Abstract
Background:
New instruments and techniques for hepatectomy have been shown to reduce blood loss during liver resection. The present study aims to evaluate the feasibility and result of our techniques of liver resection without routine inflow occlusion (the Pringle manoeuver).
Methods:
The cavitron ultrasonic surgical aspirator (CUSA) and saline-linked radio-frequency dissecting sealer (TissueLink) were used together for open hepatectomy, whereas a bipolar vessel sealing device (Ligasure) and TissueLink were used for laparoscopic hepatectomy. Between June 2003 and May 2007, 248 consecutive cases of liver resection were carried out using the above techniques without the routine Pringle manoeuver. The operative and clinical outcome data were prospectively collected and analysed.
Results:
During the study period, a total of 220 cases of open hepatectomy and 28 cases of laparoscopic hepatectomy were performed. The Pringle manoeuver was eventually applied in six patients (2.4%): two for portal vein tumour thrombus extraction and four as a result of heavy bleeding. Median blood loss was 300 ml (20–2700 ml) and the blood transfusion rate was 7.7%. In most of the cases, the liver function tests showed improvement on post-operative day 1 or 2, and the median post-operative hospital stay was 7 days. There were two post-operative deaths (0.8%). Complications occurred in 63 patients (25.4%) and most complications were minor.
Conclusions:
Refined techniques and instruments for liver resection allow hepatectomy to be done safely without using the routine Pringle manoeuver. Most patients had a quick recovery of liver function and were discharged early.
Keywords: liver resection, hepatectomy, Pringle manoeuver, inflow occlusion, hepatic pedicle clamping
Introduction
During liver resection, bleeding remains the single most important challenge. Studies have shown that bleeding and subsequent blood transfusion increased operative morbidity and mortality.1–3 Furthermore, bleeding may increase the recurrence of and reduce the survival rate for malignant diseases.3–6 Thus, a reduction in blood loss and the avoidance of a blood transfusion are important objectives of liver surgeons today. Different methods of vascular control have been developed in the past to cut down blood loss with variable success rates.7–9 Amongst these, the Pringle manoeuver still stands out as the most simple and effective method.10 This manoeuver involves the control of the hepatic vascular inflow by clamping the hepatoduodenal ligament. During hepatectomy, the Pringle manoeuver is still commonly employed today as a routine procedure worldwide.11–14 However, ischaemic insult to the remnant liver is always a concern, particularly in cirrhotic livers whenever this manoeuver is applied.15,16
In recent years, with the refinement of surgical equipments and techniques in liver resection, blood loss during such procedures has been reduced even without the use of the Pringle manoeuver.17,18 Since early 2003, we have adopted the combined use of a cavitron ultrasonic surgical aspirator (CUSA; ValleyLab, Boulder, Co, USA) and a saline-linked radio-frequency dissecting sealer (TissueLink; TissueLink Medical Inc., Dover, DE, USA) to perform liver resection.19 We found that blood loss during liverresection was low even when the Pringle manoeuver was not applied. This made us believe that the Pringle manoeuver might not be necessary as a routine adjuvant in liver resection and since June 2003 we have given up routine use of the manoeuver. Here we report the results of our liver resections and explore the feasibility and possible benefits of this technique.
Patients and methods
In a 4-year period from June 2003 to May 2007, 248 consecutive cases of liver resection were carried out in our unit without the use of the routine Pringle manoeuver. Demographic data, operative data, clinical outcomes, pathological findings and follow-up information were prospectively collected for all patients. Blood loss was calculated from the volume of the suction bottle, the suction bottle of CUSA and by weighing the soaked gauze. A transfusion was given if the patient developed haemodynamically instability as a result of blood loss or whenever the haemoglobin fell below 8 g/dl. Blood given during the operation and at any time in the post-operative period during the same admission for the operation was counted as the transfusion requirement for the operation. The recovery of liver function after hepatectomy was assessed by the occurrence of peak levels of serum bilirubin, the international normalized ratio for coagulation (INR) and alanine aminotransferase (ALT) after surgery. Liver failure was defined according to the ‘50-50 Criteria’ on post-operative day 5 (serum bilirubin > 50 µmol/l and INR > 1.7).20 Survival was measured using the Kaplan–Meier survival curve. The Mann–Whitney U-test was used for statistical analysis of blood loss, operative time and post-operative hospital stay between the different groups of patients. Fisher's exact test or χ2 test were used for statistical analysis of categorical variables of the major and minor hepatectomy group of patients. P < 0.05 was taken as the level of statistical significance.
