Abstract
Background/aims:
To evaluate the ability of the model for end-stage liver disease (MELD) in predicting the post-hepatectomy outcome for hepatocellular carcinoma (HCC).
Methods:
Between 2001 and 2004, 69 cirrhotic patients with HCC underwent hepatectomy and the results were retrospectively analysed. MELD score was associated with post-operative mortality and morbidity, hospital stay and 3-year survival.
Results:
Seventeen major and 52 minor resections were performed. Thirty-day mortality rate was 7.2%. MELD ≤ 9 was associated with no peri-operative mortality vs. 19% when MELD > 9 (P < 0.02). Overall morbidity rate was 36.23%; 48% when MELD > 9 vs. 25% when MELD ≤ 9 (P < 0.02). Median hospital stay was 12 days [8.8 days, when MELD ≤ 9 and 15.6 days when MELD > 9 (P = 0.037)]. Three-year survival reached 49% (66% when MELD ≤ 9; 32% when MELD > 9 (P < 0.01). In multivariate analysis, MELD > 9 (P < 0.01), clinical tumour symptoms (P < 0.05) and American Society of Anesthesiologists (ASA) score (P < 0.05) were independent predictors of peri-operative mortality; MELD > 9 (P < 0.01), tumour size >5 cm (P < 0.01), high tumour grade (P = 0.01) and absence of tumour capsule (P < 0.01) were independent predictors of decreased long-term survival.
Conclusion:
MELD score seems to predict outcome of cirrhotic patients with HCC, after hepatectomy.
Keywords: hepatocellular carcinoma, MELD score, hepatectomy, cirrhosis, liver resection outcome
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. Its incidence is 1:500 000 and it is strongly correlated with cirrhosis.1 The mainstay of treatment, in patients with solitary HCC and good liver function, is hepatic resection.2
Evolution in surgical techniques and peri-operative care have improved post-operative outcome, in patients with severe underlying liver disease undergoing hepatectomy. The risk of hepatic failure in a cirrhotic patient undergoing hepatectomy still remains high, as a result of compromised function of the liver remnant.3,4 Therefore, a thorough evaluation of the hepatic function reserve is necessary prior to surgical intervention, in order to select the best candidates for hepatic resection among cirrhotic patients, with reasonable post-operative morbidity and mortality.
Child–Pugh–Turcotte (CPT) classification was the first systematic approach used to determine the severity of cirrhosis and select those patients who could tolerate hepatic resection.5 CPT class C is considered an absolute contraindication for surgical treatment, whereas only few hepatectomies are performed in class B cirrhosis.5–7 CPT class A patients are generally considered good candidates for hepatic resection and good post-operative outcome is expected. More refined evaluation of the liver function reserve isoften needed, as a result of limitations in the discriminatory ability of the CPT system, as it uses subjective parameters, such as ascites and encephalopathy.8–11
Many tests have been applied for the assessment of dynamic hepatic function, such as the indocyanine green clearance test,9 lidocaine test,10 galactose elimination capacity,11 and it was shown that they could provide a more refined estimate of hepatic function than the CPT score.9
The model for end-stage liver disease (MELD) score was recently introduced to evaluate hepatic function reserve in cirrhotic patients.12–15 It has the advantage of using three objective and easily measured parameters: creatinine levels, international normalized ratio (INR) and total bilirubin.
MELD score is used for survival prediction in cirrhotic patients receiving a transjugular intrahepatic portosystemic shunt.12 It has also been used to determine priority on waiting lists for liver transplantation13 and in predicting post-operative outcome of cirrhotic patients, undergoing surgical procedures.14,15
The aim of this study was to examine whether the pre-operative MELD score can predict post-operative mortality, morbidity, hospital stay and 3-year survival in cirrhotic class A patients undergoing hepatectomy for HCC. An effort to subcategorize the low-from the high-risk class A patients is provided.
Materials and methods
We retrospectively analysed the clinical records of all patients with HCC, who underwent hepatic resection in our institution between January 2001 and January 2004. Patients who were anticoagulated and those with chronic renal insufficiency requiring haemodialysis were excluded from the study. HCC was pathologically confirmed in all patients included in the study. We identified 69 patients fulfilling the above criteria. Clinical and pathological features of the patients are reported in Tables 1 and 2.
