Abstract
Chronic angiotensin II (Ang II) infusions enhance urinary excretion of angiotensinogen suggesting augmentation of distal nephron sodium reabsorption. To assess if chronic Ang II infusions (15 ng/min for 2 weeks) enhance distal nephron sodium reabsorption, we compared sodium excretion before and following blockade of the two main distal nephron sodium transporters by iv amiloride (5 mg/kg body weight) plus bendroflumethiazide (12 mg/kg body weight) in male C57/BL6 anesthetized control mice (n=10) and in chronic Ang II-infused mice (n=8). Chronic Ang II infusions increased systolic blood pressure to 141±6 mm Hg compared to 106±4 mm Hg in control mice. After anesthesia, mean arterial pressure averaged 97±4 mm Hg in chronic Ang II-infused mice compared with 94±3 mm Hg in control mice allowing comparison of renal function at similar arterial pressures. Ang II-infused mice had lower urinary sodium excretion (0.16±0.04 versus 0.30±0.05 μEq/min, P<0.05), higher distal sodium reabsorption (1.74±0.18 versus 1.12±0.18 μEq/min, P<0.05) and higher fractional reabsorption of distal sodium delivery (91.1±1.8% versus 77.9±4.3 %, P<0.05) than control mice. Urinary Ang II concentrations, measured during distal blockade, were greater in Ang II infused mice (1235.0±277.2 versus 468.9±146.9 fmol/ml, P<0.05). In chronic Ang II-infused mice treated with spironolactone (n=5), fractional reabsorption of distal sodium delivery was similarly augmented as in chronic Ang II infused mice (94.6±1.7%, P<0.01). These data provide in vivo evidence that there is enhanced distal sodium reabsorption dependent on sodium channel and Na+-Cl− cotransporter activity and increased urinary Ang II concentrations in mice infused chronically with Ang II.
Keywords: tubular sodium reabsorption, filtered sodium, amiloride, bendroflumethiazide, renal plasma flow, glomerular filtration rate
Introduction
Chronic angiotensin II (Ang II) infusions elicit hypertension through combined effects of increases in vascular resistance, decreases in sodium excretion and suppression of the pressure natriuresis relationship (1–4). The latter effects are associated with enhanced formation and secretion of angiotensinogen by proximal tubular cells and increased angiotensinogen spillover into distal nephron segments reflected by increases in urinary excretion of angiotensinogen (5–10). Because renin in collecting duct segments is also stimulated by chronic Ang II infusions (11–12), increased spillover of angiotensinogen into distal nephron segments suggests increases in distal nephron Ang II levels leading to stimulation of sodium reabsorption (12). Several studies have demonstrated that Ang II stimulates distal sodium transport processes including the amiloride sensitive-epithelial sodium channels (ENaC) and the sodium/hydrogen exchangers (13–18). However, there is no in vivo evidence that chronic Ang II infusions lead to increases in either absolute or fractional distal nephron sodium reabsorption that may play an important role in the progressive increase in arterial pressure during chronic Ang II infusions. In Ang II infused rats, sodium excretion was lower for any given arterial pressure and there was marked suppression of pressure natriuresis (3, 19), but the data did not allow segmental localization of the changes in sodium reabsorption. In chronic Ang II infused dogs, the sodium excretion initially decreased and remained decreased if renal arterial pressure was prevented from increasing (2, 20–21).
Whereas the proximal tubule is responsible for reabsorbing the bulk of the filtered load, the distal nephron segments are ultimately responsible for the fine regulation of sodium excretion (13, 22–25). Sodium reabsorption in connecting tubule and collecting duct segments is mainly mediated by amiloride (AM) sensitive ENaC and bendroflumethiazide (BFTZ) sensitive-Na+-Cl−cotransporters (NCC) (13, 22–23, 25–26). Thus, treatment with AM plus BFTZ blocks most of sodium transport in distal nephron segments allowing sodium excretion under these conditions to provide a collective measure of sodium delivery to distal nephron segments (27–30). Sodium reabsorption by distal nephron segments can thus be determined from the difference between urinary sodium excretion (UNaV) during distal blockade and UNaV during control conditions.
In this study, we tested the hypothesis that chronic Ang II-infused mice exhibit enhanced sodium reabsorption in distal nephron segments which may contribute to sodium retention. Although technical limitations prevent direct assessment of distal nephron and collecting duct Ang II concentrations, we measured concentrations in urine samples as an index of distal nephron Ang II levels. To minimize peptide degradation, we measured the urinary Ang II concentrations and excretion rates during the diuretic treatment, conditions where the urine would more closely reflect distal nephron tubular fluid. To obtain a collective assessment of distal nephron sodium reabsorption in the total nephron population, studies were performed during control conditions and following blockade of the two major distal nephron sodium transporters in mice infused chronically with Ang II (27–30). To investigate whether the observed changes in distal nephron sodium reabsorption were mediated by aldosterone during chronic Ang II infusions, we treated a group of chronic Ang II infused mice with spironolactone.
