Abstract
Background:
Liver resection of large hepatocellular carcinomas (HCC), measuring at least 10 cm remains a controversial debate. Multiple studies on HCCs treated with surgical resection and/or ablation had shown variable results with 5-year survival rates ranging from 0% to 54.0%. The aim of this study was to evaluate the survival of patients with HCCs measuring at least 10 cm and to identify the potential prognostic variables affecting the outcome.
Methods:
Retrospective analysis was performed on the prospectively updated HCC database. A total of 44 patients with tumours measuring 10 cm or more were ‘curatively’ treated with surgical resection with or without ablation. Patient demographics, clinical, surgical, pathology and survival data were collected and analysed.
Results:
Thirty-one patients received surgical resection alone. Thirteen other patients were treated with a combination of surgical resection and ablation. The median follow-up duration was 14.5 months. The overall median survival at 1, 3 and 5 years were 66.4%, 38.1% and 27.8%, respectively. The median time to tumour recurrence was 10.7 months and the 1, 3 and 5-year disease-free survival were 49.6%, 23.9% and 19.1%, respectively.
Univariate analysis demonstrated cirrhosis, microvascular invasion, poor tumour differentiation and ethnicity to adversely affect survival. For overall survival, only cirrhosis, poor tumour differentiation and ethnicity were significant on multivariate analysis. Portal vein tumour thrombus, microvascular invasion and ethnicity were identified on univariate analysis to significantly affect disease-free survival.
Conclusion:
Surgical treatment offers good survival to patients with large HCCs (≥10 cm). Both cirrhosis and poor tumour differentiation are independent variables prognostic of adverse survival.
Keywords: hepatocellular carcinoma, large hepatocellular, hepatectomy, resection and ablation
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world. Despite advances in imaging technologies, a significant proportion of HCCs are inoperable as a result of advanced stages at time of diagnosis.1 To date, liver transplant, surgical resection and local ablative therapies (cryotherapy and radiofrequency ablation) are the most effective treatments available, offering a chance of cure to HCC patients. Other alternatives such as transarterial chemoembolization (TACE), percutaneous ethanol injection, acetic acid injection and intra-arterial lipiodol I131 are not as effective in achieving complete tumour eradication, particularly in large tumours.2,3
Tumour size plays a significant role in the decision making of treatment preference. Previously, large HCCs (>10 cm) were often not treated leading to the associated poor prognosis within this cohort of patients. Amidst the availability of effective surgical treatments, tumour size of over 10 cm precludes the eligibility for liver transplant or thermal ablative therapies. While hepatectomy of smaller tumours (<10 cm) is commonly performed with 5-yearsurvival rates ranging from 11% to 58%, surgical resection of large tumours remains controversial.4–6 Several studies have identified tumour size as prognostic of adverse survival. Others, however, showed that liver resection of non-vascular-involved solitary HCCs (Stage T1) can achieve good long-term outcome, irrespective of tumour size.7
The purpose of this study was to evaluate the outcome of therapy in patients with HCC measuring at least 10 cm, which was regarded by most surgeons as inoperable; and to identify prognostic factors for survival.8,9
Methods
This is a retrospective study of a prospectively updated database. Since January 1990, a total of 349 patients with hepatocellular carcinoma (HCC) had been looked after by the St George Hospital Liver Unit. Of these, 110 patients were ‘curatively’ treated with sole surgical resection or combined surgical resection and ablation. Treatment was regarded as potentially curative as long as the liver was macroscopically clear of tumour post-surgery; which was confirmed intra-operatively with ultrasonography. Determination of the extent of liver resection was adopted from the study by Imamura et al.10 The decision to perform synchronous liver resection and ablative therapies was based on the anatomical location of tumours; aiming to preserve sufficient post-operative liver parenchyma. Edge ablations were indicated for cases with a macroscopically close resection margin.
Our selection criteria for ‘curative’ surgical treatment included absence of extrahepatic disease and preserved liver function (Child's A and B). Extent of disease was assessed by lipiodol CT, chest/abdominal/pelvic CT, bone scans and, if required, CT angiography. Preservation of liver function was established with Child's score and ICG retention in cirrhosis. Child's score for all patients was summated based on two clinical (ascites and encephalopathy) and three biochemical factors (serum bilirubin, albumin level and international normalized ratio). Suitability of resection was further determined intra-operatively with ultrasound and palpation. Cirrhosis and involvement of portal and hepatic veins were not absolute contraindications for surgery. All surgical resections were performed using the cavitron ultrasonic aspiration (CUSA) technique. All patients were followed up with serum αFP (alpha-fetoprotein) levels, liver function tests and abdominal CT scans 1-month post-discharge and at 3-monthly intervals thereafter. Only patients treated from 1999 onwards were offered adjuvant lipiodol I131.
Patients with lesions larger than 10 cm, measured at the largest diameters, were identified and included into study. All files were reviewed up until 2nd June 2008. Data extracted for analysis included patient demographics, hepatitis status, Child–Pugh scoring, pre- and post-operative serum αFP (α-fetoprotein) levels, type of surgery, histopathology details of resected specimens, disease course and survival.