Table 1.
Univariate analysis of peri-operative mortality in patients with cirrhosis with hepatocellular carcinoma
| Variable | No. of patients | Peri-operative mortality, n (%) | P-value |
|---|---|---|---|
| Age (years) | 0.6 | ||
| ≤65 | 41 | 2 | |
| >65 | 28 | 3 | |
| Gender | 0.7 | ||
| Male | 49 | 4 | |
| Female | 20 | 2 | |
| Symptoms | <0.05 | ||
| Present | 28 | 5 | |
| Absent | 41 | 0 | |
| CPT class | 0.5 | ||
| A | 62 | 4 | |
| B | 7 | 1 | |
| MELD score | 0.01 | ||
| ≤9 | 43 | 0 | |
| >9 | 26 | 5 | |
| Tumour size | >0.05 | ||
| ≤5 | 43 | 3 | |
| >5 | 26 | 2 | |
| Grade | >0.05 | ||
| 1 | 5 | 0 | |
| 2 | 36 | 3 | |
| 3 | 28 | 2 | |
| 4 | 0 | ||
| Stage | 0.1 | ||
| 1 | 22 | 2 | |
| 2 | 26 | 2 | |
| 3 | 19 | 1 | |
| Extent of resection | 0.09 | ||
| Minor | 52 | 2 | |
| Major | 17 | 3 | |
| ASA class | <0.05 | ||
| 1 | 14 | 0 | |
| 2 | 34 | 1 | |
| 3 | 21 | 4 |
CPT, Child–Pugh–Turcotee; MELD, model for end-stage liver disease; ASA, American Society of Anesthesiologists.
Table 2.
Univariate analysis of clinicopathologic factors associated with survival after hepatectomy for hepatocellular carcinoma in patients with cirrhosis
| Variable | 1-year survival (%) | 3-year survival (%) | P-value |
|---|---|---|---|
| Age (yr) | 0.3 | ||
| ≤65 | 61 | 52 | |
| >65 | 69 | 43 | |
| Gender | 0.6 | ||
| Male | 66 | 47 | |
| Female | 64 | 58 | |
| Symptoms | 0.01 | ||
| Present | 43 | 16 | |
| Absent | 82 | 69 | |
| CTP class | 0.6 | ||
| A | 66 | 46 | |
| B | 51 | 50 | |
| MELD score | 0.01 | ||
| ≤9 | 90 | 66 | |
| >9 | 49 | 32 | |
| Tumor size | 0.01 | ||
| ≤5 | 79 | 69 | |
| >5 | 42 | 31 | |
| Grade | 0.01 | ||
| 1 | 100 | 100 | |
| 2 | 73 | 61 | |
| 3 | 52 | 32 | |
| 4 | 0 | 0 | |
| Stage | 0.6 | ||
| 1 | 72 | 65 | |
| 2 | 83 | 33 | |
| 3 | 49 | 37 | |
| Extent of resection | 0.06 | ||
| Minor | 74 | 52 | |
| Major | 43 | 31 | |
| Capsule | 0.01 | ||
| Yes | 72 | 52 | |
| No | 33 | 14 |
MELD, model for end stage liver disease.
CPT class was calculated using prothrombin time, albumin, bilirubin and clinical findings of ascites and encephalopathy.16 CPT score was stratified as class A (5–6), B (7–9) and C (10–15). Sixty two patients were classified as CPT class A (89.8%) and seven patients as CPT class B, score 7 (10.2%).
MELD score was calculated using pre-operative values of three laboratory tests: INR for prothrombin time, serum total bilirubin (TBil) and serum creatinine (Cr). MELD score was calculated using the following formula: MELD = 9.57 × loge(Cr mg/dl) + 3.78 × loge(TBil mg/dl) + 11.20 × loge(INR) + 6.43.12 We used the MELD score of the patient upon admission to our clinic, as it more accurately represents the severity of cirrhosis before surgery. Median MELD score prior to surgery was nine (range, 6–15). The distribution of MELD score in our population is shown in Fig. 1.
Figure 1.