Methods
Animals
Experiments were performed on 9 to 12-wk old male C57/BL6 mice (Jackson Laboratory, Bar Harbor, Maine) maintained on a 12:12-hr light-dark schedule (6 AM to 6 PM) at 25°C in the vivarium at Tulane University Health Sciences Center. Rodent chow containing normal salt content (0.5%) along with tap water was provided. The protocol was approved by the Institutional Animal Care and Use Committee of Tulane University Health Sciences Center.
Experimental Protocol
Chronic Ang II-infused mice were prepared by administration of Ang II (Phoenix Pharmaceuticals, Burlingame, CA) at 15 ng/min via osmotic minipump for 11–13 days to elicit a slow progressive pressor response (10, 31). Systolic blood pressure in awake mice was measured by noninvasive computerized tail-cuff plethysmography (Visitech BP2000, Apex, NC). Systolic blood pressure was monitored at 7 and 11 days after minipump implantation in chronic Ang II-infused mice. On the day of the experiment, mice were anesthetized with Inactin (thiobutabarbital sodium) injected intraperitoneally at 200 mg/kg body weight (28, 32–33). Once a stable level of anesthesia was obtained, judged by heart rate and lack of toe reflex, mice were placed on a surgical table (37°C) with servo-control of temperature to maintain body temperature at 37°C and prepared for clearance experiments as previously described (28). During surgery, an isotonic saline solution containing 6% albumin (bovine; Sigma Chemical Co., St. Louis, MO) was infused at a rate of 4μl/min. The bladder was catheterized with PE-90 tubing via a suprapubic incision for urine collections. After surgery, the intravenous infusion solution was changed to isotonic saline containing 1% albumin, 4.5% polyfructosan (Inutest (Inulin), Laevosan, Linz/Donau, Austria), and 1.5% para-aminohippurate (PAH, Merck Sharpe & Dohme, West Point, PA) and was infused at 4μl/min for a 60 minute equilibration period prior to urine collections (28, 32–33).
Renal plasma flow (RPF), glomerular filtration rate (GFR), urine flow and sodium excretion were determined in control mice (n=10), chronic Ang II-infused mice (n=8) and chronic Ang II-infused mice treated with spironolactone pellet (n=5, ~190 mg/kg BW/day) which was implanted at same time as the minipump containing Ang II using a renal clearance protocol in mice previously described (28, 32). A separate group of mice were used for determination of urinary Ang II concentrations in Ang II infused and control mice. Urine samples for urinary Ang II measurement were initially collected directly into an inhibitor cocktail (5mM EDTA, 20uM Pepstatin, 10uM PMSF, 20uM Enalaprilat, 1.25mM 1–10-phenanthroline).
In each group, clearance periods 1 and 2 were performed for control measurements without diuretic treatment. An iv dose of AM (5 mg/kg body weight) and BFTZ (12 mg/kg body weight) was then administered followed by urine collections for assessment of distal sodium delivery. The peak sodium excretion during blockade was used as an estimate of sodium delivery to the distal nephron segments. By subtracting the average sodium excretion measured during periods 1 and 2, distal nephron sodium reabsorption was determined (27–30). At the end of the experiment, an arterial blood sample was collected from the arterial catheter for measurements of plasma PAH, inulin, and sodium concentrations. To determine if the diuretic treatment alter RPF and GFR, we compared AP, RPF and GFR between a group of mice treated with AM + BFTZ (n=7) and a group of mice not treated with AM + BFTZ (n=5). We did not observe any significant differences between untreated control mice and diuretic treated mice in MAP (99±2 mm Hg versus 93±2 mm Hg, P>0.05), RPF (1.42±0.32 ml/min versus 1.33±0.30 ml/min, P>0.05) and GFR (0.25±0.05 ml/min versus 0.19±0.02 ml/min, P>0.05).
Urine and Plasma PAH, Inulin and Sodium Concentrations
Urine and plasma PAH and inulin concentrations were measured using standard colorimetric techniques as reported previously (33). RPF was estimated from the PAH clearance calculated as the ratio of urine and plasma PAH concentrations times urine flow. GFR was calculated as the ratio of urine and plasma inulin concentrations times urine flow. Urine output was determined gravimetrically assuming a density of 1g/ml. Urine and plasma sodium concentrations were measured using flame photometry (Flame Photometer IL 973, Instrumentation Laboratory, Lexington, MA).