Hospital deaths, defined as deaths resulting from post-operative complications and/or those within the same admission, were included into both overall survival and disease-free survival analysis. Survival analyses, estimated from the date of surgery, were performed with the Kaplan–Meier survival analysis and compared with univariate analysis. Multivariate analysis was performed using a Cox regression model to identify independent variables. A P-value of <0.05 was considered as significant. All statistical analyses were performed using SPSS for Windows (version 15.0; SPSS Inc., Chicago, IL, USA).
Results
Patients and treatment modalities
Since 1990, a total of 110 HCC patients had been treated with surgical resection with or without ablation by the St George Hospital Liver Unit. Of those, 44 patients were identified to have lesions of at least 10 cm in diameter, and were included into the study. A total of 31 patients were solely treated with surgical resection while 13 others had a combination of surgical resection and ablation. Ablative techniques performed included eight residual liver edge ablations with cryotherapy (7) and radiofrequency ablation (RFA) (1), two cryoablations and three alcohol injections to contralateral lobe lesions. Sixteen patients within this series were treated adjuvantly with lipiodol I131. Patient demographics and clinicopathological features of the study group are summarized in Table 1.
Table 1.
Demographics and clinicopathological characteristics
| Parameters | Study group | Non-cirrhotics(n = 29) | Cirrhotics(n = 15) |
|---|---|---|---|
| Age (years) | |||
| Median | 62.4 | 62.1 | 62.3 |
| Range | 19.7–87.5 | 19.7–80.5 | 32.7–87.5 |
| Gender | |||
| Male | 33 | 20 (60.9%) | 13 (86.7%) |
| Female | 11 | 9 (31.0%) | 12 (13.3%) |
| Aetiology | |||
| Hepatitis B | 15 | 7 | 8 |
| Hepatitis C | 3 | – | 3 |
| Alcohol | 5 | 3 | 2 |
| Haemochromatosis | 5 | 3 | 2 |
| Unknown | 16 | 16 | – |
| Child–Pugh score | |||
| A | 35 | 24 | 11 |
| B | 7 | 3 | 4 |
| Unknown | 2 | 2 | – |
| Type of resection | |||
| Right hepatectomy | 13 | 6 | 7 |
| Left hepatectomy | 4 | 3 | 1 |
| Extended right hepatectomy | 7 | 6 | 1 |
| Extended left hepatectomy | 3 | 3 | – |
| Central resection | 3 | 2 | 1 |
| Left lateral resection | 4 | 3 | 1 |
| Segmentectomy | 10 | 6 | 4 |
| Serum αFP-level (ng/ml) | |||
| Median | 33.5 | 33.5 | 29.0 |
| Range | 0.2–168000 | 2.0–168000 | 2.0–168000 |
| Tumour size (cm) | |||
| Mean (SD) | 13.5 ± 3.2 | 13.2 ± 3.0 | 14.1 ± 3.7 |
| Median | 12.4 | 12.3 | 13 |
| Range | 10.0–20.0 | 10.0–20.0 | 10.0–20.0 |
| No. of lesions | |||
| Solitary | 23 | 17 (58.6%) | 6 (40%) |
| Multiple | 21 | 12 (41.4%) | 9 (60%) |
| Lobar involvement | |||
| Unilobar | 32 | 21 (72.4%) | 11 (73.3%) |
| Bilobar | 12 | 8 (27.6%) | 4 (26.7%) |
| Ruptured tumours | 6 | 5 (17.2%) | 1 (6.7%) |
| Portal vein thrombus | 5 | 1 (3.4%) | 4 (26.7%) |
| Microvascular invasion | 27 | 16 (48.3%) | 11 (73.3%) |
| Positive margins | 22 | 13 (44.8%) | 9 (60.0%) |
| Histology | |||
| Fibrolamellar | 8 | 7 | 1 |
| Well differentiated | 8 | 6 | 2 |
| Moderately differentiated | 21 | 10 | 11 |
| Poorly differentiated | 7 | 6 | 1 |
Survival analysis
After a median follow-up duration of 14.5 months (range, 0.03–169.9 months), two patients were lost to follow up at 76 and 110 months, and were censored in analyses. At the time of analysis, disease had recurred in 24 patients and 27 had died. The overall median survival was 21.5 months (range, 0.03–169.9 months), and the 1-, 3- and 5-year survival rates were 66.4%, 38.1% and 27.8%, respectively (Fig. 1a).
Figure 1.