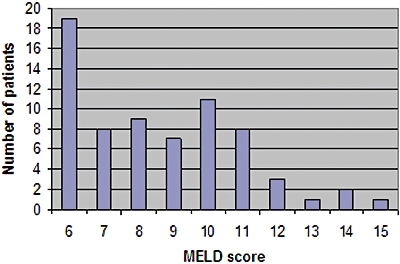
Distribution of model for end-stage liver disease (MELD) in patients with cirrhosis
Patients with CPT class A cirrhosis showed a median MELD score of 8, ranging from 6 to 14, significantly lower than patients with CTP class B cirrhosis who had a median MELD score of 11 (range, 9–15, P < 0.05).
During hospitalization and prior to surgery, the majority of the patients received blood products such as fresh frozen plasma (FFP) in order to improve their laboratory values. Hepatitis activity and cirrhosis were evaluated using the Isaak fibrosis score.17 Necrosis and inflammatory changes characteristic of hepatitis were scored as mild (0–5), moderate (6–12) or severe (13–18), and fibrosis was scored as cirrhosis vs. Non-cirrhosis. Operative data are shown in Tables 1 and 2. Major hepatic resection was defined as the removal of three or more segments.18 Portal vein embolization (PVE) was performed 6 to 8 weeks before surgery whenever the future liver remnant (FLR) was expected to be less than 40% of the total liver volume (TLV) with the tumour volume subtracted (as calculated using three-dimensional CT volumetry). Tumour histological grading was assessed according to the criteria of Edmondson and Steiner based on the areas of the tumour with the highest grade.19 Tumours were classified accordingly to the sixth edition of AJCC Cancer staging manual.20
Post-operative mortality was defined as any death occurring within 30 days after surgery. The primary end point of the study was the investigation of the relationship between the pre-operative MELD score and the development of irreversible liver failure after hepatectomy in cirrhotic patients. It was defined as a growing impairment of liver function after resection, which led to the death of the patient or required transplantation. The relationship between the MELD score and post-operative complications (morbidity), length of hospital stay and 3-year patient survival represented secondary end points. Post-operative jaundice was defined as a serum bilirubin level above 5 mg/dL, alteration of coagulation factors was defined as considerable or severe, when a FFP infusion was required in order for these to be corrected, and renal impairment was defined as an increase in blood urea nitrogen above 2 g/l and/or an increase in serum creatinine above 2 mg/dl.
Hospital stay was computed from the date of the operation, until discharge at home. Patient survival was computed from the date of the operation, until the most recent follow-up. Controls and patients still alive after the first year after surgery were censored at this time point; patients transplanted for post-operative liver failure were censored the day prior to transplantation, and patients who died from causes not related to liver failure were censored the day prior to the event.
Long-term follow-up included serum α-fetoprotein (AFP) and CT scan of the abdomen every 3 months during the first year after surgery and at 6-month intervals thereafter. CT, MRI and PET scan or angiography were performed selectively when recurrence was suspected.
Statistical analysis
Continuous variables were expressed as median and range. The values in the different subgroups were compared using the Kruskal–Wallis test. Normal distribution could not be proven for the clinical variables available (Kolmogorov–Smirnov test, P < 0.05).
Non-parametric tests were applied to all the data analysis. Categorical variables were expressed as prevalence and subgroups were compared using the χ2 test with Yates's correction. Survival probabilities were constructed using Kaplan–Meier survival estimates and compared using the log-rank test.
The prognostic value of MELD in predicting post-operative liver failure and complications was assessed using receiver-operating characteristic (ROC) curve analysis. A significance level of 0.05 was used in all analyses. ROC analysis was performed using MedCalc Version 7.2.1.0 (Med-Calc Software, Mariakerke, Belgium). The statistical analysis was done using SPSS Version 10.0 software (SPSS, Chicago, IL, USA).
Results
Surgery consisted of 52 (75.36%) minor hepatic resections and 17 (24.64%) major hepatic resections. Serum AFP was elevated in 61% of patients with a mean level of 2211 ng/ml (range 1–25 000 ng/ml). Six right hepatectomies (8.6%), 11 left hepatectomies (16%), 23 wedge resections (33.33%), 21 segmentectomies (30.4%) and 8 left lateral sectionectomies (11.6%) were performed.
