers who observed a decrease in genioglossus activity during transition from wakefulness to pentobarbital anesthesia3, we tested by RMAOVA the secondary hypothesis that pentobarbital decreases phasic genioglossus activity when compared with wakefulness. Finally, RAMOVA was used for comparing the effects on respiratory genioglossus activity of pentobarbital with those of normal REM and non-REM sleep. Schefe's tests were used for post-hoc testing throughout.
Calculation of the sample size for testing the primary criterion in the rat studies was based on the results of Younes and co-workers8, who found a 20% increase in phasic genioglossus activation (standard deviation: 10%) and a 15% difference (SD: 7%) in the magnitude of pentobarbital's effect at different muscles (genioglossus versus diaphragm) (alpha-error=0.01, power: 99%). For testing the secondary hypothesis that pentobarbital decreases phasic genioglossus activity when compared with wakefulness, we expected a 10 percent difference (standard deviation: 5%; alpha-error: 0.01, power: 99%)3. Finally, for comparison of the effects of pentobarbital on respiratory genioglossus activity with normal REM and non-REM sleep we expected a 10 percent difference (standard deviation: 5%) between pentobarbital and REM sleep (alpha-error: 0.01, power: 99%), as well as a 10 percent difference (standard deviation: 5%) pentobarbital and non-REM sleep. We calculated that a total sample size of 21 rats would provide a sufficient power to detect a significant difference in the primary, secondary, and exploratory hypotheses (power1×2×3×4×5= 0.9, alpha<0.01). The results are expressed as the mean ± SD unless indicated otherwise.
SPSS Version 11.0 (SPSS Inc, Chicago, IL) as well as Sigma Stat Version 3.0 (SPSS Inc) were used for statistical analysis.
RESULTS
Protocol 1: Acute experiments in rats
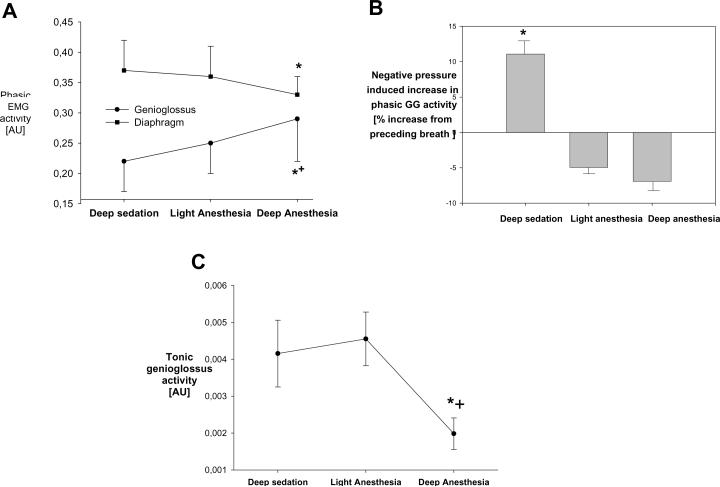
The amplitude of the respiratory (phasic) genioglossus electromyogram increased significantly with pentobarbital dose, whereas the diaphragmatic electromyogram decreased (figure 4A). During deep sedation, the negative pressure reflex was maintained (figure 4B), but it was abolished during anesthesia. The amplitude of the tonic genioglossus electromyogram was unaffected at the two lower pentobarbital doses (p=0.7), but it was significantly lower during `deep anesthesia' (figure 4C).
Figure 4.
Protocol 1: Effects of three different pentobarbital doses on respiratory muscle function. Means ± SEM.
A. Phasic activity of genioglossus (circles) and diaphragm (squares). * p<0.05 versus deep sedation, same muscle. + p<0.05 versus diaphragm.
B. Negative pressure reflex: Increase in phasic genioglossus electromyogram in response to negative pharyngeal pressure application. * p<0.05 versus preceding breath.
C. Tonic genioglossus activity. * p<0.05 versus deep sedation, + p<0.05 versus light anesthesia.
EMG=electromyogram, AU=arbitrary units, GG=genioglossus.