Operative techniques
We performed open hepatectomies via right subcostal incisions with upward midline extensions, and in some cases with left subcostal extensions. In all cases, operative ultrasound (Aloka, Tokyo, Japan) was used to define the tumour, to exclude pre-operatively undetected lesions and to mark the plane for liver parenchymal transection. To reduce venous bleeding during liver transection, the central venous pressure was kept below 5 cm H2O using fluid restriction. In most cases the liver was mobilized in the standard fashion before transection, whereas in the rest, we adopted the anterior approach or the hanging technique for hemi-hepatectomy. The Pringle manoeuver was not applied unless heavy or uncontrolled bleeding was encountered during liver resection or the extraction of the portal vein tumour thrombus was required. The decision to apply the Pringle manoeuver relied on the discretion of the operating surgeons. The heaviness of the bleeding would be dependent on both the rate as well as the volume of blood loss. For major hepatectomy, if possible, hilar dissection was performed to ligate the hepatic artery and the portal vein branch to the lobe of liver that was removed. Otherwise, the hepatic artery and portal vein branches were ligated intra-hepatically during parenchymal transection. Hilar dissection was not performed for non-anatomical hepatectomy. The liver parenchyma was divided by CUSA which was operated by one surgeon while the other surgeon held the saline-linked radio-frequency dissecting sealer for coagulation and haemostasis (Fig. 1). The dissecting sealer delivered radiofrequency energy via a continuous stream of saline dripped from the device tip, so as to prevent char formation. The device was efficient in controlling the small bleeding vessels within the liver parenchyma.19 Larger vessels were either ligated or clipped. The major hepatic veins were usually divided using endovascular staplers (Tyco Healthcare, Norwalk, CT, USA). In most cases, tissue glue (Tisseel; Baxter, Vienna, Austria) was sprayed onto the liver resection surface to augment haemostasis and prevent bile leak. The placement of drains was left to the discretion of the operating surgeons.
Figure 1.
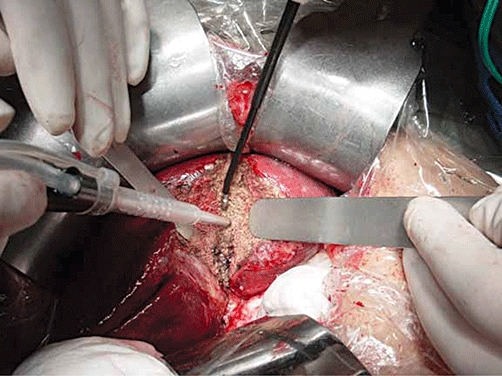
Combined use of the cavitron ultrasonic surgical aspirator (CUSA) and saline-linked radio-frequency dissecting sealer for liver parenchymal transaction
Laparoscopic liver resection was performed without the application of a hand port.21 In most cases, three to five laparoscopic ports (5 to 12 mm in diameter) were placed according to the location of the tumour. Laparoscopic ultrasound (Aloka) was routinely performed. The Pringle manoeuver was not used in any of the laparoscopic resections. The liver was transected with a bipolar vessel sealing device (Ligasure; Valleylab) and haemostasis was achieved by using the laparoscopic TissueLink device. Radiofrequency ablation-assisted (Cool-Tip; Tyco Healthcare) laparoscopic liver resection was used in one case. Endovascular staplers were used to divide the large vascular pedicles whereas the smaller vessels were controlled by metal clips. Tissue glue was routinely applied, and all specimens were retrieved in sterile plastic bags through an extended port site, usually at the umbilicus.
Results
There were 152 men and 96 women in this series. The median age was 54 years (range 22 to 80 years). Seventy-seven patients (31%) had liver cirrhosis on histology. Most of the patients (96%) suffered from Child's A cirrhosis, whereas nine patients suffered from Child's B and one patient from Child's C cirrhosis. One hundred and four patients (42%) had one or more co-morbidities including hypertension, diabetes mellitus, renal impairment or ischaemic heart disease. Regarding the American Society of Anesthesiologists score (ASA), most of the patients (146) were ASA II, and the rest were either ASA I or III. No patients belonged to ASA IV or V.