Urine Ang II Radioimmunoassay Measurement
The detailed procedure is available in the online supplement at http://www.hypertensionaha.org.
Calculations
UNaVc designates the control urinary sodium excretion (the average of periods 1 and 2). UNaVAM+BFTZ (distal sodium delivery, DSD) was based on the peak urinary sodium excretion after administration of AM + BFTZ (period 3). Calculated distal sodium reabsorption (DSR) was determined from UNaVAM+BFTZ − UNaVc. Fractional reabsorption of distal sodium delivery (FRDSD) was calculated from (UNaVAM+BFTZ − UNaVc)/UNaVAM+BFTZ.
Statistical Analysis
The statistical analysis was performed by one-way ANOVA with Bonferroni’s multiple comparison post hoc tests using the GraphPad PRISM program (GraphPad, San Diego, CA). The results are presented as mean ± SE. Significance was set at P<0.05.
Results
Systolic Blood Pressure and AP
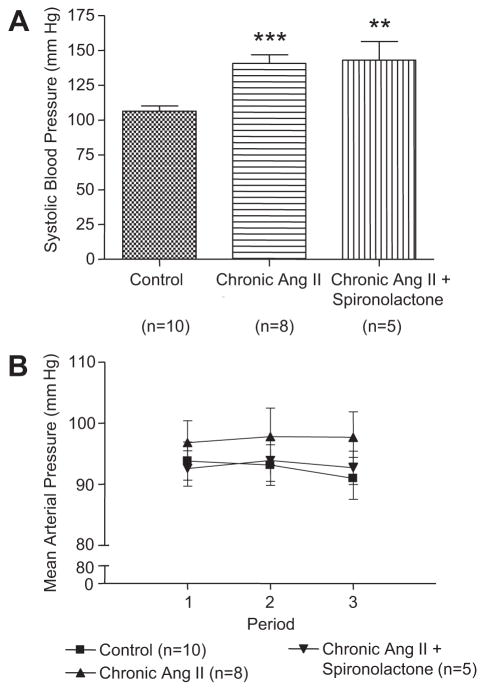
As shown in Figure 1A, systolic blood pressure measured in awake mice was increased after 2 weeks of chronic Ang II infusions (10) as compared with control mice (141±6 mm Hg versus 106±4 mm Hg, P<0.001). In chronic Ang II-infused mice treated with spironolactone, systolic blood pressure was also higher as compared with control mice (143±13 mm Hg, P<0.01). As shown in Fig. 1B, after anesthesia, mice infused chronically with Ang II had MAP averaging 97±4 mm Hg. MAP in normal mice studied so far are generally lower averaging around 94±3 mm Hg. In the group of chronic Ang II-infused mice treated with spironolactone, MAP averaged 93±3 mm Hg.
Figure 1.
Effects of chronic Ang II infusions for 2 weeks on systolic blood pressure and AP. Values are means ± SE for the representative groups. As compared with control, *, P<0.05; **, P<0.01; ***, P<0.001.
RPF and GFR
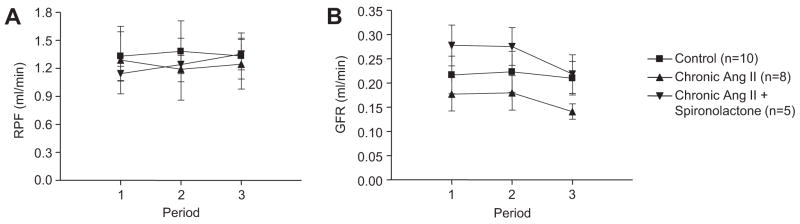
As shown in Figure 2, RPF and GFR were not significantly different among the three groups and remained stable during the clearance periods. Although GFR tended to be slightly lower in the Ang II infused groups, the differences are not statistically significant.
Figure 2.
PAH clearance and GFR in control mice, chronic Ang II-infused mice and chronic Ang II-infused mice treated with spironolactone. Values are means ± SE for the representative groups.