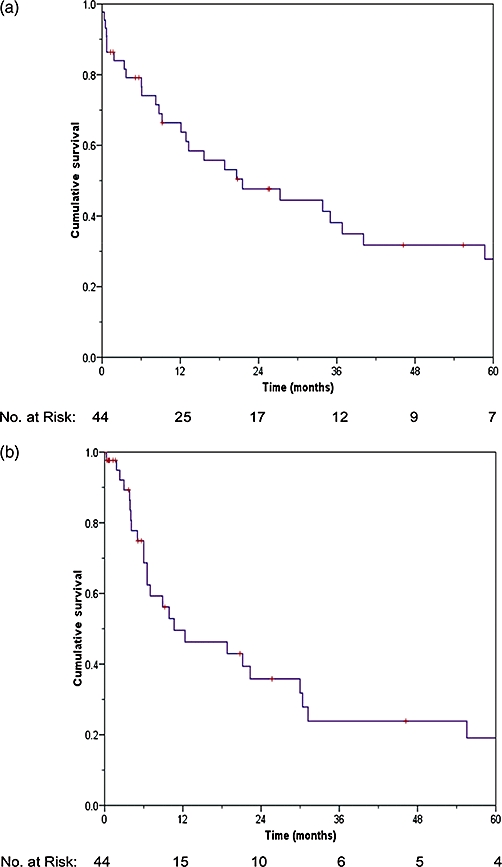
Survival analysis. (a) Overall survival of large hepatocellular carcinomas (HCCs) after surgical treatment; (b) disease-free survival of large HCCs after surgical treatment
Disease-free survival (DFS) rates at 1-, 3- and 5-years were 49.6%, 23.9% and 19.1%, respectively (Fig. 1b); with a median time to tumour recurrence of 10.7 months (range, 0.3–169.6 months). Liver recurrence occurred in 13 patients, three of them at the resection margin or ablation site. Treatment of liver recurrence (12) was by lipiodol I131 (5), ablation (3), resection (1) and chemotherapy (1). Extrahepatic recurrence (3) occurred in the lungs (2), and peritoneum (1). Patients with recurrence both in the liver and another site (7) were treated by resection (3) and chemotherapy (2).
The survival difference between the resection only and synchronous resection and ablation groups was insignificant.
Hospital mortalities
There were a total of eight hospital deaths (18.2%). The causes of deaths were liver failure in three patients, sepsis and liver failure in two, hypovolaemic shock in one and non-disease related in two others. Of the non-disease-related mortalities, one was as a result of refusal of amputation because of a gangrenous foot. The second patient died of hypovolaemic shock following removal of a chest drain after resolution of a pleural effusion.
Prognostic factors
Fourteen potential prognostic factors were analysed in univariate analysis for overall survival (Table 2). The presence of cirrhosis (P = 0.014), microvascular invasion (P = 0.032), poor tumour differentiation (P < 0.001) and ethnicity (P < 0.001) were significantly associated with adverse survival (Fig. 2). In multivariate analysis, three factors were found to be independently associated with poorer survival – cirrhosis (hazard ratio, 4.4; 95% CI, 1.078–9.961; P = 0.036), poor tumour differentiation (hazard ratio, 10.128; 95% CI, 2.510–48.000; P = 0.001) and ethnicity (hazard ratio, 8.805; 95% CI, 1.547–8.448; P = 0.003). Evaluation of the same variables identified three factors that significantly reduced DFS – the presence of portal vein tumour thrombus (P = 0.003), microvascular invasion (P = 0.038) and ethnicity (P = 0.003).
Table 2.
Univariate analysis
| Variables | Frequency | Overall survival |
Disease-free survival |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1-year (%) | 3-year (%) | 5-year (%) | P-value | 1-year (%) | 3-year (%) | 5-year (%) | P-value | ||
| Gender | 0.345 | 0.418 | |||||||
| Male | 33 | 62.1 | 35.1 | 21.1 | 46.7 | 22.3 | 14.9 | ||
| Female | 11 | 81.8 | 46.8 | 46.8 | 60.0 | 30.0 | 30.0 | ||
| Age | 0.176 | 0.075 | |||||||
| <65 years | 25 | 70.4 | 49.4 | 38.4 | 56.7 | 36.0 | 36.0 | ||
| >65 years | 19 | 61.5 | 23.5 | 11.7 | 40.9 | 9.1 | 0.0 | ||
| Cirrhosis | 0.014 | 0.286 | |||||||
| Yes | 15 | 44.0 | 12.2 | 0.0 | 50.0 | 15.0 | 0.0 | ||
| No | 29 | 77.8 | 48.5 | 39.7 | 48.4 | 27.2 | 27.2 | ||
| Child–Pugh score | 0.438 | 0.569 | |||||||
| A | 35 | 70.2 | 37.5 | 28.5 | 50.9 | 26.3 | 19.7 | ||
| B | 7 | 51.4 | 34.3 | 17.1 | 40.0 | 20.0 | 20.0 | ||
| Serum αFP-level | 0.711 | 0.486 | |||||||
| <400 ng/ml | 28 | 73.2 | 41.3 | 25.8 | 48.8 | 25.3 | 19.0 | ||
| ≥400 ng/ml | 12 | 58.3 | 24.3 | 24.3 | 43.6 | 14.5 | 14.5 | ||
| No. of lesions | 0.490 | 0.570 | |||||||
| 1 | 23 | 76.0 | 31.3 | 31.3 | 51.7 | 26.2 | 26.2 | ||