The types of hepatectomy performed are shown in Table 1. Seventy-five (30%) of the liver resections were major hepatectomy (a resection of three segments or more). Within this group, hilar dissection was performed in 68 patients (90.7%). Twenty-eight patients (11.3%) underwent laparoscopic liver resection, two of which were converted to open hepatectomy as a result of bleeding in one case and a lack of progress in the other. Excluding cholecystectomy, concomitant procedures were carried out in 41 patients (16.5%), including radiofrequency ablation of other liver lesions, bile duct exploration, bile duct resection, porta hepatic lymphatic dissection and bowel resection, etc.
Table 1.
Types of hepatectomy performed in the 248 patients
| Type of hepatectomy | Number of patients | Percentage |
|---|---|---|
| Open hepatectomy | ||
| Right hepatectomy | 47 | 19.0% |
| Extended right hepatectomy | 5 | 2.0% |
| Left hepatectomy | 21 | 8.5% |
| Extended left hepatectomy | 2 | 0.8% |
| Left lateral sectionectomy | 58 | 23.4% |
| Bisegmentectomy | 32 | 12.9% |
| Segmentectomy | 39 | 15.7% |
| Wedge resection | 16 | 6.5% |
| Laparoscopic hepatectomy | ||
| Left lateral sectionectomy | 13 | 5.2% |
| Wedge resection | 15 | 6.0% |
| Total | 248 | 100.0% |
The final diagnoses in these 248 cases are shown in Table 2. Hepatocellular carcinoma (HCC) was the commonest indication for hepatectomy (52.4%). Eleven of the HCC patients presented with acute rupture or a history of rupture. The size of the liver lesions, if applicable, ranged from 8 mm to 20 cm (median: 4.5 cm). Multiple lesions (more than one) were found in 50 patients. The median resection margin was 1.2 cm (range: 0–6.5 cm). Eight patients had histological involvement of the resection margin.
Table 2.
Histological diagnosis in the 248 patients
| Histological diagnosis | Number of patients | Percentage |
|---|---|---|
| Hepatocellular carcinoma | 130 | 52.4% |
| Colorectal liver metastasis | 47 | 19.0% |
| Metastasis from other organs | 8 | 3.2% |
| Cholangiocarcinoma | 11 | 4.4% |
| Carcinoma of gallbladder | 7 | 2.8% |
| Recurrent pyogenic cholangitis | 25 | 10.1% |
| Liver haemangioma | 5 | 2.0% |
| Focal nodular hyperplasia | 5 | 2.0% |
| Hepatic adenoma/cystadenoma | 4 | 1.6% |
| Liver abscess | 2 | 0.8% |
| Others | 4 | 1.6% |
| Total | 248 | 100.0% |
The Pringle manoeuver was applied during the operation on six patients (2.4%), with a clamping duration from 5 to 20 min (median: 7.5 min). In two cases, a hilar clamp was used for the extraction of the portal vein tumour thrombus, and in the remaining four, it was used to control the bleeding. Blood loss in these six patients ranged from 200 to 1800 ml (median: 710 ml). All six patients recovered without complications.
The main operative outcomes are summarized in Table 3. There were two operative mortalities (0.8%). One patient died of myocardial infarction on post-operative day 5 and the other died of liver failure on post-operative day 23. A total of 82 complications occurred in 63 patients (25.4%). In order of decreasing frequency, these complications were wound infections (19), pleural effusion (18), ascites (14), intra-abdominal collection (13), atrial fibrillation (4), bile leak (2), liver failure (3) and others (9). Of the three patients who developed liver failure, one subsequently died, whereas the other two who received a right and an extended right hepatectomy, respectively, and stayed in hospital for 25 and 28 days post-operatively. One patient needed re-operation to control bleeding from the right adrenal gland. Nineteen patients (8 in the major hepatectomy group and 11 in the minor hepatectomy group) required percutaneous drainage procedures for intra-abdominal collections or pleural effusion. The median operative blood loss was 300 ml (range: 20 to 2700 ml). Nineteen patients (7.7%) required blood transfusion. These included five right hepatectomies, three left hepatectomies, four left lateral sectionectomies, three bisegmentectomies, one segmentectomy and three wedge resections. Most of the transfusions were given during the operation because of heavy blood loss (up to 2700 ml). The rest were given in the post-operative period. Further analysis of the blood loss between the cirrhotic and non-cirrhotic groups of patients revealed no statistical differences in either major or minor hepatectomy (Table 4). The median operative time was 240 min (range 70 to 490 min). The median peak level of serum bilirubin and INR occurred on post-operative day 2, whereas the median peak level of serum ALT occurred on post-operative day 1 (Fig. 2). The median post-operative hospital stay was 7 days (range: 2 to 47 days). The longest hospital stay belonged to a 71-year-old patient who received a left lateral sectionectomy and bile duct exploration for recurrent pyogenic cholangitis with background cirrhosis and had complications of infected ascites.