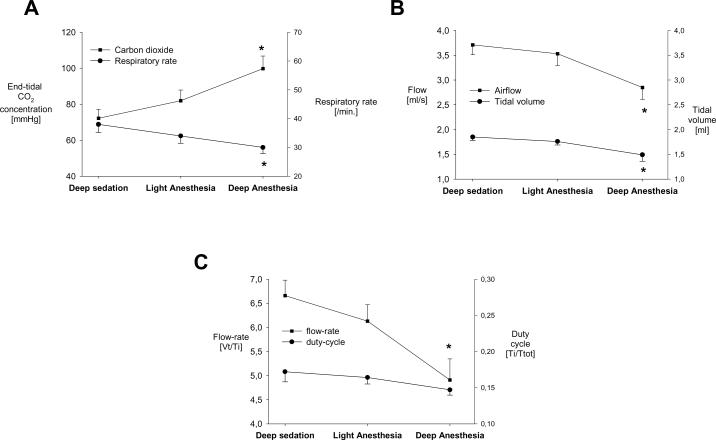
Pentobarbital induced significant dose-dependent decreases in respiratory rate, tidal volume, flow-rate and duty-cycle. End-tidal carbon dioxide concentration increased, as expected (figure 5). Pentobarbital dose-dependently decreased heart rate; 320±4 bpm, 307±4, and 283±3 bpm during deep sedation, light anesthesia, and deep anesthesia, respectively. Mean arterial pressure also decreased with pentobarbital dose, (92±8 mmHg, 80±7, and 67±4 mm Hg, respectively).
Figure 5.
Protocol 1: Effects of three different pentobarbital doses on breathing. Means±SEM.
A. End-tidal CO2 concentration (squares) increased and respiratory rate (circles) decreased with increasing pentobarbital doses.
* p<0.05 versus deep sedation.
B. Air-flow (squares) and tidal-volume (circles) decreased with increasing pentobarbital doses. * p<0.05 versus deep sedation.
C. Flow rate (Vt [tidal volume]/TI [inspiration time]) decreased with increasing pentobarbital doses, whereas duty-cycle (Ti [inspiratory time] and Ttot [total cycle-time]) did not significantly change.
* p<0.05 versus deep sedation.
CO2= carbon dioxide, Vt=tidal volume, Ti=inspiration time, Ttot=total respiratory cycle duration.
Light and deep levels of anesthesia were achieved after administration of a total pentobarbital dose of 68±3.5 and 84±3.7 mg, respectively.
Protocol 2: Chronically instrumented rats
Both of the pentobarbital doses produced anesthesia. Injection of 10 or 20 mg/kg, i.p. abolished the response to tail clamping after 18.3±1.6 minutes and 6.2±0.6 minutes, respectively. Compared to the awake state, both the phasic and tonic genioglossus electromyograms were significantly decreased, and the peak depression of muscle electromyograms did not differ when pentobarbital 10 versus 20 mg/kg were applied (Figure 6).
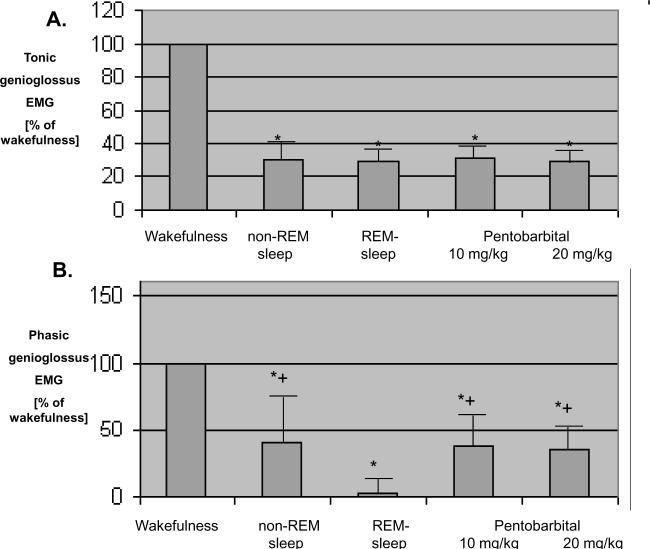
Figure 6.
Comparison of the effects of pentobarbital and normal sleep on genioglossus muscle activity in chronically instrumented rats. Means ± SEM.
A. Nadir tonic (non-respiratory) genioglossus muscle activity during wakefulness, sleep, and pentobarbital anesthesia. Compared to wakefulness activity, nadir genioglossus activity is significantly lower during sleep and pentobarbital anesthesia, but values observed during pentobarbital anesthesia did not differ from those observed during non REM and REM sleep. *p<0.05 versus wakefulness.
B. Phasic (respiratory) genioglossus activity during wakefulness, sleep, and pentobarbital anesthesia. Compared to wakefulness activity, phasic genioglossus activity was significantly lower during sleep and pentobarbital anesthesia. Phasic (respiratory) genioglossus activity during REM-sleep was significantly lower compared to non-REM sleep and pentobarbital anesthesia at a dose-level of 10 mg/kg.