Resection was performed using the conventional method with hepatic inflow dissection and selective vascular pedicle ligation followed by outflow short hepatic vein ligation in a piggyback fashion. Intra-operative ultrasound was performed routinely in patients undergoing hepatectomy. Median operating time was 195 min (range 150 to 310 min). In nine patients, ischaemic preconditioning was performed by vascular inflow occlusion during resection.
The mean size of HCC was 5.6 cm (range 1.5 to 14 cm). AJCC stage was I in 22 patients, II in 26 patients and III in 19 patients.
All resections were performed with a tumour-free margin of at least 1 cm.
Five patients (7.24%) developed irreversible post-operative liver failure and died within 30 days after surgery. Four of these patients were classified as CPT class A: two underwent a left hepatectomy, one a right hepatectomy and one a right posterior sectionectomy. One patient, classified as CTP class B, developed post-operative liver failure after a wedge resection.
Patients who experienced post-operative liver failure had a median MELD score of 10 (range, 9–15), significantly higher in comparison to patients where this event did not occur (median, 7; range, 6–13; P = 0.01). ROC analysis identified a MELD score above nine as a satisfactory cut-off value for predicting post-operative liver failure [area under the curve (AUC) = 0.92, 95% CI = 0.87–0.96; sensitivity = 81%; specificity = 87%] (Fig. 2).
Figure 2.
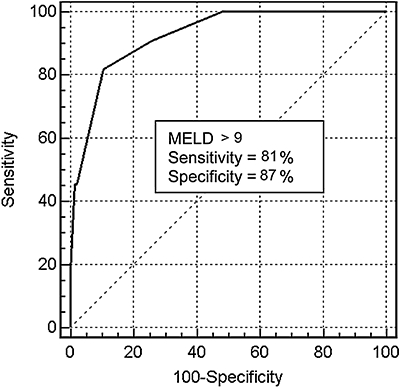
Receiver-operating characteristic (ROC) curve of the model for end-stage liver disease (MELD) score in predicting post-operative liver failure after hepatic resection in patients with cirrhosis (AUC = 0.92, 95% CI = 0.87–0.96)
Univariate analysis identified a MELD score > 9 (P < 0.01), presence of clinical tumour symptoms (P < 0.05) and ASA score (P < 0.05) as significantly associated with peri-operative mortality. The peri-operative mortality was significantly higher when the MELD score was above nine (P = 0.02). Other patients' demographics and pathological factors were not associated with peri-operative mortality (Table 1). Multivariate analysis demonstrated that a MELD score > 9 (P < 0.01), clinical tumour symptoms (P < 0.01) and ASA score (P < 0.051) are independent predictors of peri-operative mortality.
Other less life-threatening complications than post-operative liver failure included the occurrence of intractable ascites, requiring intensive therapy with diuretics for remission, elevation of INR > 2; 24-h post-surgery requiring a FFP transfusion and elevation of total bilirubin >5 mg/dl. Twenty-five patients (36.23%) experienced at least one post-operative complication. Refractory ascites developed in 10 cases (14.5%), jaundice in 7 cases (10.1%), and alteration of coagulation factors in 12 cases (17.4%).
Patients who experienced post-operative complications, had higher MELD scores (median, 10; range, 7–15) in comparison to patients who did not experience any complication (median, 8; range, 6–11; P = 0.001). ROC analysis again identified a MELD score above nine as the best cut-off value for predicting occurrence of post-operative complications (AUC 0.84, 94% CI = 0.77–0.88; sensitivity = 85%; specificity = 61%) (Fig. 3).
Figure 3.