Table 3.
Summary of the operative outcomes
| Overall (n = 248) | Major hepatectomy (n = 75) | Minor hepatectomy (n = 173) | P-value | |
|---|---|---|---|---|
| Operative mortality | 0.8% | 1.3% (n = 1) | 0.6% (n = 1) | 0.51 |
| Operative morbidity | 25.4% | 30.7% (n = 23) | 23.1% (n = 40) | 0.21 |
| Conversion to use of Pringle manoeuver | 2.4% | 4.0% (n = 3) | 1.7% (n = 3) | 0.37 |
| Operative blood loss | 300 ml (20–2700) | 460 ml (100–2400) | 297 ml (20–2700) | <0.001* |
| Blood transfusion rate | 7.7% | 10.7% (n = 8) | 6.4% (n = 11) | 0.24 |
| Operative time | 240 min (70–490) | 300 min (70–475) | 225 min (80–490) | <0.001* |
| Post-operative hospital stay | 7 days (2–47) | 8 days (5–36) | 7 days (2–47) | 0.001* |
Values expressed as median (range).
denote statistical difference between major and minor hepatectomy (P-value < 0.05).
Table 4.
Comparison of blood loss between cirrhotic and non-cirrhotic livers in major and minor hepatectomy
| Cirrhotic(n = 77) | Non-cirrhotic(n = 171) | P-value | |
|---|---|---|---|
| Major hepatectomy (n = 75) | 300 ml (100–1600) (n = 17) | 475 ml (100–2400) (n = 58) | 0.196 |
| Minor hepatectomy (n = 173) | 250 ml (20–2000) (n = 60) | 300 ml (20–2700) (n = 113) | 0.168 |
Blood loss expressed in median (range).
Figure 2.
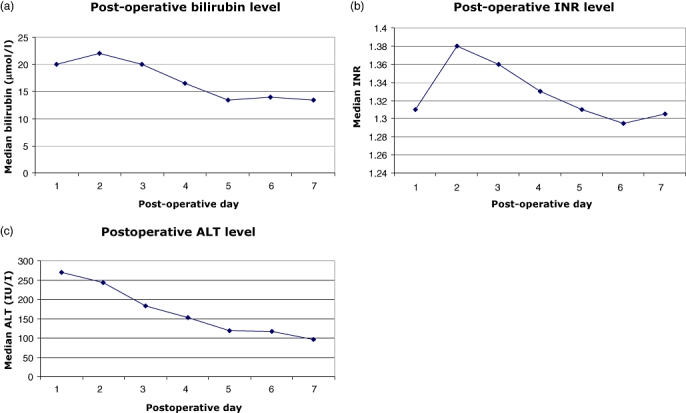
Trend of liver function tests in the early post-operative period (a) bilirubin, (b) international normalized ratio (INR) and (c) alanine aminotransferase (ALT)
The operative outcomes of the 75 patients undergoing major hepatectomy were compared against the 173 patients undergoing minor hepatectomy (Table 3). Patients undergoing major hepatectomy had higher operative blood loss (median: 460 ml vs. 297 ml), longer operating time (median: 300 min vs. 225 min) and longer post-operative hospital stay (median: 8 days vs. 7 days). There was no statistical difference in the operative mortality, morbidity, conversion to the use of the Pringle manoeuver and the blood transfusion rate between the two groups.