| >1 | 21 | 56.1 | 44.9 | 24.1 | 46.8 | 19.5 | 0.0 | ||
| Lobar involvement | 0.670 | 0.658 | |||||||
| Unilobar | 32 | 66.8 | 31.8 | 27.3 | 42.8 | 22.5 | 22.5 | ||
| Bilobar | 12 | 64.8 | 54.0 | 28.8 | 66.7 | 26.7 | 13.3 | ||
| Portal vein thrombus | 0.157 | 0.003 | |||||||
| Yes | 5 | 20.0 | 20.0 | 20.0 | 0.0 | 0.0 | 0.0 | ||
| No | 39 | 72.7 | 40.3 | 28.3 | 54.3 | 26.2 | 20.9 | ||
| Rupture | 0.123 | 0.945 | |||||||
| Yes | 6 | 66.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| No | 38 | 66.8 | 41.7 | 30.4 | 51.8 | 24.9 | 19.9 | ||
| Microvascular invasion | 0.032 | 0.038 | |||||||
| Yes | 27 | 57.5 | 22.5 | 22.5 | 33.3 | 13.9 | 13.9 | ||
| No | 17 | 80.7 | 64.5 | 38.7 | 71.8 | 36.5 | 24.3 | ||
| Margin involvement | 0.565 | 0.744 | |||||||
| Yes | 22 | 51.6 | 36.1 | 36.1 | 39.7 | 29.8 | 29.8 | ||
| No | 22 | 81.1 | 41.6 | 22.3 | 58.7 | 19.6 | 13.0 | ||
| Histology | <0.001 | 0.093 | |||||||
| Fibrolamellar | 8 | 87.5 | 58.3 | 58.3 | 50.0 | 33.3 | 33.3 | ||
| Well differentiated | 8 | 83.3 | 66.7 | 50.0 | 66.7 | 50.0 | 50.0 | ||
| Moderately differentiated | 21 | 65.8 | 32.5 | 16.2 | 55.4 | 10.1 | 0.0 | ||
| Poorly differentiated | 7 | 28.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Surgical modality | 0.161 | 0.775 | |||||||
| Resection only | 28 | 77.0 | 41.1 | 35.9 | 49.9 | 23.3 | 23.3 | ||
| Resection and ablation | 16 | 50.0 | 34.3 | 12.9 | 50.0 | 26.7 | 0.0 | ||
| Ethnicity | <0.001 | 0.003 | |||||||
| Asians | 13 | 38.5 | 7.7 | 0.0 | 11.4 | 0.0 | 0.0 | ||
| Non-Asians | 31 | 79.3 | 53.5 | 42.5 | 64.2 | 33.4 | 26.7 | ||
Figure 2.
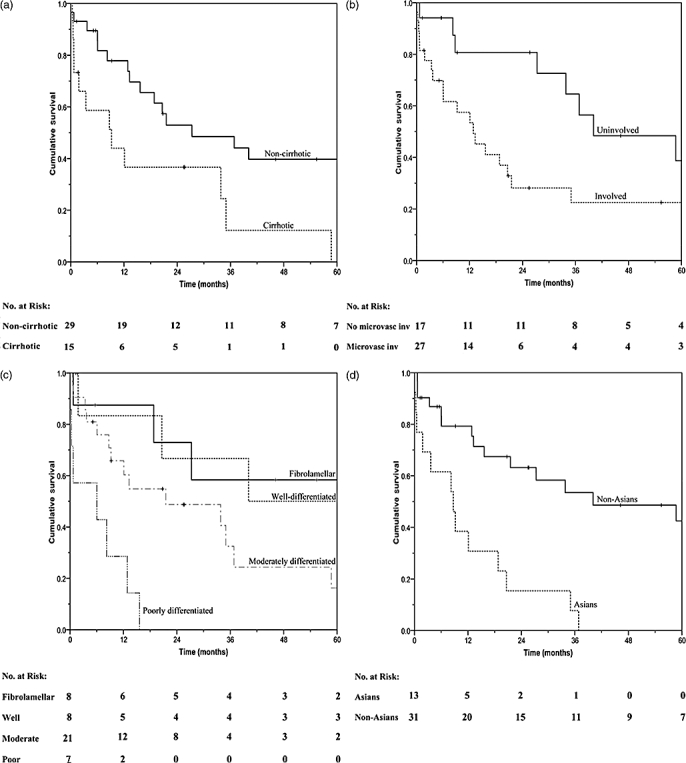
Univariate analysis. (a) Influence of cirrhosis on overall survival; (b) influence of microvascular invasion on overall survival; (c) influence of tumour histological on overall survival; (d) influence of ethnicity on overall survival.
Analysis of both cirrhotics and non-cirrhotics showed that the drastic difference in survival rates between groups occurred early, mainly within the first 3 months post-surgery (Fig. 2a). All five early post-operative deaths within the cirrhotics were because of surgical mortalities. One case had intercurrent pulmonary metastasis, developed 2 weeks post-operatively. This patient had no evidence of extrahepatic disease on complete pre-operative workup with CT chest/abdominal/pelvis, lipiodol CT and bone scans. Comparatively, only two non-cirrhotic patients died within this period – one from severe blood loss and another from refusal of gangrenous foot amputation. Further comparison between groups showed a higher prevalence of vascular invasion and multinodularity within cirrhotics (Table 1).
Twenty-seven HCCs were identified with microvascular involvement. A tumour recurred in 16 of them with 50% occurrence within the first post-operative year. Five cases also had apparent portal vein thrombus. Four of which also had a background of cirrhosis. None of them survived past 1 year. Causes of deaths were as a result of early disease recurrence in two patients, liver failure in one and disseminated intravascular coagulation in another patient. The other case of portal vein tumour invasion developed in a fibrolamellar HCC. Disease recurred within the liver and lymph nodes 6 months post-operatively. This patient received further surgical intervention and is alive until the date after 8 years from time of first surgery.
