Abstract
Objectives:
Pancreatic fistula (PF) predicts mortality and morbidity in patients undergoing pancreaticoduodenectomy (PD). This study aimed to assess whether isolated Roux loop pancreaticojejunostomy (IPJ) is superior to conventional pancreaticojejunostomy (CPJ).
Methods:
Between September 2003 and July 2007, we performed 108 PDs. All patients underwent classical Kausch–Whipple PD with pancreaticojejunostomy (PJ). Patients were divided into two groups based on the type of PJ. Patients in group 1 underwent IPJ and those in group 2 underwent CPJ. A retrospective analysis of prospectively maintained data was performed to compare outcomes in the two groups.
Results:
There were 53 patients in group 1 and 55 in group 2. The two groups were comparable in both pre- and intraoperative parameters. The overall incidence of PF was 10.1% (five cases in group 1 vs. six in group 2). The course of clinically significant PF was similar in both groups in terms of fistula behaviour, management and the duration of spontaneous closure. Two patients in each group died. Overall complications, mortality and length of hospital stay were also similar; however, duration of surgery was significantly higher in group 1 vs. group 2 (442 min and 370 min, respectively; P= 0.005).
Conclusions:
Isolated Roux loop pancreaticojejunostomy is not superior to conventional PJ; instead, it increases the duration of surgery.
Keywords: pancreaticoduodenectomy, pancreaticojejunostomy
Introduction
Pancreaticoduodenectomy (PD) has evolved over time to become a safe and acceptable therapeutic option for patients with pancreatic and periampullary tumours. Although the operative mortality of PD has reduced, morbidity remains high.1,2 Leakage from the pancreaticoenteric anastomosis (PEA) remains the principal factor for procedure-related morbidity. A variety of techniques have been described to reduce the chances of formation of a pancreatic fistula (PF).3 Despite the diverse range of methods described for PEA, the incidence of PF has not changed significantly over time (9.9%–28.5%).4,5 One of the proposed methods of reconstruction after PD is to perform an isolated Roux loop pancreaticojejunostomy (IPJ). Proponents of this technique believe that the diversion of bile away from the pancreaticojejunostomy (PJ) site minimizes the pancreatic enzyme activation and this reduces the risk of PF formation.6 Another argument cited in favour of using a Roux loop in PJ relies on the belief that, if a PF fistula forms, it will be a ‘pure’ pancreatic fistula and these fistulae cause lesser complications compared with complex PF, in which the bile activates the pancreatic juice, with further repercussions.
Although some series have reported good results with IPJ, there has been no comparative study to assess the superiority of this method of reconstruction over conventional pancreaticojejunostomy (CPJ) in which the same jejunal loop is used for the pancreatic, biliary and gastric anastomosis. We have used bothmethods of reconstruction and this paper reports our experience with them.
Materials and methods
This report is a retrospective review of 108 consecutive PD procedures performed by a single surgical team in a tertiary care centre between September 2003 and July 2007. Detailed information on these patients was maintained on a prospective database. Patient demographics and characteristics were carefully recorded. Preoperative imaging included a combination of abdominal ultrasound, contrast-enhanced computed tomography (CT) or magnetic resonance imaging of the abdomen and endoscopic retrograde cholangiopancreatography. Intraoperative features, such as tumour size, pancreatic duct diameter, consistency of pancreas, type of PJ anastomosis, use of pancreatic stent, duration of surgery and blood loss, were carefully documented. We divided the patients into two groups based on the type of PJ. In the initial 53 patients, operated between September 2003 and July 2005 (group 1), PJ was constructed with an isolated loop of jejunum (IPJ). In the 55 patients operated between August 2005 and July 2007 (group 2), conventional PJ (CPJ) with a single loop represented the method of reconstruction. Apart from the type of reconstruction, the operative procedure was similar in both groups.