The 248 patients were followed up for a median period of 16.1 months and the 1-year, 3-year and 5-year overall survival rates were 90.2%, 66.7% and 56.5%, respectively. The 130 patients with HCC were followed up for a median period of 13.0 months. The overall survival rates at 1, 3, 5 years were 85.9%, 61.9% and 58.1%, respectively, and their disease-free survival rates at 1, 3, 5 years were 65.6%, 42.3% and 36.2%, respectively.
Discussion
The Pringle manoeuver is routinely applied during liver resection in many centres in the world today as various retrospective studies have shown that this manoeuver helps to reduce blood loss, with or without the delayed recovery of liver function.22,23 To our knowledge, only two prospective randomized trials have been performed in the past to test the effectiveness of the Pringle manoeuver. One prospective randomized trial that compared liver resection with or without the Pringle manoeuver showed that the application of inflow occlusion resulted in less blood loss.24 Furthermore, the same study revealed that the liver function was better preserved in the Pringle group, as shown by a lower serum bilirubin level in the early post-operative period. The authors attributed this to less haemodynamic disturbance induced by the bleeding. However, the other more recent prospective randomized study reported that there was no difference in terms of blood loss, rate of blood transfusion, mortality or morbidity for liver resection with or without the Pringle manoeuver.25 Thus it seems that the value of the Pringle maneuver in reducing blood loss during liver resection is still controversial, especially in this era of improving surgical dissecting techniques and equipments in liver surgery.
We believe that factors other than inflow occlusion also play significant roles in the control of bleeding during hepatectomy. Amongst these are the extra-parenchymal ligation of the inflow and/or outflow vessels to the resected segment/lobe of the liver,25 maintaining central venous pressure below 5 cm H2O,26–28 refined surgical instruments and meticulous dissection techniques. We intended to achieve low central venous pressure anaesthesia in all our cases, and in major hepatectomy hilar dissection to achieve ligation of inflow vessels was done in 90.7% of cases. Of the different dissection instruments, we have found that the combined use of CUSA and the saline-linked radio-frequency dissecting sealer particularly effective in achieving an almost bloodless transection. This technique has been shown by another group to reduce the duration of inflow occlusion, blood loss and operative time as compared with the use of CUSA alone.19 Such a technique was also reported to be safe and effective without the need for portal triad clamping.17 We have been accustomed to using this technique since 2003 and we confidently abandoned the routine Pringle manoeuver in June of that year. Giving up this routine manoeuver has allowed us to transect the liver more meticulously without worrying about the clamp time. Surgeons can take their time in the transection of the liver, as they do not have to hastily transect the liver with the vascular clamp on, and surgical trainees can develop their skill in the procedure more easily.
The median blood loss in this series was 300 ml with a blood transfusion rate of 7.7%. These figures compare favourably with other large series of liver resections where inflow occlusion was commonly used, in which the median blood loss was reported to be greater than 500 ml with transfusion rates of 6.2% to 49%.11–14 In this series, although the major hepatectomy group had a significantly higher blood loss, longer operating time and longer post-operative hospital than the minor hepatectomy group, the difference is relatively small considering the larger transection areas and smaller parenchymal volume of the remnant livers in patients undergoing major hepatectomies. More importantly, these differences did not translate into differences in operative mortality, operative morbidity, conversion to the use of the Pringle manoeuver and the rate of blood transfusion.
Our series confirms that relatively low levels of blood loss can equally be achieved in hepatectomy without the Pringle manoeuver. Furthermore, no increase in blood loss was observed in the cirrhotic patients, as compared with the non-cirrhotic patients in either minor or major hepatectomy (Table 4). Although cirrhosis and more extensive resections have been shown to be associated with increased rates of blood transfusion,29 we have not found this to be the case. A low operative mortality rate of 0.8% was achieved in this consecutive series, which is also consistent with the low mortality rates (<5%) that have been achieved in well-respected centres.11–14 Although the morbidity rate is not very low (25.4%), it should be noted that most of these were minor complications. Only one patient required re-operation and 19 others required percutaneous drainage of an intra-abdominal collection or pleural effusion. More importantly, most patients had a quick recovery of liver function, except for the three patients who developed post-operative liver failure.