Urine Flow and Urinary Sodium Excretion
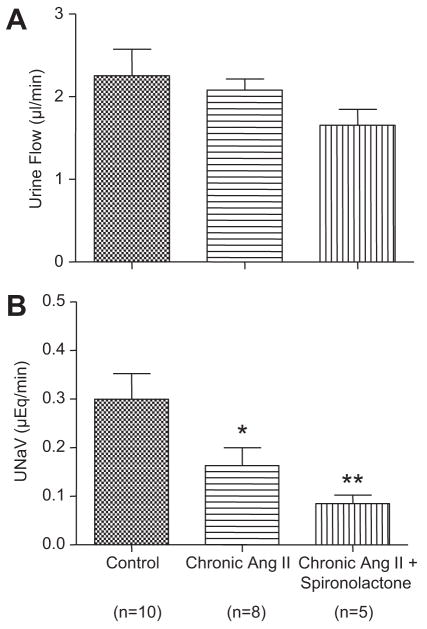
As shown in Figure 3, urine flow was slightly lower in the chronic Ang II-infused mice, but this was not statistically different from urine flow in control mice. However, urinary sodium excretion rate in the chronic Ang II-infused mice was lower compared to control (0.16±0.04 μEq/min versus 0.30±0.05 μEq/min, P<0.05). Following dual distal nephron blockade with AM plus BFTZ, the fractional urinary sodium excretion rates increased markedly to 4.5±0.6% in control and 7.5±1.3% in Ang II-infused mice thus reflecting the fractional sodium delivery to the terminal nephron segments. In the group of chronic Ang II-infused mice treated with spironolactone, urine flow was not statistically different as compared with control mice, whereas urinary sodium excretion (0.08±0.02 μEq/min, P<0.01) was lower. However, urine flow and urinary sodium excretion were not statistically different as compared with chronic Ang II-infused mice.
Figure 3.
Urine flow and urinary sodium execration in control mice, chronic Ang II-infused mice and chronic Ang II-infused mice treated with spironolactone. Values are means ± SE for the representative groups. As compared with control, *, P<0.05; **, P<0.01.
Distal Sodium Delivery and Sodium Reabsorption in Distal Nephron Segments
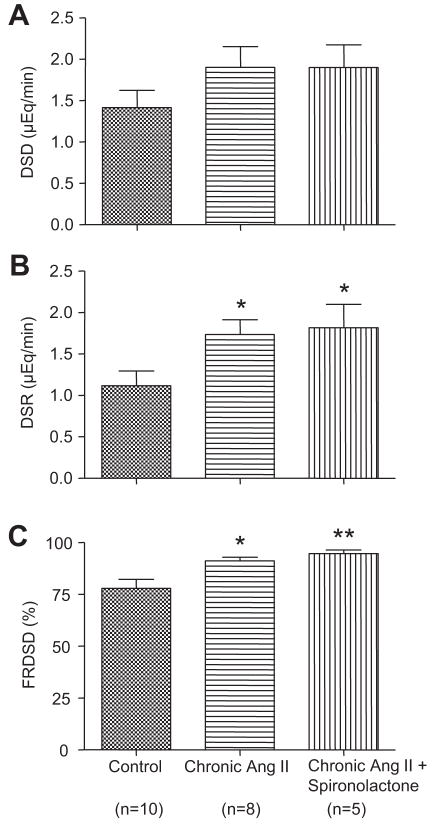
As shown in Figure 4, distal sodium delivery values were not significantly different between chronic Ang II-infused mice and control mice (1.90±0.25 μEq/min versus 1.42±0.21 μEq/min, P>0.05). In contrast, distal sodium reabsorption in the chronic Ang II-infused mice was greater as compared with control mice (1.74±0.18 μEq/min versus 1.12±0.18 μEq/min, P<0.05). Likewise, the fractional reabsorption of distal sodium delivery values were augmented in the chronic Ang II-infused mice (91.1±1.8% versus 77.9±4.3%, P<0.05). In chronic Ang II-infused mice treated with spironolactone, distal sodium reabsorption (1.82±0.28 μEq/min, P<0.05) and the fractional reabsorption of distal sodium delivery values (94.6±1.7%, P<0.01) were greater than in control mice, whereas distal sodium delivery values were not significantly different between chronic Ang II-infused mice treated with spironolactone and control mice (1.90±0.27 μEq/min, P>0.05). However, the distal sodium reabsorption and the fractional reabsorption of distal sodium delivery values were not statistically different as compared with chronic Ang II-infused mice.
Figure 4.
Distal sodium delivery and sodium reabsorption in distal nephron segments in control mice, chronic Ang II-infused mice and chronic Ang II-infused mice treated with spironolactone. Values are means ± SE for the representative groups. As compared with control, *, P<0.05; **, P<0.01.