Operative details
All patients underwent classical Kausch–Whipple PD. Duct-to-mucosa pancreaticojejunostomy was performed using 5/0 poplydioxanone monofilament interrupted sutures for the pancreatic duct and jejunal mucosa and 3/0 interrupted silk sutures between the capsule of pancreas and the seromuscular layer of jejunum. An internal pancreatic stent was placed when the pancreatic duct diameter was <3 mm. In group 1 (IPJ), a 40-cm isolated loop of jejunum was passed through the mesocolon for PJ. The remaining reconstruction was completed by end-to-side bilioenteric anastomosis followed by gastrojejunostomy from the distal loop of jejunum and the jejunojejunostomy was constructed after gastrojejunostomy (Fig. 1). In group 2 (CPJ), the reconstruction was performed using a single, retrocolic jejunal loop. Pancreaticojejunostomy was performed first, followed by hepaticojejunostomy and posterior gastrojejunostomy at a distance of 15–20 cm each (Fig. 2). All patients in both groups received octreotide 150 µg i.v. bolus at the time of constructing the PJ, followed by 150 µg administered subcutaneously at 8-hourly intervals and continued for the following 3 days until enteral feeding began. In patients with clinical evidence of PF, octreotide was continued further based on the clinical response. Octreotide was discontinued if the fistula output did not reduce or any definitive intervention was performed.
Figure 1.
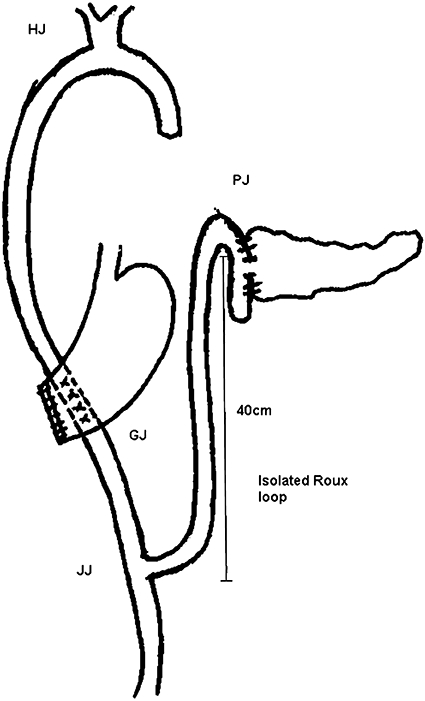
Pancreaticoenteric reconstruction in group 1. PJ, pancreaticojejunostomy; HJ, hepaticojejunostomy; GJ, gastrojejunostomy; JJ, jejunojejunostomy
Figure 2.
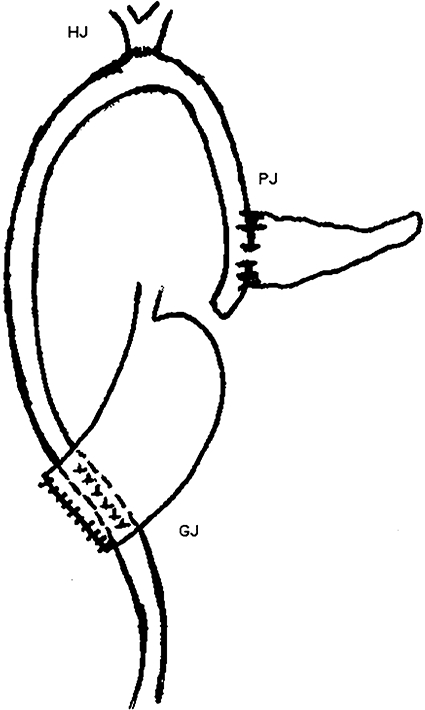
Pancreaticoenteric reconstruction in group 2. PJ, pancreaticojejunostomy; HJ, hepaticojejunostomy; GJ, gastrojejunostomy
All postoperative complications were recorded. Pancreatic fistula was defined as drainage of any measurable volume of fluid with fluid amylase more than or equal to three times the upper limit of normal serum value on postoperative day 3 or later.5 We routinely measure drain fluid amylase on postoperative days 1, 3 and 7. The clinical behaviour of patients with PF was entered prospectively into the database, with specific focus on the nature of PF, and its presentation, management and outcome.
The International Study Group on Pancreatic Fistula (ISGPF) definition for PF5 was used retrospectively in patients operated before July 2005 on the basis of the essential information held in our prospective database. All cases of postoperative PF were identified as representative of either pure or complex fistulae depending on the nature of the draining fluid. The presence of bile or enteric contents suggested a complex fistula, whereas the presence of clear, water-like fluid suggested a pure PF. In any suspicion of intra-abdominal sepsis, CT scans were liberally performed. Delayed gastric emptying (DGE) was defined as the need for nasogastric intubation for >10 postoperative days, inability to resume enteral feed at 14 days after surgery, need of prokinetics for >10 days or re-insertion of the nasogastric tube.7 Length of hospital stay was defined as the period from the first postoperative day until discharge from hospital. Death within the same hospital admission or within 30 days of surgery was considered as operative mortality.