Although the operative time was relatively long (median 240 min), it did not adversely affect the post-operative outcomes. The median post-operative hospital stay was only 7 days. Liver function, as measured by serum aminotransferase, bilirubin and the clotting profile, improved on post-operative day 1 or day 2. As a previous study has shown that liver function recovered after post-operative day 2,30 we believe that the avoidance of inflow vascular occlusion accounts for the earlier recovery in our series. Obviously, other factors may also account for the favourable results in this series. For example, most of the patients in the series had Child's A cirrhosis, and only 30% of the resections were major hepatectomies. Also, only a small proportion of patients required major concomitant procedures.
The Pringle manoeuver was used in six of the patients in this series, all of whom were in the open hepatectomy group. In two of these patients, the manoeuvers were performed intentionally to extract the portal vein tumour thrombus. The other four patients had uncontrolled bleeding during liver parenchymal transection and the Pringle manoeuver was used temporarily for haemostasis. The total clamping time ranged from 5 to 20 min only and such durations should be tolerated by even the worst cirrhotic liver.31
The overall survival rate for this group of patients was satisfactory. The group of patients with HCC also achieved comparable overall and disease-free 5-year survival rates (58.1% and 36.2 % respectively) as reported in other series where inflow occlusion was used selectively.32–34 However, in view of the relatively short period of follow-up, a valid conclusion on the relationship between survival and the no-clamp technique cannot be drawn.
Conclusion
The Pringle manoeuver is still routinely used by many liver surgeons in liver resection. However, we believe that if we can control blood loss without applying the Pringle manoeuver, we can avoid ischaemic insult to the remnant liver and can lower the chance of post-operative liver failure. We have shown that, with the application of new and effective instruments, liver resection is safe without routine inflow occlusion at the liver hilum. This applies to the cirrhotic liver as well. Our series of no-clamp hepatectomy has shown comparable rates of blood loss, morbidity and mortality to those reported in other series where inflow occlusion was commonly used. Nevertheless, whether adding the Pringle manoeuver on top of our liver transection techniques is harmful or beneficial remains unknown, and prospective randomized trials should be carried out to address this issue. Furthermore, it should be noted that liver surgeons should be familiar with the Pringle manoeuver, as it may be required if massive bleeding is encountered during no-clamp hepatectomy.
Conflicts of interest
None declared.
References
- 1.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei AC, Poon TPR, Fan ST, Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33–41. doi: 10.1002/bjs.4018. [DOI] [PubMed] [Google Scholar]
- 3.de Boer MT, Molenaar IQ, Porte RJ. Impact of blood loss on outcome after liver resection. Dig Surg. 2007;24:259–264. doi: 10.1159/000103656. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303–309. [PubMed] [Google Scholar]
- 5.Asahara T, Katayama K, Itamoto T, Yano M, Hino H, Okamoto Y, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg. 1999;23:676–680. doi: 10.1007/pl00012367. [DOI] [PubMed] [Google Scholar]
- 6.Hanazaki K, Kajikawa S, Shimozawa N, Matsushita A, Machida T, Shimada K, et al. Perioperative blood transfusion and survival following curative hepatic resection for hepatocellular carcinoma. Hepatogastroenterology. 2005;52:524–529. [PubMed] [Google Scholar]
- 7.Abdalla EK, Noun R, Belghiti J. Hepatic vascular occlusion: which technique? Surg Clin North Am. 2004;84:563–585. doi: 10.1016/S0039-6109(03)00231-7. [DOI] [PubMed] [Google Scholar]
- 8.Smyrnitotis V, Farantos C, Kostopanagiotou G, Arkadopoulos N. Vascular control during hepatectomy: review of methods and results. World J Surg. 2005;29:1384–1396. doi: 10.1007/s00268-005-0025-x. [DOI] [PubMed] [Google Scholar]
- 9.Lai PB, Lee KF, Wong J, Li AK. Techniques for liver resection: a review. Surgeon. 2007;5:166–174. doi: 10.1016/s1479-666x(07)80044-8. [DOI] [PubMed] [Google Scholar]