Urinary Ang II level
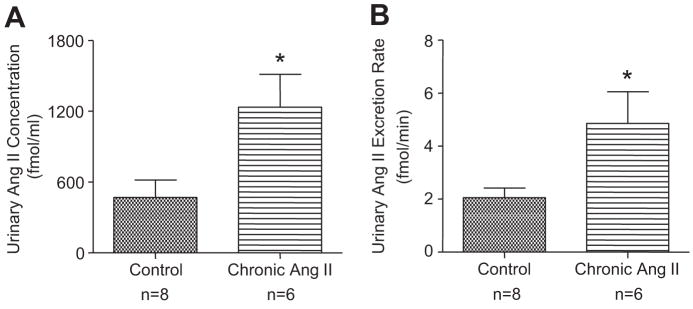
As shown in Figure 5, urinary Ang II concentrations (1235.0±277.2 fmol/ml versus 468.9±146.9 fmol/ml, P<0.05) and urinary Ang II excretion rates (4.86±1.19 fmol/min versus 2.06±0.36 fmol/min, P<0.05) were significantly higher in the chronic Ang II-infused mice during administration of AM+BFTZ as compared with control.
Figure 5.
Urinary Ang II concentrations and excretion rates in control mice and chronic Ang II-infused mice during administration of AM + BFTZ. Values are means ± SE for the representative groups. As compared with control, *, P<0.05.
Discussion
Various studies have demonstrated that, in addition to its actions on earlier nephron segments, Ang II can stimulate transport activity in distal nephron segments (13, 16–18, 26, 34–35). In particular, intraluminal Ang II can stimulate NHE, NCC, ENaC, potassium channel and H+-ATPase as well (16–18, 26, 36–38). Furthermore, luminal application of Ang II directly stimulates ENaC activity in the cortical collecting duct (13–15), even at a low concentration of 10−12M (13). These studies demonstrate that Ang II directly stimulates sodium reabsorption in distal nephron segments; nevertheless, in vivo quantitative data in support of enhanced distal sodium transport in chronic Ang II infused models of hypertension have been difficult to obtain. While micropuncture and isolated perfused tubule and cell studies provide direct data indicating the actions of Ang II on the transport mechanisms (17–18, 36–38), they do not provide quantitative estimates of the collective effect on distal nephron reabsorption rate. It is recognized that the present approach using transport inhibitors that block the distal nephron sodium transport systems provides only an indirect estimate of distal sodium reabsorption, nevertheless, it is an effective means of providing a collective quantitative estimate of the net sodium rabsorption in the distal nephron segments (28–30).
The present study provides in vivo evidence that chronic Ang II infusions elicit a chronic stimulatory influence on sodium reabsorptive processes in distal nephron segments. These results are consistent with the hypothesis that augmentation of distal Ang II levels stimulates distal sodium reabsorption thus contributing to the progressive hypertension that develops during chronic Ang II infusions. Previous studies have shown that chronic Ang II infused rats have a rightward shift of the pressure-natriuresis relationshift caused primarily by a decrease in fractional excretion of sodium (3–4, 19–20, 39–40). In chronic Ang II-infused rats and mice, circulating Ang II levels increase within a few days and lead to augmentation of intrarenal angiotensinogen and Ang II that reduces sodium excretion resulting in progressive increases in arterial pressure (1, 6–10, 36, 41). Hall et al (20) reported that Ang II infusions caused sodium retention in dogs when renal arterial pressure was prevented from rising with a servo-controlled arotic occluder. Chronic Ang II infusions caused sodium retention for several days before sodium balance was achieved at an elevated MAP in dogs (21).
Although inappropriate stimulation of sodium reabsorption in any segments of the nephron can lead to excess sodium retention and hypertension (42), various studies have emphasized the important role of sodium transport in the terminal distal nephron segments, in particular the collecting duct system (12, 16, 23, 25). Several monogenetic mutations in human subjects with hypertension involve overactivation of distal sodium transport systems (24, 43) including activation of ENaC and NCC which are found in the distal convoluted tubule and collecting duct segments (16, 23, 25–26). In addition, chronic Ang II infusions have been shown to upregulate ENaC expression in collecting duct (16) and Ang II has been shown to enhance trafficking of the distal tubule NCC (26) and to activate ENaC (13, 15). These and related findings discussed earlier support an enhanced distal nephron sodium reabsorption in Ang II dependent hypertension.
In the present study, RPF and GFR were not significantly different in chronic Ang II-infused mice and control mice as previously reported by Cervenka et al (44). These results suggest that GFR is less responsive in mice than rats since previous studies in rats showed that GFR is significantly decreased by chronic Ang II infusions, however, RPF was not different consistent with the present results (3, 45). Notably, renal vascular Ang II type 1 (AT1) receptor expression is maintained in chronic Ang II-infused rats (46), and losartan prevents the decreases of GFR in chronic Ang II-infused rats (3). Furthermore, Ang II mediated increases in oxidative stress may be involved in the regulation of arterial pressure and renal vascular resistance in chronic Ang II infused mice (31).