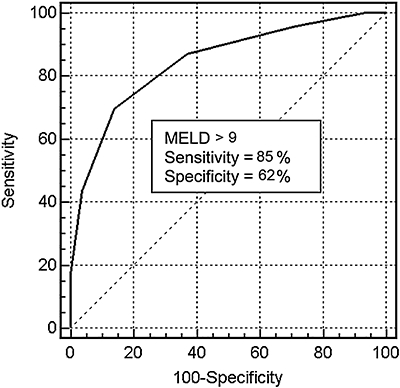
ROC curve of the MELD score in predicting occurrence of post-operative complication after hepatic resection in patients with cirrhosis [area under the curve (AUC) = 0.85, 95% CI = 0.78–0.89]
Patients were divided according to the cut-offs of the MELD scores obtained by ROC analysis in two groups: MELD below or equal to 9 and MELD above 9. MELD score was ≤9 in 43 patients (62.3%) and >9 in 26 patients (37.7%). The prevalence of post-operative liver failure and the morbidity in relationship with the MELD score prior to surgery as well as 3-year survival rates are reported in Tables 1 and 2. Patients with a MELD score below or equal to nine did not experience post-operative liver failure, they had zero 30-day mortality and showed the lowest morbidity 25% in contradiction to patients with MELD > 9, who had the highest prevalence of post-operative liver failure and 30-day mortality (19%) and the highest morbidity (48%) (P < 0.02 in both cases).
Mean hospital stay was 12 days. Patients with a MELD score below or equal to nine were discharged from the hospital after a median of 8.8 days (range, 6–15 days). When MELD score was above nine, the median hospital stay reached 15.6 days (range 9–27 days; P = 0.037). The 3-year survival for the entire cohort reached 49%. The 3-year survival was 66% when MELD ≤ 9 and 32% when MELD > 9; P < 0.01 (Table 2).
Multivariate analysis revealed that MELD > 9 (P < 0.01), tumour size > 5 cm (P < 0.01), high tumour grade (P = 0.01) and absence of tumour capsule (P < 0.01) are independent predictors of decreased long-term survival (Table 2).
Discussion
Liver failure after hepatectomy is one of the most severe complications of liver resection. In patients with liver cirrhosis, liver failure after hepatectomy is even more common because of the fact that resection removes functional liver tissue, from an organ that is already functioning marginally.
Pre-operative assessment of liver function and prediction of post-operative functional reserve are of paramount importance to minimize surgical risk.
CPT is not always a reliable indicator of hepatic reserve and has a limited role in predicting post-operative outcome.21
The MELD score seems to have the ability to categorize cirrhotic patients more accurately.22 A cirrhotic patient eligible for resection on the basis of CTP score may not be resectable on the basis of MELD. Such patients should be referred to non-surgical approaches such as radiofrequency ablation or transarterial chemoembolization. In our study most patients were class A (62 out of 69 patients: 89.8%) according to the CPT system. However, MELD classification managed to identify pre-operatively those class A patients with a higher 30-day mortality rate, greater risk of developing a post-hepatectomy complication and having a longer hospital stay and poorer long-term outcome.
Our study indicated that patients who underwent hepatectomy, with a pre-operative MELD score > 9, had elevated 30-day mortality rate (19% vs. 0%), high incidence of post-operative complications (48% vs. 25%), longer hospital stay (15.6 vs. 8.8 days) and worse 3-year survival (32% vs. 66%) in comparison to patients with a MELD score ≤ 9.
Conversely, patients with MELD scores below or equal to 9, who represent the 62.3% of cirrhotic patients in the present study, showed no post-operative liver failure, as well as low morbidity, shorter hospital stays and a 100% survival rate, proving that a good outcome can be achieved in these patients through hepatic resection.
Several reports from different groups, including ours, consider partial hepatectomy as a good option in well-compensated CPT class A patients.23–26 In addition, liver resection has been considered as a bridge to liver transplantation in order to reduce the dropout rate in patients with HCC on cirrhosis on the waiting list.27
Patients with a MELD score equal to or above 11 probably represent the ideal patients to refer for non-surgical approaches such as thermal ablation or chemoembolization and, whenever possible, for liver transplantation.
In particular, in the setting of liver transplantation, Merion et al.28 showed that a threshold score of 11 patients in whom, independent of HCC, transplantation is justified (above 11) or futile (below 11); in particular, cirrhotic patients with a MELD score between 6 and 11, were shown to have a post-transplant mortality significantly higher than waiting list mortality. Therefore, the results from recent studies may give support to a systematic transplantation policy in small HCC patients with a MELD score exceeding 11 as well as partial hepatectomy in patients with lower MELD scores.22 Even in patients with a MELD score between 9 and 10, carefully consideration must be given in intra-operative and post-operative management and major hepatectomies should be avoided.