Further investigation on the correlation of αFP-level with tumour histology was performed. No relationship, however, was demonstrated (Fig. 3).
Figure 3.
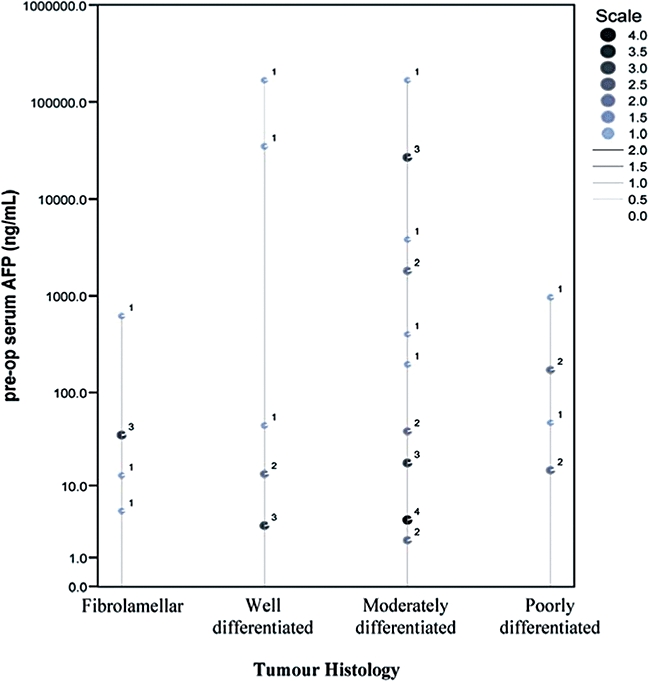
Correlation of serum αFP-level with histological subtypes
Discussion
Multiple studies on radical extirpation of large (>10 cm) tumours have had variable outcomes. The literature on large HCC includes 14 reports including 2749 patients, with 5-year survival rates ranging from 0% to 54.0%.1,11–23 However, three of these excluded hospital mortality. All, except one, of these studies looked at patients treated with surgical resection alone.1,11–22 Pandey et al. included in their study, patients treated with RFA within the same surgical procedure.23 Zhou et al. had previously shown survival of patients treated with resection and cryotherapy were similar to those treated with resection alone.24 Our series also included a mixed group of patients surgically treated with resection and/or ablation. A 5-year survival rate of 27.8%, similar to the other large HCC studies identified was achieved.
Cirrhosis was identified to independently affect survival of HCC patients. As most large tumours require major hepatectomy, the impossibility of liver resection in the presence of cirrhosis substantiates.25 In addition, the presence of cirrhosis in large HCCs denies patients of consideration for aggressive management as a result of an increased risk of post-operative hepatic insufficiency.14 Even when these lesion(s) are amenable to surgical resection, prognosis is generally poorer.26 In fact, our 5-year survival rates in cirrhotics and non-cirrhotics were 0% and 39.7%, respectively. The eminent difference between groups was observed in the first 3 months post-treatment, mainly because of post-operative complications. This concurs with the expected high post-operative mortality in cirrhotic liver resection in other studies.14,25 In the contrary, Torzilli et al. reported zero mortality in resection of cirrhotic livers.27
A large proportion of venous involvement is usually found in multinodular disease and large tumours.28 This feature was noted within the cirrhotic group.26,29 The involvement of microvascular invasion commonly present even at early stages of disease increases the risk of microtumour embolization.28 Recurrence risk also escalates with evidence of gross vascular involvement, which indicates advanced stages of disease according to the step-by-step dissemination theory.28 Five out of the 27 microvascular-involved tumours had concomitant portal vein thrombus. Both these factors – microvascular invasion and portal vein thrombus – were identified to adversely affect disease-free survival. Resection of tumours in the presence of portal vein tumour thrombus is also associated with poorer survival, which may explain the much poorer survival in our cirrhotic group.28–30 Except for the fibrolamellar variant, none of the patients with portal vein thrombus survived past 1 year. Despite these findings, the number comprising this subgroup may have been too small to have influenced significance on survival in this study.
Ethnicity was identified to independently affect survival. Regardless of the statistics, reasons for this association are rather ambiguous and may possibly be influenced by non-disease-related factors such as socioeconomic factors.31,32 Hence, further studies may, therefore, be required to establish this causal relationship.