The sodium excretion responses to chronic Ang II infusions are complex and depend on the dose and duration of the Ang II infusions as well as the magnitude of the blood pressure responses. In this study, urinary sodium excretion in the anesthetized chronic Ang II-infused mice was significantly lower than in control mice thus suggesting that the higher arterial pressures measured in vivo are required to maintain sodium balance in the awake mice (2, 20). These studies suggest initial sodium retention during chronic Ang II infusions with restoration of sodium balance achieved at the elevated arterial pressures (21). Following dual distal nephron blockade with AM plus BFTZ, the fractional sodium excretion rates increased to 4.5% in control and 7.5% in chronic Ang II-infused mice thus reflecting the fractional sodium delivery to the terminal nephron segments. These data support our basic rationale that the dual distal blockade effectively inhibits the bulk of the sodium reabsorbed by the distal nephron segments. Furthermore, absolute distal sodium delivery was similar suggesting that sodium loadsarriving at distal nephron segments were similar. These results thus suggest that much of the augmented sodium reabsorption responsible for the reduced sodium excretion in the chronic Ang II-infused mice occurred in distal nephron segments.
Chronic Ang II infusions stimulate production and release of aldosterone, which activates the mineralocorticoid receptors in principal cells of collecting ducts and thus augment the abundance of Na/K ATPase, ENaC and potassium channel (47–50). However, in the present study, there was a trend toward a lower urinary sodium excretion which was not statistically significant. Also, fractional reabsorption of distal sodium delivery between chronic Ang II-infused mice treated with spironolactone and chronic Ang II-infused mice was not significantly different. These results suggest that increased aldosterone is not responsible for the enhanced distal sodium reabsorption in chronic Ang II-infused mice and are consistent with the recent study by Ortiz, et al (51).
While proximal tubule fluid Ang II concentrations have been measured (35–36), it has not been possible to obtain an index of the Ang II concentrations in distal nephron tubular and collecting duct fluid. Due to major technological issues, micropuncture (34) and microcatheterization procedures are not feasible and would require extensive surgical procedures and unrealistic collection periods. We reasoned that the markedly increased urine output occurring during dual distal blockade would provide samples that more closely reflect distal tubular fluid or collecting duct fluid and might have Ang II concentrations approaching those existing in the tubular fluid. We found significant increases in Ang II concentrations as well as in Ang II excretion rates in the chronic Ang II infused mice. These data provide in vivo evidence that tubular fluid Ang II concentrations in distal nephron segments are indeed higher in the chronic Ang II infused mice.
Perspective
While elevated intrarenal Ang II levels may augment sodium reabsorption in multiple nephron segments, the augmentation of distal Ang II levels may play a particularly important role in the progressive hypertension during chronic Ang II infusions. These effects to stimulate the distal sodium reabsorption leading to reabsorption of 90% of the distal sodium delivery provide the final influence to achieve marked sodium retention which leads to progressive hypertension. The efficacy of thiazide diuretics and amiloride in certain hypertensive cases may be due to their ability to counteract the enhanced distal sodium reabsorption. The noninvasive approach utilized in the present study is applicable to studies in transgenic mice and also to translational experiments which could test this hypothesis in human subjects.
Acknowledgments
Sources of Fundings
This work was supported by National Heart, Lung, and Blood Institute Grant HL-26731, by a Health Excellence Fund grant from the Louisiana Board of Reagents and by the COBRE grant P20RR0117659 from the Institutional Development Award Program of NCRR.