REM=rapid-eye-movement, EMG=electromyogram.
The genioglossus electromyogram was assessed during REM and NREM sleep and after each doses of pentobarbital. Under each of these conditions, the tonic genioglossus electromyogram was depressed by approximately the same amount compared to the awake state (Figure 6A). The phasic genioglossus electromyogram was also decreased during both normal sleep and anesthesia (Fig 6B), but the depression was most marked during REM sleep. The depression during REM was significantly greater than NREM or following pentobarbital.
DISCUSSION
In this study we found that anesthetic doses of pentobarbital impaired respiratory genioglossus activity compared to waking, but activity still exceeded that observed during natural REM sleep. During anesthesia, increasing doses of pentobarbital increased the phasic genioglossus electromyogram despite a decreased phasic diaphragmatic electromyogram. As a technical note, this dose-dependency was observed under steady-state conditions in which pentobarbital was given as an infusion over half an hour. By contrast, we observed a ceiling effect of pentobarbital in the protocol when single bolus doses of pentobarbital were given to chronically instrumented rats. The difference in pentobarbital's effects on the genioglossus muscle between our two protocols might be explained by a time-dependent physiological effect such as different levels of hypercarbia being reached in the two protocols.
It is well known that a decrease in genioglossus tone occurs during the onset of normal sleep due to the loss of the wakefulness stimulus14, but this does not typically produce airway collapse unless there is an underlying anatomical abnormality. By contrast anesthetics produce airway collapse. One possibility is that anesthetics produce more loss of upper airway muscle tone than what occurs during normal sleep. Orem and co-workers have studied the laryngeal abductors (the posterior cricoarytenoid muscles) during sleep, wakefulness, and barbiturate anesthesia in cats. The authors found that barbiturate anesthesia produced laryngeal abductor activity patterns characteristic of sleep15. In the present study, as expected, phasic genioglossus activity was abolished by REM sleep, whereas with the anesthetic doses of pentobarbital applied to chronically instrumented rats, there was still some phasic genioglossus activity present. Moreover, our first protocol showed that pentobarbital increases phasic genioglossus activity when the effect due to sleep is eliminated. The explanation for this surprising finding is unclear.
One possibility is that there is a mismatch between measured electromyographic activity and muscle function. Depending on the load to the muscle, increased electromyogram activity may be associated with increased tension in the muscle or shortening of the fibres, or both, such that no linear relation exists between genioglossus electromyogram and airway collapsibility. Nonetheless since the effects of sleep and anesthesia on genioglossus and diaphragm activity were measured with the same technique we feel these comparisons are valid. Phasic genioglossus activation increases the size of the airway and decreases collapsibility, and it may be of particular importance under conditions when protective reflexes (e.g. the negative pressure reflex) are impaired. However, future studies will be required to show whether or not pentobarbital's activating effects on the genioglossus electromyogram translate to a more stable upper airway particularly since this may not offset the abolition of genioglossus responses to negative pharyngeal pressure during pentobarbital anesthesia. And, indeed, blockade of this important reflex may be the key mechanism responsible for anesthetics airway-collapsing effects.
Probably the most interesting finding of this study relates to the dissociation of pentobarbital's respiratory effects on the genioglossus (activation) and breathing (inhibition of diaphragmatic activity and consequent hypercarbia), which we observed in our acute experiments in the absence of airflow. This is in contrast to isoflurane's increasing effects on genioglossal activity which are positively correlated with improved flow-rate and tidal volume (and also diaphragmatic activity [unpublished data, Eikermann M, MD, PhD and Chamberlin NL, PhD, Boston, MA]). Thus, the effects of isoflurane and pentobarbital on respiratory muscle function must be in part mediated by different mechanisms. The effects of isoflurane could be explained by a simple increase in inspiratory drive; however this is not the case with pentobarbital.