Survival of patients stratified according to tumour histological type demonstrated correlation of poor tumour differentiation with poorer survival (Fig. 2c). Patients with fibrolamellar variant tend to do much better than the classic HCCs.33 Our result also demonstrated comparable 5-year survival rates with well-differentiated tumours (50.0%) and fibrolamellar variant (58.3%). Of the eight well-differentiated tumours, only two arose on a background of cirrhosis. Nagorney et al. showed that in the absence of cirrhosis, classic HCCs can result in similar outcome to a fibrolamellar variant.34 In addition, moderately-differentiated tumours had a 3-year survival of 32.5%. The survival of poorly differentiated tumour was much worse compared with all other histological types, and is found to be significant on multivariate analysis. All seven patients with poorly-differentiated tumours died within 16 months post-surgery. This may be as a result of the increased risk of microvascular invasion in higher-grade HCCs.35 Oishi et al. described the association of high-grade HCCs with larger proportions of high (>400 ng/ml) αFP-secretors, early extrahepatic recurrences and better liver function.36 These features, however, did not accurately describe our poorly-differentiated HCCs. Six out of the seven patients had Child's A and serum αFP-levels less than 400 ng/ml. Only one patient had an αFP-level of 963 ng/ml and the Child's score was unestablished. A total of four patients died of the disease, which recurred within the first post-operative year but only two had extrahepatic (lungs) involvements.
The significantly worse prognosis in poorly differentiated HCCs may imply sensibility in withholding operative treatment within this subgroup of patients. Hence, the ability to identify tumour differentiation via pre-operative assessments may be useful. Currently, imagings with computed tomography and ultrasonography are capable of recognizing well-differentiated HCCs but discerning moderate from poorly differentiated tumours remains a difficulty.37,38 Percutaneous tumour biopsy may be the only available technique providing definitive diagnosis of high-grade tumours but is associated with needle tract implantation.39 This may jeopardize the supposedly better outcome in other histological subtypes. In our study, correlation of serum αFP-levels with histological subtypes did not yield significant results (Fig. 3).
Other factors such as tumour rupture and positive resection margins on histological examination have also been described by others to adversely affect survival.30,40,41 The prognostic significance of ruptured tumours, however, may have been undervalued by its low incidence within this series. Although proven safer with staged liver resection, the outcome of treated ruptured HCCs is generally poor, particularly after intra-abdominal spread manifests.33,40 Four out of six ruptured tumours were dead at the time of analysis. Two patients died within the same admission from post-operative coagulapathy and refusal of ischaemic leg amputation, and two of disease recurrence. One recurrence involved intra-abdominal manifestation of disease while the other involved the lungs. Lai and colleagues showed that a resection margin of more than 0.5 cm significantly reduces histological margin infiltration, hence a reduction in both disease-free and overall survival.41 Within this study, all hepatectomies were performed using CUSA transaction, which allows a potential margin of at least 1.0 cm.42 In addition, suspicion of margin involvement immediately post-resection was further treated with edge ablation. This may in part explain our good 5-year survival rate (36.1%) which did not differ from the 3-years; within patients with microscopic margin involvement.
Despite surgical treatment, prognosis of HCCs is still poor as a result of high rates of recurrence. Various agents have thus been trialled along with surgical resection to improve disease-free survival and long-term outcome.43–46 A randomized, controlled trial with adjuvant lipiodol I131 after resection of smaller tumours was shown to achieve better prognosis in surgically treated HCCs, with 10-year overall and disease-free survival rates of 52.4% and 47.6%, respectively.47 A total of 16 patients in this study group were identified to have received adjuvant lipidol I131. The 5-year survival rates in the treated and non-treated groups were 35.5% and 14.7%, respectively (P = 0.009). Another agent (PI-88) currently under trial as an adjuvant treatment was shown to have similar potential benefits.48
Long-term survival can be achieved with surgical treatment of large HCCs (>10 cm). In addition, the combination of surgical resection and ablation can achieve similar outcome to treatment with resection alone, providing a chance for curative treatment in ‘inadequate’ margins and bilobar-involved disease. After surgical treatment, the administration of adjuvant lipiodol I131 is also effective, even in large HCCs. Both cirrhosis and poor tumour differentiation were independently associated with adverse survival. Further studies to identify poor histological subtype of HCCs in the pre-operative setting may be useful.
Conflicts of interest
None declared.