The primary endpoint of assessment concerned the presence or absence of pancreas-specific complications, which included death or leakage of pancreatoenteric or bilioenteric anastomosis. The main focus was on the incidence, type, behaviour and management of the PF in the two groups. Secondary endpoints included duration of surgery and length of postoperative hospital stay.
Statistical methods
spss (Version 10.0; SPSS, Inc., Chicago, IL, USA) was used for data analysis. To test significance, Student's t-test was used for continuous variables and chi-squared or Fischer's exact test was used for categorical variables. A P-value of <0.05 was considered significant.
Results
The two groups were comparable in terms of baseline clinicopathological parameters (Table 1). Subjects included 81 men and 27 women, with a mean age of 54.3 years (standard deviation [SD]± 11.2 years, range 18–80 years). The indications for PD in order of frequency were ampullary adenocarcinoma, carcinoma in the head of pancreas, duodenal adenocarcinoma, lower-end common bile duct (CBD) cholangiocarcinoma, carcinoid tumour, and others, which included three patients in group 1 (chronic pancreatitis, n= 2; cystic tumour of the pancreas, n= 1) and five patients in group 2 (chronic pancreatitis, n= 3; cystic tumour, n= 1; chronic inflammation with lymphocytic infiltration, n= 1). Intraoperative findings were comparable in the two groups for tumour size, consistency of pancreas and pancreatic duct diameter (Table 1).
Table 1.
Clinicopathological parameters of patients (n= 108) undergoing pancreaticoduodenectomy
| Preoperative parameters | Group 1 (IPJ) (n= 53) (range) ± SD | Group 2 (CPJ) (n= 55) (range) ± SD | P-value |
|---|---|---|---|
| Age, years | 53.3 (32–80) ± 12.1 | 53.5 (18–74) ± 10.1 | 0.931 |
| Sex, male/female | 40 (75.4%)/13 (24.6%) | 41 (74.5%)/14 (25.6%) | 0.955/0.980 |
| Diagnosis | |||
| Ampullary carcinoma | 24 (45.3%) | 25 (45.5%) | 0.978 |
| HOP carcinoma | 12 (22.6%) | 13 (23.6%) | 0.918 |
| Duodenal carcinoma | 3 (5.7%) | 6 (10.9%) | 0.345 |
| Lower-end CBD carcinoma | 10 (18.9%) | 4 (7.3%) | 0.082 |
| Carcinoid tumour | 1 (1.9%) | 2 (3.6%) | 0.583 |
| Others | 3 (5.7%) | 5 (9.1%) | 0.513 |
| Endobiliary stent | |||
| Yes | 14 (26.4%) | 17 (30.9%) | 0.662 |
| No | 39 (73.6%) | 38 (69.1%) | 0.782 |
| Haemoglobin (g/dl) | 11.0 (6.0–15.6) ± 2.0 | 11.7 (7.0–14.2) ± 1.7 | 0.602 |
| WBC count, 103/mm3 | 11.1 (9.6–14.1) ± 3.6 | 10.9 (6.36–13.1) ± 3.1 | 0.782 |
| Bilirubin, mg/dl | 7.4 (0.3–24.1) ± 5.2 | 5.6 (0.4–29) ± 4.9 | 0.835 |
| Intraoperative parameters | |||
| Tumour size, cm | 2.96 (0.5–10) ± 1.8 | 2.66 (1–8) ± 1.5 | 0.371 |
| MPD diameter, mm | 5.41 (2–9) ± 2.5 | 5.11 (2–8) ± 2.3 | 0.546 |
| Pancreatic consistency | |||
| Soft | 35 (66.0%) | 41 (74.6%) | 0.598 |
| Hard | 18 (34.0%) | 14 (25.4%) | 0.419 |
| PJ stent | |||
| Yes | 14 (26.4%) | 17 (30.9%) | 0.515 |
| No | 39 (73.6) | 38 (69.1%) | 0.919 |
| PJ anastomosis | |||
| Duct to mucosa | 50 (94.3%) | 49 (89.0%) | 0.812 |
| Dunking | 03 (05.7%) | 06 (11.0%) | 0.098 |