We speculate that the most likely explanation is that pentobarbital has differential effects on hypoglossal and phrenic premotoneurons and that the effect is via disinhibition. Barbiturates are well-known to potentiate gamma-aminobutyric-acid (GABA)-ergic inhibition by increasing chloride influx through GABAA channels. Therefore, direct effects of barbiturates on motoneurons would be expected to be inhibitory. Indeed, Park et al have shown that administration of several common hypnotic GABAA-ergic agents into the hypoglossal motor nucleus decreased genioglossus activity. By contrast, systemic administration of the same drugs increases genioglossus but not diaphragmatic activity16. Thus, the activating effects of GABAA-ergic drugs on respiratory genioglossus activity cannot be explained by direct effects on hypoglossal motoneurons. Rather, both pentobarbital's increasing effects on phasic genioglossus activity and its decreasing effects on the genioglossus negative pressure reflex could be mediated by inhibition of inhibitory neurons located in the periobex that relay negative pressure-related stimuli to hypoglossal motoneurons from the nucleus of the solitary tract17. In fact, pentobarbital's effects resemble those observed after microinjection of the GABAA agonist muscimol into the periobex17, an effect we suspect to be due to disinhibition of hypoglossal motoneurons. The findings of Park et al suggest16 that the effects of pentobarbital on genioglossus muscle activation are not specific to this agent but rather represent a GABAA receptor agonist class effect. It will be interesting to study the effects of short acting barbiturates on genioglossus muscle control.
Although we suspect that the major effect of pentobarbital on genioglossus activity is mediated by disinhibition, it is also possible that hypercapnia plays a role. Pentobarbital inhibits ventilatory responses to carbon dioxide resulting in hypercapnia. The genioglossus is more sensitive than the pump muscles to several conditions including hypercapnia, behavioral state, and vagotomy18,19. To the extent that chemoreflex activation of the genioglossus muscle may occur independently of ventilatory drive, it is possible that elevated carbon dioxide may partially account for on the divergence between the genioglossus and diaphragm activities under pentobarbital anesthesia18.
Differential effects of pentobarbital on respiratory pump versus airway muscles have been reported before20, 8. Warner and coworkers showed in pentobarbital-anesthetized dogs that phasic electrical activity increased over time in the triangularis sterni, transversus abdominis, and external oblique muscles (expiratory muscles), whereas electrical activity of the costal diaphragm, crural diaphragm, and parasternal intercostal muscles (inspiratory muscles) was unchanged20. Younes and co-workers found in rats a trend towards lower minute diaphragmatic activity and a higher genioglossus activity at the time of carbon dioxide induced arousal when pentobarbital was given8. Our study confirms and extends these data by showing dose-dependency in an isolated upper airway in the absence of air flow. We also show this effect is selective for phasic respiratory activity as tonic and negative pressure reflex activities were inhibited.
Our results are at odds with Hwang and co-workers who reported that pentobarbital depresses hypoglossal output more than phrenic. These results may be due to the fact that they used animals that were decerebrated and vagotomized 1. In fact, the vagus nerve is important for mediating the interplay between lung volume and upper airway muscle activity, and lung inflation decreases genioglossus muscle activity, an effect that is mediated by the vagus nerve as the afferent pathway21. The effects of anesthetics on respiratory muscles differ between species22, and it is unclear whether our findings, translate to humans. Comparative studies in humans of the differential effects of various anesthetics on pharyngeal mechanics are clearly needed. In summary, pentobarbital in rats impairs respiratory genioglossus activity compared to the awake-state, but the decrease is no greater than seen during natural sleep. During anesthesia, phasic genioglossus activity is increased in a dose-dependent fashion.
Acknowledgement
We are grateful to Quan Ha, Research Assistant, Beth Israel Deaconess Med. Ctr., Department of Neurology, Boston, MA, for excellent technical support.
This work was supported by grants from the National Institute of Health (Bethesda, MD, USA, P50 HL060292, RO1-HL73146, R01-HL085188, AG024837), an Established Investigator Award from the AHA and a grant from the German Research Council (DFG-EI684/2-1, Bonn, Germany).
Footnotes
Summary statement: Increasing pentobarbital doses decrease the amplitude of the phasic diaphragmatic electromyogram but increase phasic genioglossus electromyogram.