Footnotes
Conflict of Interest/Disclosure Statement: None
References
- 1.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall JE, Granger JP, Hester RL, Coleman TG, Smith MJ, Jr, Cross RB. Mechanisms of escape from sodium retention during angiotensin II hypertension. Am J Physiol. 1984;246:F627–F634. doi: 10.1152/ajprenal.1984.246.5.F627. [DOI] [PubMed] [Google Scholar]
- 3.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell KD, Braam B, Navar LG. Hypertensinogenic mechanisms mediated by renal actions of renin-angiotensin system. Hypertension. 1992;19:I18–I27. doi: 10.1161/01.hyp.19.1_suppl.i18. [DOI] [PubMed] [Google Scholar]
- 5.Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol. 1992;263:E863–E869. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- 6.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal Angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, Katsurada A, Tran DV, Kobori H, Navar LG. Intrarenal Angiotensin II and Angiotensinogen Augmentation in Chronic Angiotensin II-infused mice. Am J Physiol Renal Physiol. 2008;295:F772–F779. doi: 10.1152/ajprenal.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 14.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 15.Burns KD, Li N. The role of angiotensin II-stimulated renal tubular transport in hypertension. Curr Hypertens Rep. 2003;5:165–171. doi: 10.1007/s11906-003-0074-1. [DOI] [PubMed] [Google Scholar]
- 16.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003;41:1143–1150. doi: 10.1161/01.HYP.0000066129.12106.E2. [DOI] [PubMed] [Google Scholar]
- 17.Barreto-Chaves ML, Mello-Aires M. Effect of luminal angiotensin II and ANP on early and late cortical distal tubule HCO3- reabsorption. Am J Physiol. 1996;271:F977–F984. doi: 10.1152/ajprenal.1996.271.5.F977. [DOI] [PubMed] [Google Scholar]
- 18.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol. 1996;271:F143–F149. doi: 10.1152/ajprenal.1996.271.1.F143. [DOI] [PubMed] [Google Scholar]
- 19.van der Mark J, Kline RL. Altered pressure natriuresis in chronic angiotensin II hypertension in rats. Am J Physiol. 1994;266:R739–R748. doi: 10.1152/ajpregu.1994.266.3.R739. [DOI] [PubMed] [Google Scholar]
- 20.Hall JE, Guyton AC, Brands MW. Control of sodium excretion and arterial pressure by intrarenal mechanisms and the renin-angiotensin system. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis and Management. New York: Raven Press; 1990. pp. 1451–1475. [Google Scholar]
- 21.Lohmeier TE, Hildebrandt DA. Renal nerves promote sodium excretion in angiotensin-induced hypertension. Hypertension. 1998;31:429–434. doi: 10.1161/01.hyp.31.1.429. [DOI] [PubMed] [Google Scholar]
- 22.Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev. 2007;87:1083–1112. doi: 10.1152/physrev.00053.2006. [DOI] [PubMed] [Google Scholar]
- 23.Pratt JH. Central role for ENaC in development of hypertension. J Am Soc Nephrol. 2005;16:3154–3159. doi: 10.1681/ASN.2005050460. [DOI] [PubMed] [Google Scholar]
- 24.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 25.McCormick JA, Yang CL, Ellison DH. WNK kinases and renal sodium transport in health and disease: an integrated view. Hypertension. 2008;51:588–596. doi: 10.1161/HYPERTENSIONAHA.107.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+-Cl− cotransporter to apical membrane. Am J Physiol Renal Physiol. 2007;293:F662–F669. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- 27.Majid DS, Navar LG. Blockade of distal nephron sodium transport attenuates pressure natriuresis in dogs. Hypertension. 1994;23:1040–1045. doi: 10.1161/01.hyp.23.6.1040. [DOI] [PubMed] [Google Scholar]
- 28.Zhao D, Navar LG. Acute angiotensin II infusions elicit pressure natriuresis in mice and reduce distal fractional sodium reabsorption. Hypertension. 2008;52:137–142. doi: 10.1161/HYPERTENSIONAHA.108.111435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan O, Riazi S, Hu X, Song J, Wade JB, Ecelbarger CA. Regulation of the renal thiazide-sensitive Na-Cl cotransporter, blood pressure, and natriuresis in obese Zucker rats treated with rosiglitazone. Am J Physiol Renal Physiol. 2005;289:F442–F450. doi: 10.1152/ajprenal.00335.2004. [DOI] [PubMed] [Google Scholar]
- 30.Kleinman LI, Banks RO. Segmental nephron sodium and potassium reabsorption in newborn and adult dogs during saline expansion. Proc Soc Exp Biol Med. 1983;173:231–237. doi: 10.3181/00379727-173-41637. [DOI] [PubMed] [Google Scholar]
- 31.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol. 2002;13:2860–2868. doi: 10.1097/01.asn.0000035087.11758.ed. [DOI] [PubMed] [Google Scholar]
- 32.Cervenka L, Mitchell KD, Navar LG. Renal function in mice: effects of volume expansion and angiotensin II. J Am Soc Nephrol. 1999;10:2631–2636. doi: 10.1681/ASN.V10122631. [DOI] [PubMed] [Google Scholar]