Our findings show that a MELD score > 9 is strongly predictive of increased peri-operative mortality in patients with cirrhosis undergoing hepatic resection for HCC. No other clinical and pathological factors except for clinical tumour symptoms and ASA score were predictive of peri-operative mortality. This is in agreement with other reports in the field.21
Univariate analysis demonstrated that a MELD score > 9, tumour size > 5, high tumour grade, micro-vascular invasion, hepatitis C cirrhosis and the number of lesions are independent predictors of decreased long-term survival. Moreover, subset analysis of survival using the MELD score and tumour size identify patients who would potentially derive significant benefit from resection despite the background cirrhosis. Interestingly we documented the MELD score utility in assessing long-term survival of patients with HCC size > 5 cm as a major factor influencing operative intervention.
The high recurrence rate in patients with higher MELD scores can be explained by the different immunological status of these patients (cytokines).29–31
In our study, all patients had histological proved cirrhosis. The MELD score's reliability in predicting morbidity and mortality after elective liver resection has been criticized in patients with minimal or no evidence of liver disease.32
It is worth noting that there is a number of patients who, in spite of a low MELD score, have advanced liver disease using clinical evaluation and CTP score and therefore cannot be candidates for resection. These are patients with intractable ascites, who are malnourished and with encephalopathy, who have a very low MELD score, but clearly in whom surgery would be too dangerous. CPT stage and clinical evaluation should always be the initial consideration that precedes the MELD calculations. We must remember that only CPT class A and B patients should be considered for resection in the first place.
Conclusion
MELD score can accurately predict mortality, morbidity and long-term survival in patients with HCC and cirrhosis, undergoing hepatic resection. Cirrhotic patients with a high MELD score have an increased risk of post-operative liver failure and complications, they are expected to have poorer long-term survival after liver resection and should be referred for other treatments. Cirrhotic patients with a low MELD score treated with minor hepatic resections achieve zero 30-day mortality and low morbidity rates, whereas expected long-term survival is promising. Application of a MELD score in the pre-operative assessment of liver function prior to hepatic resection is recommended, as it facilitates identification of high-risk class A patients prior to hepatic resection and selection of the best candidates for hepatectomy. A multi-institutional study is required to better define the selection criteria for hepatic resection in HCC patients with cirrhosis.
Role of the funding source
All authors declare that no sponsors had any involvement in the study.
Conflicts of interest
All authors declare to have no financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
References
- 1.El-serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87–107. doi: 10.1016/s1089-3261(05)70155-0. [DOI] [PubMed] [Google Scholar]
- 2.Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belghiti J, Regimbeau JM, Durand F, Kianmanesh AR, Dondero F, Terris B. Resection of hepatocellular carcinoma: a European experience on 328 cases. Hepatogastroenterology. 2002;49:41–46. [PubMed] [Google Scholar]
- 4.Bilimoria MM, Lauwers GY, Doherty DA, Nagorney DM, Belghiti J, Do KA. Underlying liver disease, not tumor factors, predicts long-term survival after resection of hepatocellular carcinoma. Arch Surg. 2001;136:528–535. doi: 10.1001/archsurg.136.5.528. [DOI] [PubMed] [Google Scholar]
- 5.Franco D, Capussoti L, Smadja C, Bouzari H, Meakins J, Kemeny F. Resection of hepatocellular carcinomas. Results in 72 European patients with cirrhosis. Gastroenterology. 1990;98:733–738. [PubMed] [Google Scholar]
- 6.Paquet KJ, Gad HA, Lazar A, Koussouris P, Mercado MA, Heine WD. Analysis of factors affecting outcome after hepatectomy of patients with liver cirrhosis and small hepatocellular carcinoma. Eur J Surg. 1998;164:513–519. doi: 10.1080/110241598750005868. [DOI] [PubMed] [Google Scholar]
- 7.Wayne JD, Lauwers GY, Ikai I, Doherty DA, Belghiti J, Yamaoka G. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;235:722–731. doi: 10.1097/00000658-200205000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169:589–594. doi: 10.1016/s0002-9610(99)80227-x. [DOI] [PubMed] [Google Scholar]