- 10.Pringle JH. Notes on the arrest of hepatic haemorrhage due to trauma. Ann Surg. 1909;48:541–549. doi: 10.1097/00000658-190810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 12.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397–407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 14.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–710. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin XY, Lai PB, Lee JF, Lau JW. Effects of hepatic blood inflow occlusion on liver regeneration following partial hepatectomy in an experimental model of cirrhosis. Br J Surg. 2000;87:1510–1515. doi: 10.1046/j.1365-2168.2000.01574.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim YI. Ischemia-reperfusion injury of the human liver during hepatic resection. J Hepatobiliary Pancreat Surg. 2003;10:195–199. doi: 10.1007/s00534-002-0730-x. [DOI] [PubMed] [Google Scholar]
- 17.Khan AZ, Bann SD, Pitsinis V, McCall J, Mudan SS. Refining the technique of hepatic parenchymal transection: combined saline-linked radiofrequency precoagulation and ultrasonic aspiration. Hepatogastroenterology. 2007;54:1167–1169. [PubMed] [Google Scholar]
- 18.Fan ST. Protection of the liver during partial hepatectomy. Hepatobiliary Pancreat Dis Int. 2004;3:490–494. [PubMed] [Google Scholar]
- 19.Aloia TA, Zorzi D, Abdalla EK, Vauthey JN. Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg. 2005;242:172–177. doi: 10.1097/01.sla.0000171300.62318.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The 50-50 criteria on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–829. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee KF, Cheung YS, Chong CN, Tsang YY, Ng WW, Ling E, et al. Laparoscopic versus open hepatectomy for liver tumours: a case control study. Hong Kong Med J. 2007;13:442–448. [PubMed] [Google Scholar]
- 22.Nuzzo G, Giuliante F, Giovannini I, Vellone M, De Cosmo G, Capelli G. Liver resection with or without pedicle clamping. Am J Surg. 2001;181:238–246. doi: 10.1016/s0002-9610(01)00555-4. [DOI] [PubMed] [Google Scholar]
- 23.Chau GY, Lui WY, King KL, Wu CW. Evaluation of effect of hemihepatic vascular occlusion and the Pringle manoeuvre during hepatic resection for patients with hepatocellular carcinoma and impaired liver function. World J Surg. 2005;29:1374–1383. doi: 10.1007/s00268-005-7766-4. [DOI] [PubMed] [Google Scholar]
- 24.Man K, Fan ST, Ng IOL, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704–713. doi: 10.1097/00000658-199712000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capussotti L, Muratore A, Ferrero A, Massucco P, Ribero D, Polastri R. Randomized clinical trial of liver resection with and without hepatic pedicle clamping. Br J Surg. 2006;93:685–689. doi: 10.1002/bjs.5301. [DOI] [PubMed] [Google Scholar]
- 26.Johnson M, Mannar R, Wu AV. Correlation between blood loss and inferior vena cava pressure during liver resection. Br J Surg. 1998;85:188–190. doi: 10.1046/j.1365-2168.1998.00570.x. [DOI] [PubMed] [Google Scholar]
- 27.Smyrniotis V, Kostopanagiotou G, Theodoraki K, Tsantoulas D, Contis JC. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg. 2004;187:398–402. doi: 10.1016/j.amjsurg.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 2006;12:935–939. doi: 10.3748/wjg.v12.i6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulitano C, Arru M, Bellio L, Rossini S, Ferla GA. A risk score for predicting perioperative blood transfusion in liver surgery. Br J Surg. 2007;94:860–865. doi: 10.1002/bjs.5731. [DOI] [PubMed] [Google Scholar]
- 30.Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, et al. Continuous versus intermittent portal triad clamping for liver resection. A controlled study. Ann Surg. 1999;229:369–375. doi: 10.1097/00000658-199903000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishizaki Y, Yoshimoto J, Miwa K, Sugo H, Kawasaki S. Safety of prolonged intermittent pringle manoeuvre during hepatic resection. Arch Surg. 2006;141:649–653. doi: 10.1001/archsurg.141.7.649. [DOI] [PubMed] [Google Scholar]
- 32.Hanazaki K, Kajikawa S, Shimozawa N, Mihara M, Shimada K, Hiraguri M, et al. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381–388. doi: 10.1016/s1072-7515(00)00700-6. [DOI] [PubMed] [Google Scholar]
- 33.Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tralhao JG, Kayal S, Dagher I, Sanhueza M, Vons C, Franco D. Resection of hepatocellular carcinoma: the effect of surgical margin and blood transfusion on long-term survival. Analysis of 209 consecutive patients. Hepatogastroenterology. 2007;54:1200–1206. [PubMed] [Google Scholar]