- 33.Shi SJ, Vellaichamy E, Chin SY, Smithies O, Navar LG, Pandey KN. Natriuretic peptide receptor A mediates renal sodium excretory responses to blood volume expansion. Am J Physiol Renal Physiol. 2003;285:F694–F702. doi: 10.1152/ajprenal.00097.2003. [DOI] [PubMed] [Google Scholar]
- 34.Wang CT, Navar LG, Mitchell KD. Proximal tubular fluid angiotensin II levels in angiotensin II-induced hypertensive rats. J Hypertens. 2003;21:353–360. doi: 10.1097/00004872-200302000-00027. [DOI] [PubMed] [Google Scholar]
- 35.Navar LG, Prieto-Carrasquero MC, Kobori H. Molecular aspects of the renal renin-angiotensin system. In: Re RN, Dipette DJ, Schilffrin EL, Sowers JR, editors. Molecular Mechanisms in Hypertension. United Kingdom: Taylor and Francis; 2006. pp. 3–14. [Google Scholar]
- 36.Wei Y, Wang WH. Angiotensin II stimulates basolateral K channels in rat cortical collecting ducts. Am J Physiol Renal Physiol. 2003;284:F175–F181. doi: 10.1152/ajprenal.00211.2002. [DOI] [PubMed] [Google Scholar]
- 37.Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II activates H+-ATPase in type A intercalated cells. J Am Soc Nephrol. 2008;19:84–91. doi: 10.1681/ASN.2007030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiner ID, New AR, Milton AE, Tisher CC. Regulation of luminal alkalinization and acidification in the cortical collecting duct by angiotensin II. Am J Physiol. 1995;269:F730–F738. doi: 10.1152/ajprenal.1995.269.5.F730. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell KD, Navar LG. Intrarenal actions of angiotensin II in the pathogenesis of experimental hypertension. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management. New York: Raven; 1995. pp. 1437–1450. [Google Scholar]
- 40.Navar LG, Hamm LL. The kidney in blood pressure regulation. In: Wilcox CS, editor. Atlas of Disease of the Kidney, Hypertension and the Kidney. Philadelphia: Current Medicine; 1999. pp. 1–22. [Google Scholar]
- 41.Zou LX, Imig JD, von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal angiotensin II augmentation in angiotensin II-infused rats. Hypertension. 1996;28:669–677. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]
- 42.Coffman TM, Crowley SD. Kidney in hypertension: guyton redux. Hypertension. 2008;51:811–816. doi: 10.1161/HYPERTENSIONAHA.105.063636. [DOI] [PubMed] [Google Scholar]
- 43.Johnson RJ, Feig DI, Nakagawa T, Sanchez-Lozada LG, Rodriguez-Iturbe B. Pathogenesis of essential hypertension: historical paradigms and modern insights. J Hypertens. 2008;26:381–391. doi: 10.1097/HJH.0b013e3282f29876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cervenka L, Maly J, Karasova L, Simova M, Vitko S, Hellerova S, Heller J, El-Dahr SS. Angiotensin II-induced hypertension in bradykinin B2 receptor knockout mice. Hypertension. 2001;37:967–973. doi: 10.1161/01.hyp.37.3.967. [DOI] [PubMed] [Google Scholar]
- 45.Alexander BT, Cockrell KL, Rinewalt AN, Herrington JN, Granger JP. Enhanced renal expression of preproendothelin mRNA during chronic angiotensin II hypertension. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1388–1392. doi: 10.1152/ajpregu.2001.280.5.R1388. [DOI] [PubMed] [Google Scholar]
- 46.Harrison-Bernard LM, El-Dahr SS, O’Leary DF, Navar LG. Regulation of angiotensin II type 1 receptor mRNA and protein in angiotensin II-induced hypertension. Hypertension. 1999;33:340–346. doi: 10.1161/01.hyp.33.1.340. [DOI] [PubMed] [Google Scholar]
- 47.Welling PA, Caplan M, Sutters M, Giebisch G. Aldosterone-mediated Na/K-ATPase expression is alpha 1 isoform specific in the renal cortical collecting duct. J Biol Chem. 1993;268:23469–23476. [PubMed] [Google Scholar]
- 48.McEneaney V, Harvey BJ, Thomas W. Aldosterone regulates rapid trafficking of epithelial sodium channel subunits in renal cortical collecting duct cells via protein kinase D activation. Mol Endocrinol. 2008;22:881–892. doi: 10.1210/me.2007-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Subramanya AR, Yang CL, McCormick JA, Ellison DH. WNK kinases regulate sodium chloride and potassium transport by the aldosterone-sensitive distal nephron. Kidney Int. 2006;70:630–634. doi: 10.1038/sj.ki.5001634. [DOI] [PubMed] [Google Scholar]
- 50.Chun TY, Pratt JH. Mineralocorticoid receptors. In: Izzo JL, Black HR, Sica DA, editors. Hypertension Primer: The Essentials of High Blood Pressure. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 64–65. [Google Scholar]
- 51.Ortiz RM, Graciano ML, Seth D, Awayda MS, Navar LG. Aldosterone receptor antagonism exacerbates intrarenal angiotensin II augmentation in ANG II-dependent hypertension. Am J Physiol Renal Physiol. 2007;293:F139–F147. doi: 10.1152/ajprenal.00504.2006. [DOI] [PubMed] [Google Scholar]