REFERENCES
- 1.Hwang JC, John WM, Bartlett D., Jr. Respiratory-related hypoglossal nerve activity: influence of anesthetics. J Appl Physiol. 1983;55:785–92. doi: 10.1152/jappl.1983.55.3.785. [DOI] [PubMed] [Google Scholar]
- 2.Ochiai R, Guthrie RD, Motoyama EK. Differential sensitivity to halothane anesthesia of the genioglossus, intercostals, and diaphragm in kittens. Anesth Analg. 1992;74:338–44. doi: 10.1213/00000539-199203000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Drummond GB. Influence of thiopentone on upper airway muscles. Br J Anaesth. 1989;63:12–21. doi: 10.1093/bja/63.1.12. [DOI] [PubMed] [Google Scholar]
- 4.Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology. 2005;103:470–7. doi: 10.1097/00000542-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Eastwood PR, Szollosi I, Platt PR, Hillman DR. Collapsibility of the upper airway during anesthesia with isoflurane. Anesthesiology. 2002;97:786–93. doi: 10.1097/00000542-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Norton JR, Ward DS, Karan S, Voter WA, Palmer L, Varlese A, Rackovsky O, Bailey P. Differences between midazolam and propofol sedation on upper airway collapsibility using dynamic negative airway pressure. Anesthesiology. 2006;104:1155–64. doi: 10.1097/00000542-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Roda F, Pio J, Bianchi AL, Gestreau C. Effects of anesthetics on hypoglossal nerve discharge and c-Fos expression in brainstem hypoglossal premotor neurons. J Comp Neurol. 2004;468:571–86. doi: 10.1002/cne.10974. [DOI] [PubMed] [Google Scholar]
- 8.Younes M, Park E, Horner RL. Pentobarbital sedation increases genioglossus respiratory activity in sleeping rats. Sleep. 2007;30:478–88. doi: 10.1093/sleep/30.4.478. [DOI] [PubMed] [Google Scholar]
- 9.Eikermann M FP, Malhotra A, Jordan AS, Zaremba S, Gautam S, White DP, Chamberlin NL. Differential effects of isoflurane and propofol on genioglossus muscle function and breathing. Anesthesiology. 2008;108:897–906. doi: 10.1097/ALN.0b013e31816c8a60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orth M, Barter L, Dominguez C, Atherley R, Carstens E, Antognini JF. Halothane and propofol differentially affect electroencephalographic responses to noxious stimulation. Br J Anaesth. 2005;95:477–84. doi: 10.1093/bja/aei208. [DOI] [PubMed] [Google Scholar]
- 11.Kissin I, Stanski DR, Brown PT, Bradley EL., Jr. Pentobarbital-morphine anesthetic interactions in terms of intensity of noxious stimulation required for arousal. Anesthesiology. 1993;78:744–9. doi: 10.1097/00000542-199304000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 13.Eikermann M, Fassbender P, Malhotra A, Takahashi M, Kubo S, Jordan AS, Gautam S, White DP, Chamberlin NL. Unwarranted Administration of Acetylcholinesterase Inhibitors Can Impair Genioglossus and Diaphragm Muscle Function. Anesthesiology. 2007;107:621–9. doi: 10.1097/01.anes.0000281928.88997.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RA, Eikermann M, Schory K, Dover L, White DP. Influence of wakefulness on pharyngeal airway muscle activity. Thorax. 2007;62:799–805. doi: 10.1136/thx.2006.072488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orem J, Lydic R. Upper airway function during sleep and wakefulness: experimental studies on normal and anesthetized cats. Sleep. 1978;1:49–68. doi: 10.1093/sleep/1.1.49. [DOI] [PubMed] [Google Scholar]
- 16.Park E, Younes M, Liu H, Liu X, Horner RL. Systemic vs. central administration of common hypnotics reveals opposing effects on genioglossus muscle activity in rats. Sleep. 2008;31:355–65. doi: 10.1093/sleep/31.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol. 2007;579:515–26. doi: 10.1113/jphysiol.2006.121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs.diaphragm muscle response to CO(2) in rats. J Appl Physiol. 2002;92:878–87. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- 19.Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO(2) on upper airway and respiratory pump muscle activity in the rat. J Physiol. 2001;532:525–34. doi: 10.1111/j.1469-7793.2001.0525f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warner DO, Joyner MJ, Rehder K. Electrical activation of expiratory muscles increases with time in pentobarbital-anesthetized dogs. J Appl Physiol. 1992;72:2285–91. doi: 10.1152/jappl.1992.72.6.2285. [DOI] [PubMed] [Google Scholar]
- 21.van Lunteren E, Strohl KP, Parker DM, Bruce EN, Van de Graaff WB, Cherniack NS. Phasic volume-related feedback on upper airway muscle activity. J Appl Physiol. 1984;56:730–6. doi: 10.1152/jappl.1984.56.3.730. [DOI] [PubMed] [Google Scholar]
- 22.Warner DO, Joyner MJ, Ritman EL. Anesthesia and chest wall function in dogs. J Appl Physiol. 1994;76:2802–13. doi: 10.1152/jappl.1994.76.6.2802. [DOI] [PubMed] [Google Scholar]