IPJ, isolated Roux loop pancreaticojejunostomy; CPJ, conventional pancreaticojejunostomy; SD, mean standard deviation; HOP, head of pancreas; CBD, common bile duct; WBC, white blood cell; MPD, main pancreatic duct; PJ, pancreaticojejunostomy
The mean duration of surgery was 442 min (SD ± 32.0 min, range 300–510 min) in group 1 vs. 370 min (SD ± 38.5 min, range 240–500 min) in group 2 (P= 0.005, with 95 % confidence interval [CI] 57.0–84.1) (Table 2). Intraoperative blood loss was similar in the two groups. Mean recorded blood loss was 350 ml (SD ± 78 ml, range 150–650 ml) and 330 ml (SD ± 89 ml, range 125–730 ml) in groups 1 and 2, respectively (P= 0.780). Two patients in each group required a transfusion of 1 unit of packed red cells and only one patient in each group needed ≥2 units. Octreotide was discontinued once enteral feed was started, except in patients with PF (five in group 1, six in group 2), who received octreotide for longer. The mean duration of octreotide use in patients with PF was 11 ± 4.6 days in group 1 vs. 12.8 ± 3.1 days in group 2; this difference did not attain statistical significance (P= 0.523).
Table 2.
Comparison of clinical outcomes between the two groups (n= 108)
| Outcome | Total (n= 108) | Group 1 (IPJ) n= 53 | Group 2 (CPJ) n= 55 | P-value (group 1 vs. group 2) |
|---|---|---|---|---|
| Overall complications | 32 (29.6%) | 17 (32%) | 15 (27.3%) | 0.674 |
| Nature of complications | ||||
| Pancreatic fistula | 11 (10.1%) | 5 (9.4%) | 6 (10.9%) | 0.800 |
| DGE | 9 (8.3%) | 5 (9.4%) | 4 (7.2%) | 0.739 |
| Wound infection | 8 (7.4%) | 5 (9.4%) | 3 (5.4%) | 0.484 |
| Bleeding | 4 (3.7%) | 2 (3.7%) | 2 (3.6%) | 1.000 |
| Operative mortality | 4 (3.7%) | 2 (3.7%) | 2 (3.6%) | 1.000 |
| Mean duration of surgery, min (95% CI, OR) | 442 (300–510) ± 32.0 | 370 (240–500) ± 38.5 | 0.005 | |
| Mean hospital stay, days (95% CI, OR) | 10.1 (5–27) ± 3.7 | 9.5 (4–26) ± 5.0 | 0.483 | |
IPJ, isolated Roux loop pancreaticojejunostomy; CPJ conventional pancreaticojejunostomy; DGE, delayed gastric emptying; CI, confidence interval; OR, odds ratio
A total of 32 of 108 (29.6%) patients developed some form of postoperative complication. The complications ranged from PF to DGE, intra-abdominal bleeding and wound infection. The incidence and nature of postoperative complications were similar in the two groups (Table 2).
We followed the clinical course of PF in both groups of patients. Rates of incidence of PF were similar in both groups (five patients in group 1 and six in group 2; P= 0.800, odds ratio [OR] 0.85, 95% CI 0.24–2.97) and followed a similar clinical course (Fig. 3). There were no differences in the incidence of pure or complex fistulae or in the duration of their spontaneous closure (Fig. 3). Four patients developed intra-abdominal bleeding postoperatively (two in each group; P= 1.000). One patient in each group presented with blood in the abdominal drain. Angiography was suggestive of bleed from the PJ site in both cases. These patients underwent laparotomy (Fig. 3). Of the remaining two patients with intra-abdominal bleed, one patient in group 1 bled from hepaticojejunostomy and one patient in group 2 bled from gastrojejunostomy. Both these patients were managed conservatively.
Figure 3.