References
- 1.Poon RTP, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg. 2002;194:592–602. doi: 10.1016/s1072-7515(02)01163-8. [DOI] [PubMed] [Google Scholar]
- 2.Raoul JL, Guyader D, Bretagne JF, Heautot JF, Duvauferrier R, Bourguet P, et al. Prospective randomized trial of chemoembolization versus intra-arterial injection of I-labelled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997;26:1156–1161. doi: 10.1002/hep.510260511. [DOI] [PubMed] [Google Scholar]
- 3.Lau WY, Leung TWT, Yu SCH, Ho SKW. Percutaneous local ablative therapy for hepatocellular carcinoma. A review and look into the future. Ann Surg. 2003;237:171–179. doi: 10.1097/01.SLA.0000048443.71734.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai ECS, Ng IOL, You KT, Fan ST, Mok FPT, Tan ESY, et al. Hepatic resection for small hepatocellular carcinoma: the Queen Mary Hospital experience. World J Surg. 1991;15:654–659. doi: 10.1007/BF01789219. [DOI] [PubMed] [Google Scholar]
- 5.Chu F, Morris DL. Single centre experience of liver resection for hepatocellular carcinoma in patients outside transplant criteria. Eur J Surg Oncol. 2006;32:568–572. doi: 10.1016/j.ejso.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Ng KK, Vauthey JN, Pawlik TM, Lauwers GY, Regimbeau JM, Belghiti J, et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2004;12:1–10. doi: 10.1245/ASO.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 8.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–151. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery. 1991;100:726–735. [PubMed] [Google Scholar]
- 10.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Takayama T, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 11.Lee NH, Chau GY, Lui WY, King KL, Tsay SH, Wu CW. Surgical treatment and outcome in patients with a hepatocellular carcinoma greater than 10 cm in diameter. Br J Surg. 1998;85:1654–1657. doi: 10.1046/j.1365-2168.1998.00918.x. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Wahab M, Sultan A, El-Ghawalby A, Fathy O, El-Ebidy G, Abo-Zeid M, et al. Is resection for large hepatocellular carcinoma in cirrhotic patients beneficial? Study of 38 cases. Hepatogastroenterology. 2001;48:757–761. [PubMed] [Google Scholar]
- 13.Hanazaki K, Kajikawa S, Shimozawa N, Shimada K, Hiraguri M, Koide N, et al. Hepatic resection for hepatocellular carcinoma in diameter of = 10 cm. Hepatogastroenterology. 2002;49:518–523. [PubMed] [Google Scholar]
- 14.Yeh CN, Lee WC, Chen MF. Hepatic resection and prognosis for patients with hepatocellular carcinoma large than 10 cm: two decades of experience at Chang Gung Memorial Hospital. Ann Surg Oncol. 2002;10:1070–1076. doi: 10.1245/aso.2003.03.072. [DOI] [PubMed] [Google Scholar]
- 15.Zhou XD, Tang ZY, Ma ZC, Wu ZQ, Fan J, Qin LX, et al. Surgery for large primary liver cancer more than 10 cm in diameter. J Cancer Res Clin Oncol. 2003;129:543–548. doi: 10.1007/s00432-003-0446-6. [DOI] [PubMed] [Google Scholar]
- 16.Mok KT, Wang BW, Lo GH, Liang HL, Liu SI, Chou NH, et al. Multimodality management of hepatocellular carcinoma larger than 10 cm. Am Coll Surg. 2003;197:730–738. doi: 10.1016/j.jamcollsurg.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Chen XP, Qiu FZ, Wu ZD, Zhang BX. Chinese experience with hepatectomy for huge hepatocellular carcinoma. Br J Surg. 2004;91:322–326. doi: 10.1002/bjs.4413. [DOI] [PubMed] [Google Scholar]
- 18.Liau KH, Ruo L, Shia J, Padela A, Gonen M, Jarnagin WR, et al. Outcome of partial hepatectomy for large (>10 cm) hepatocellular carcinoma. Cancer. 2005;104:1948–1955. doi: 10.1002/cncr.21415. [DOI] [PubMed] [Google Scholar]
- 19.Pawlik TM, Poon RT, Abdalla EK, Zorzi D, Ikai I, Curley SA, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005;140:450–458. doi: 10.1001/archsurg.140.5.450. [DOI] [PubMed] [Google Scholar]
- 20.Nagano Y, Tanaka K, Togo S, Matsuo K, Kunisaki C, Sugita M, et al. Efficacy of hepatic resection for hepatocellular carcinoma larger than 10 cm. World J Surg. 2005;29:66–71. doi: 10.1007/s00268-004-7509-y. [DOI] [PubMed] [Google Scholar]
- 21.Chen XP, Qiu FZ, Qu ZD, Zhang BX. Hepatectomy for huge hepatocellular carcinoma in 634 cases. World J Gastroenterol. 2006;12:4652–4655. doi: 10.3748/wjg.v12.i29.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah SA, Wei AC, Cleary SP, Yang H, McGilvray ID, Gallinger S, et al. Prognosis and results after resection of very large (=10 cm) hepatocellular carcinoma. J Gastrointest Surg. 2007;11:589–595. doi: 10.1007/s11605-007-0154-7. [DOI] [PubMed] [Google Scholar]
- 23.Pandey D, Lee KH, Wai CT, Wagholikar G, Tan KC. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol. 2007;14:2817–2823. doi: 10.1245/s10434-007-9518-1. [DOI] [PubMed] [Google Scholar]
- 24.Zhou L, Rui JA, Wang SH, Chen SG, Qu Q, Chi TY, et al. Outcomes and prognostic factors for cirrhotic patients with hepatocellular carcinoma after radical major hepatectomy. World J Surg. 2007;31:1782–1787. doi: 10.1007/s00268-007-9029-z. [DOI] [PubMed] [Google Scholar]
- 25.Grazi GL, Ercolani G, Pierangeli F, Gaudio MD, Cescon M, Cavallari A, et al. Improved results of liver resection for hepatocellular carcinoma on cirrhocic give the procedure added value. Ann Surg. 2001;234:71–78. doi: 10.1097/00000658-200107000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh CN, Chen MF, Lee WC, Jeng LB. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: univariate and multivariate analysis. J Surg Oncol. 2002;81:195–202. doi: 10.1002/jso.10178. [DOI] [PubMed] [Google Scholar]