- 9.Lau H, Man K, Fan ST, Yu WC, Lo CM, Woug J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255–1259. [PubMed] [Google Scholar]
- 10.Ercolani G, Grazi GL, Calliva R, Pierangeli F, Cescon M, Cavallari A. The lidocaine (MEGX) test as an index of hepatic function: its clinical usefulness in liver surgery. Surgery. 2000;127:464–471. doi: 10.1067/msy.2000.104743. [DOI] [PubMed] [Google Scholar]
- 11.Radaelli CA, Dufour JF, Wagner M, Schilling M, Hüsler J, Krähenbühl L. Preoperative galactose elimination capacity predicts complications and survival after hepatic resection. Ann Surg. 2002;235:77–85. doi: 10.1097/00000658-200201000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjujalar intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 13.Freeman RB, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 14.Northup PG, Wanamaker RC, Lee VD, Adams RB, Berg CL. Model for end-stage liver disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann Surg. 2005;242:244–251. doi: 10.1097/01.sla.0000171327.29262.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Befeler AS, Palmer DE, Hoffman M, Longo W, Solomon H, Di Bisceglie AM. The safety of intra-abdominal surgery in patients with cirrhosis: model for end-stage liver disease is superior to Child-Turcotte-Pugh classification in predicting outcome. Arch Surg. 2005;140:650–654. doi: 10.1001/archsurg.140.7.650. [DOI] [PubMed] [Google Scholar]
- 16.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 17.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 18.Belghiti J, Clavien P-A, Gadzijev E, Garden JO, Lau WY, Makuuchi M. The Brisbane 2000 terminology of liver anatomy and resections terminology committee of the international hepato-pancreato-biliary association: Chairman, SM Strasberg (USA) HPB (Oxford) 2000;2:333–339. [Google Scholar]
- 19.Edmondson HA, Steiner PE. Primary carcinoma of the liver. A study of 100 cases among 48 900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6th. New York: Springer; 2002. pp. 145–153. [Google Scholar]
- 21.Teh SH, Christein J, Donohue J, Que F, Kendrick M, Farnell M. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: model for end-stage liver disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9:1207–1215. doi: 10.1016/j.gassur.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, La Barba G. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12:966–971. doi: 10.1002/lt.20761. [DOI] [PubMed] [Google Scholar]
- 23.Grazi GL, Ercolani G, Pierangeli F, Del Gaudio M, Cescon M, Cavallari A. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2000;234:71–78. doi: 10.1097/00000658-200107000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl. 2004;10(Suppl):S39–S45. doi: 10.1002/lt.20040. [DOI] [PubMed] [Google Scholar]
- 25.Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127(Suppl):S248–S260. doi: 10.1053/j.gastro.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 26.Ikai I, Arii S, Kojiro M, Ikida T, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796–802. doi: 10.1002/cncr.20426. [DOI] [PubMed] [Google Scholar]
- 27.Belghiti J, Cortes A, Abdalla EK, Regimbeau JM, et al. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg. 2003;238:925–928. doi: 10.1097/01.sla.0000098621.74851.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, et al. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–312. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 29.Imai H, Nakano Y, Kiyosawa K, Tan EM. Increasing titers and changing specificities of antinuclear antibodies in patients with chronic liver disease who develop hepatocellular carcinoma. Cancer. 1993;71:26–35. doi: 10.1002/1097-0142(19930101)71:1<26::aid-cncr2820710106>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Ledesma F, Echevarria S, Casafont F, Lozano JL, et al. Natural killer cell activity in alcoholic cirrhosis: influence of nutrition. Eur J Clin Nutr. 1990;44:733–740. [PubMed] [Google Scholar]
- 31.Pang RW, Joh JW, Johnson PJ, Monden M, et al. Biology of hepatocellular carcinoma. Ann Surg Oncol. 2008;15:962–971. doi: 10.1245/s10434-007-9730-z. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder RA, Marroquin CE, Bute BP, Khuri S, et al. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243:373–379. doi: 10.1097/01.sla.0000201483.95911.08. [DOI] [PMC free article] [PubMed] [Google Scholar]