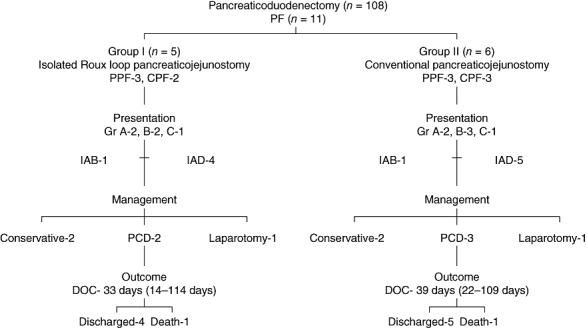
Clinical course of pancreatic fistulae in groups 1 (isolated Roux loop pancreaticojejunostomy) and 2 (conventional pancreaticojejunostomy). PF, pancreatic fistula; PPF, pure pancreatic fistula; CPF, complex pancreatic fistula; Gr, grade; IAB, intra-abdominal bleed; IAD, increased abdominal drainage; PCD, percutaneous drainage; DOC, duration of spontaneous closure
Four (3.2%) patients died, two in each group (P= 1.000). In group 1, death resulted from intra-abdominal bleeding from the PJ site secondary to PF in one case and cardiac arrhythmia in the other. In group 2, death was caused by intra-abdominal bleed from the PJ site secondary to PF in one case and chest infection in the other. The mean length of hospital stay was similar in the two groups, at 10.1 days (SD ± 3.7 days, range 5–27 days) in group 1and 9.5 days (SD ± 5.0 days, range 4–26 days) in group 2 (P= 0.483) (Table 2).
Discussion
Until recently, the reported incidence of PF following PD varied greatly because of the lack of an accepted definition of PF. Since the publication of a definition by the ISGPF,5 there has been some uniformity in this regard. Using the current definition of PF, the incidence of PF has been found to range between 9.9% and 28.5%.4 Certainly, attempts to reduce the incidence and complications of PF after PD remain challenging.
Consistency of the pancreas, size of the pancreatic duct8 and the experience of the operating team9 determine the formation of PF after PD. Many high-volume centres have reported low incidences of PF after PD, but this is more a reflection of the experience of the surgical team than it is a testimony of a particular method's superiority over others. There have been few randomized trials10–12 comparing different methods of performing pancreaticoenteric anastomosis and these have produced variable results. Thus, it appears to be familiarity with a particular technique that gives good results rather than the inherent superiority of a particular method.
Ideally, a reconstructive technique should not only minimize the risk of PF formation, but should also ensure that, should a PF form, its complications are averted or minimized. An isolated jejunal loop for PEA is theoretically expected to achieve these desired endpoints. As shown in previous studies, using an isolated jejunal loop for PEA can minimize the risk of PF, although its effect in terms of reducing PF-related morbidity is not clear.13–19 In view of these expected advantages, we started using an isolated Roux loop of jejunum for performing pancreaticojejunostomy, but over time we noticed that this did not reduce the incidence of PF and these fistulae were not totally benign. Analysis of our data showed that PF formed with equal frequency in both groups and PF after IPJ were not always innocent. Complication rates, chances of PF and duration of spontaneous closure of fistulae did not differ between the treatment groups. The PF were of same grade in the two groups and one patient in each group died (Fig. 3). As the isolated Roux loop PJ offered no advantage and the process of fashioning an isolated loop of jejunum made the entire procedure longer and more complex, we reverted to the classical method of reconstruction using a single loop.
Why does IPJ fail to live up to expectations? Healing of the PEA is dependent on various factors and the diversion of bile may not be one of them. Even the activation of leaking pancreatic juice is not solely dependent on the presence of bile: intestinal juices themselves can activate pancreatic secretions. In two of our patients with IPJ, the PF was complex in nature; a possible explanation for this may involve the disruption of biliary or gastric anastomosis by pancreatic enzymes from the leaking PJ.
The only difference between the two groups concerned duration of surgery. Duration of surgery in group 1 was significantly greater than in group 2. This is understandable because group 1 surgery required a 40-cm jejunal loop and an additional anastomosis (i.e. jejunojejunostomy) to be constructed.
Although this is not a randomized study, our observations suggest that isolated Roux loop pancreaticojejunostomy does not offer any advantage over conventional reconstruction in PD and the difference in operative time is related to the construction of isolated loop itself.
Conflicts of interest
None declared.