- 27.Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugwara Y, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients. Is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984–992. doi: 10.1001/archsurg.134.9.984. [DOI] [PubMed] [Google Scholar]
- 28.Tsai TJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, et al. Clinical significance of microscopic tumour venous invasion in patients with respectable hepatocellular carcinoma. Surgery. 2000;127:603–608. doi: 10.1067/msy.2000.105498. [DOI] [PubMed] [Google Scholar]
- 29.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IOL, Yamaoka Y, et al. Tumour size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma liver. Transplantation. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 30.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IOL, Yamaoka Y, et al. Tumour size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma liver. Transplantation. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 31.Chin PL, Chu DZJ, Clarke KG, Odom-Maryon T, Yen Y, Wagman LD. Ethnic differences in the behaviour of hepatocellular carcinoma. Cancer. 1999;85:1931–1936. [PubMed] [Google Scholar]
- 32.Liu JH, Chen PW, Asch SM, Busuttil RW, Ko CY. Surgery for hepatocellular carcinoma: does it improve survival? Ann Surg Oncol. 2004;11:298–303. doi: 10.1245/aso.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 33.Okuda K. Natural history of hepatocellular carcinoma including fibrolamellar and hepato-cholangiocarcinoma variants. J Gastroenterol Hepatol. 2002;17:401–405. doi: 10.1046/j.1440-1746.2002.02734.x. [DOI] [PubMed] [Google Scholar]
- 34.Nagorney DM, Adson MA, Weiland LH, Knight CD, Jr, Smalley SR, Zinsmeister AR. Fibrolamellar hepatoma. Am J Surg. 1985;149:113–119. doi: 10.1016/s0002-9610(85)80019-2. [DOI] [PubMed] [Google Scholar]
- 35.Esnaola NF, Lauwers GY, Mirza NQ, Nagorney DM, Doberty D, Ikai I, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6:224–232. doi: 10.1016/s1091-255x(01)00015-4. [DOI] [PubMed] [Google Scholar]
- 36.Oishi K, Itamoto T, Amano H, Fukuda S, Ohdan H, Tashiro H, et al. Clinicopathologic features of poorly differentiated hepatocellular carcinoma. J Surg Oncol. 2007;95:311–316. doi: 10.1002/jso.20661. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa S, Kumara T, Toyoda H, Ichikawa H, Kawachi T, Otobe K, et al. Evaluation of pathological features of hepatocellular carcinoma by contrast-enhanced ultrasonography: comparison with pathology on resected specimen. Eur J Radiol. 2006;59:74–81. doi: 10.1016/j.ejrad.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi M, Matsui O, Ueda K, Kawamori Y, Kadoya M, Yoshikawa J, et al. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. Am J Roentgenol. 1999;172:969–976. doi: 10.2214/ajr.172.4.10587130. [DOI] [PubMed] [Google Scholar]
- 39.Kim SH, Lim HK, Lee WJ, Cho JM, Jang HJ. Needle-tract implantation in hepatocellular carcinoma: frequency and CT findings after biopsy with a 19.5-gauge automated biopsy gun. Abdom Imaging. 2000;25:246–250. doi: 10.1007/s002610000025. [DOI] [PubMed] [Google Scholar]
- 40.Lai ECH, Lau WY. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006;141:191–198. doi: 10.1001/archsurg.141.2.191. [DOI] [PubMed] [Google Scholar]
- 41.Lai ECS, Ng IOL, You KT, Choi TK, Fan ST, Mok FPT, et al. Hepatectomy for large hepatocellular carcinoma: the optimal resection margin. World J Surg. 1991;15:141–145. doi: 10.1007/BF01658988. [DOI] [PubMed] [Google Scholar]
- 42.Hou RM, Chu F, Zhao J, Morris DL. The effects of surgical margin and edge cryotherapy after liver resection for colorectal cancer metastases. HPB. 2007;9:201–207. doi: 10.1080/13651820701275113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takenaka K, Yoshida K, Nishizaki T, Korenaga D, Hiroshige K, Ikeda T, et al. Postoperative prophylactic lipiodolization reduces the intrahepatic recurrence of hepatocellular carcinoma. Am J Surg. 1995;169:400–405. doi: 10.1016/s0002-9610(99)80184-6. [DOI] [PubMed] [Google Scholar]
- 44.Izumi R, Shimizu K, Iyobe T, Ii T, Yagi M, Matsui O, et al. Postoperative adjuvant hepatic arterial infusion of lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology. 1994;20:295–301. [PubMed] [Google Scholar]
- 45.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomized trial. Lancet. 2000;356:802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 46.Lo CM, Liu CL, Chan SC, Lam CM, Poon RT, Ng IO, et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245:837–842. doi: 10.1097/01.sla.0000245829.00977.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lau WY, Lai ECH, Leung TWT, Yu SCH. Adjuvant intra-arterial iodine-131-labeled lipiodol for respectable hepatocellular carcinoma: a prospective randomized trial – upate on 5-year and 10-year survival. Ann Surg. 2008;247:43–48. doi: 10.1097/SLA.0b013e3181571047. [DOI] [PubMed] [Google Scholar]
- 48.Gautam AM, Wilson EA, Chen PJ, Lee PH, Lin DY, Wu CC, et al. A novel heparanase inhibitor, as adjuvant therapy for hepatocellular carcinoma: a large randomized phase II clinical trial. Proc AACR Ann Meet. 2007;232:2650. [Google Scholar]