References
- 1.Miedema BW, Sarr MG, van Heerdeen JA, Nagorney DM, McIlrath DC, Ilstrup D. Complications following pancreaticoduodenectomy. Current management. Arch Surg. 1992;127:945–950. doi: 10.1001/archsurg.1992.01420080079012. [DOI] [PubMed] [Google Scholar]
- 2.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Tralamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications and outcomes. Ann Surg. 1997;226:248–260. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrikhande SV, Qureshi SS, Rajneesh N, Shukla PJ. Pancreatic anastomoses after pancreaticoduodenectomy: do we need further studies? World J Surg. 2005;29:1642–1649. doi: 10.1007/s00268-005-0137-3. [DOI] [PubMed] [Google Scholar]
- 4.Bassi C, Butturini G, Molinari E, Mascetta G, Salvia R, Falconi M, et al. Pancreatic fistula rate after pancreatic resection. The importance of definitions. Dig Surg. 2004;21:54–59. doi: 10.1159/000075943. [DOI] [PubMed] [Google Scholar]
- 5.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Machado MC, Monterio da Cunha JE, Bachella T, Bove P. A modified technique for the reconstruction of the alimentary tract after pancreatoduodenectomy. Surg Gynecol Obstet. 1976;143:271–273. [PubMed] [Google Scholar]
- 7.van Berge Henegouwen MI, Van Gulik TM, De Wit LT, Allema JH, Rauws EA, Obertop H, et al. Delayed gastric emptying after standard pancreaticoduodenectomy vs. pylorus preserving pancreaticoduodenectomy: an analysis of 200 consecutive patients. J Am Coll Surg. 1997;185:373–379. doi: 10.1016/s1072-7515(97)00078-1. [DOI] [PubMed] [Google Scholar]
- 8.Yang YM, Tian XD, Zhuang Y, Wang WM, Wan YL, Huang YT. Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 2005;11:2456–2461. doi: 10.3748/wjg.v11.i16.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pen SY, Wang JW, Lau WY, Cai XJ, Mou YP, Liu YB, et al. Conventional vs. binding pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg. 2007;245:692–698. doi: 10.1097/01.sla.0000255588.50964.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winter JM, Cameron JL, Campbell KA, Chang DC, Riall TS, Schulick RD, et al. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2006;10:1280–1290. doi: 10.1016/j.gassur.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Yeo CJ, Cameron JL, Maher MH, Maher MM, Sauter PK, Zahurak ML, Talamini MA, et al. A prospective randomized trial of pancreaticogastrostomy vs. pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–588. doi: 10.1097/00000658-199510000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan AW, Aggarwal AK, Davidson BR. Isolated Roux loop duct to mucosa pancreaticojejunostomy avoids pancreatic leaks in pancreaticoduodenectomy. Dig Surg. 2002;19:199–204. doi: 10.1159/000064213. [DOI] [PubMed] [Google Scholar]
- 14.Sutton CD, Garcea G, White SA, O'Leary E, Marshall LJ, Berry DP, et al. Isolated Roux loop pancreaticojejunostomy: a series of 61 patients with zero postoperative pancreaticoenteric leaks. J Gastrointest Surg. 2004;8:701–705. doi: 10.1016/j.gassur.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Funovics JM, Zöch G, Wenzl E, Schulz F. Progress in reconstruction after resection of the head of the pancreas. Surg Gynecol Obstet. 1987;164:545–548. [PubMed] [Google Scholar]
- 16.Kingsnorth AN. Safety and function of isolated Roux loop pancreaticojejunostomy after Whipple's pancreaticoduodenectomy. Ann R Coll Surg Engl. 1994;76:175–179. [PMC free article] [PubMed] [Google Scholar]
- 17.Albertson DA. Pancreaticoduodenectomy with reconstruction by Roux en Y pancreaticojejunostomy: no operative mortality in a series of 25 cases. South Med J. 1994;87:197–201. doi: 10.1097/00007611-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Papadimitriou JD, Fotopoulos CA, Smyrniotis B, Prahalias AA, Kostopanagiotou G, Papadimitriou LJ. Subtotal pancreatoduodenectomy. Use of a defunctionalized loop for pancreatic stump drainage. Arch Surg. 1999;134:135–139. doi: 10.1001/archsurg.134.2.135. [DOI] [PubMed] [Google Scholar]
- 19.Jover JM, Carabias A, Feurte S, Ríos R, Ortega I, Limones M. Results of defuntionalized jejunal loop after pancreaticoduodenectomy. Cir Esp. 2006;80:373–377. doi: 10.1016/s0009-739x(06)70990-3. [DOI] [PubMed] [Google Scholar]


