Abstract
Background:
Effective bile duct drainage is crucial to the health-related quality of life of patients with jaundice caused by obstruction of the bile duct by inoperable malignant tumours.
Methods:
All patients who were treated at Uppsala University Hospital, Sweden with percutaneous stenting between 2000 and 2005 were identified retrospectively. Data on the location of the obstruction and type of stent used, date and cause of death and date of stent failure were abstracted from the patients' notes. Stent patency was defined as the duration from the insertion of the stent to the date of failure. In cases in which the cause of death was directly related to failure of the stent, the date of death was defined as the patency endpoint.
Results:
A total of 64 patients (34 women, 30 men) were identified. Their mean age was 71 years (standard deviation 11 years). The median length of patency was 11.4 months. Stent diameter >10 mm and distal stricture were found to be associated with significantly longer patency time in univariate Cox proportional hazard analysis. In multivariate Cox proportional hazard analysis, only location of the stricture was found to be independently and significantly associated with patency time.
Discussion:
Percutaneous stenting is a good alternative for patients with obstructive jaundice and a life expectancy ≤1 year. It may give instant relief from the symptoms associated with jaundice. Patency time may be prolonged by using stents with a diameter ≥10 mm. However, patency time was found to be lower for hilar tumours.
Keywords: cholangiocarcinoma, pancreatic cancer, jaundice, stenting, patency
Introduction
The majority of malignant tumours that cause bile duct obstruction, particularly pancreatic cancer, papillary cancer, biliary duct cancer or gall bladder cancer, are inoperable at the time of diagnosis.1,2 Furthermore, as these tumours usually occur in the elderly population, the advanced surgery required for radical management is often futile. The main objective for these patients is to improve their health-related quality of life. The factor that most greatly limits quality of life, especially early on, is the development of obstructive jaundice. Effective drainage of bile is therefore crucial. This may be achieved through percutaneous transhepatic biliary drainage (PTBD),3 endoscopic stenting,4 surgical bilioenteric bypass 5 or percutaneous transhepatic biliary stenting.6–8 Percutaneously inserted transhepatic biliary self-expanding metal stents (SEMS) may provide effective drainage of the bile ducts and relief of jaundice, without the problems and daily dressing requirements associated with PTBD, and catheter changes can be planned or performed as required. In most cases, PTBD is associated with a much lower morbidity than surgical bilioenteric bypass and may be more easily achieved than endoscopic stenting, especially in patients with obstructions close to or above the bile duct bifurcation.9 However, remaining life expectancy must be taken into account when deciding on percutaneous transhepatic biliary stenting because these stents inevitably cease to function at some stage as a result of tumour overgrowth orbecause the stent becomes clogged or fractured. If the subject's life expectancy is considerably longer than the expected patency time of the stent, repeated measures to drain the bile may be required. This may cause the patient unnecessary episodes of jaundice or cholangitis, so reliable knowledge of stent patency and factors associated with stent failure are therefore of great importance. The way in which the SEMS is inserted and how it is located in relation to the stricture are considered crucial to successful drainage because a major cause of stent failure is tumour overgrowth.7
The aims of the present study were to assess the patency of SEMS inserted via a percutaneous transhepatic biliary approach and to explore factors predicting patency, especially with regard to the type of SEMS and its location.
Materials and methods
All patients who were treated at Uppsala University Hospital with percutaneous transhepatic biliary stenting for malignant bile duct obstruction during the period 2000–2005 were identified retrospectively. A total of 64 patients, including 34 women and 30 men, were identified. Their mean age was 71 years (standard deviation 11 years). Data on the location of the obstruction and type of stent used, date and cause of death and, if applicable, date of stent failure, were abstracted from the patients' notes. Stent failure was defined as persistent or recurrent jaundice. Proximal direction was defined as towards the liver and distal as towards the intestine.
All patients received PTBD for initial drainage. After an initial period of 2–6 weeks, the PTBD was changed to an SEMS. Insertion of the stent was performed under fluoroscopy over a guidewire through an 8-Fr, 30-cm-long introducer (William Cook Europe Aps, Bjaeverskov, Denmark) after the PTBD had been removed. All stents were allowed to pass through the papilla of Vater about 1–2 cm into the duodenum. The length of the stent was chosen according to the length of the stricture so that the stent extended ≥2 cm beyond the tumour margin. The stent was dilated if it was compressed by the tumour, usually with an 8-mm balloon catheter. Function was checked by injecting contrast and observing its passage through the stent. If the passage of contrast into the duodenum showed no sign of obstruction, the guidewire and introducer were removed. If not, a temporary drainage catheter was left in place and tested after a few days and then removed if the passage of contrast was satisfactory. The stents used were the Wallstent™ (Boston Scientific Corp., Natick, MA, USA), the ZA™ (Cook Medial, Möenchengladbach, Germany), the Luminexx™ (Bard, Karlsruhe, Germany), and the Sinus Flex™ (Optimed GmbH, Ettlingen, Germany). Six patients with hilar tumours received double Luminexx™ stents. The median length of the stents was 80 mm (range 60–100 mm). The median diameter was 10 mm (range 6–12 mm).
Patency was followed until the patient developed signs of stent failure (i.e. jaundice or cholangitis). If the patient developed clinical signs of stent failure, he or she was evaluated using non-invasive imaging techniques, usually computed tomography or ultrasound. In most cases, efforts were made to re-establish stent function after occlusion, usually through a new percutaneous procedure. Only patency until first failure is considered in this report; patency after re-intervention is not included in the analysis.
All procedures were carried out in accordance with the Helsinki Declaration of 1983.
Statistics
Stent patency was defined as the duration from the insertion of the stent until the date of failure. If the cause of death was directly related to stent failure, the date of death was defined as the patency endpoint. If no stent failure occurred, stent patency was considered as censored at the date of death or at the end of the study period (17 March 2006). Age, gender, type of stricture and size and location of the stent were included as covariates in univariate and multivariate Cox proportional hazard analysis, with patency as outcome measure. The multivariate model was constructed by stepwise selection of the covariates, with entry testing based on the significance of the score statistic, and removal testing based on the probability of a likelihood ratio statistic based on the maximum partial likelihood estimates.
Results
Diagnoses and stricture levels are presented in Table 1. Fourteen patients had strictures at the biliary bifurcation. The strictures were characterized according to the Bismuth–Corlette classification10 as hilar type 1 (n= 2) and hilar type 4 (n= 12). The Bismuth–Corlette classification is shown in Fig. 1. Fifty patients had a more distal stricture. In two cases the stricture was located at the junction of the hepatic duct and common bile duct. In 48 cases the stricture was located near or above the papilla of Vater.
Table 1.
Diagnoses and stricture levels
| Stricture level | n |
|---|---|
| Hilar type 110 | |
| Metastasis from ovarian cancer | 1 |
| Gall bladder cancer | 1 |
| Hilar type 410 | |
| Gall bladder cancer | 4 |
| Bile duct cancer | 5 |
| Metastasis from small bowel cancer | 1 |
| Hilar metastasis of unknown origin | 1 |
| Pancreatic cancer | 1 |
| Junction between hepatic duct and common bile duct | |
| Gall bladder cancer | 1 |
| Pancreatic cancer | 1 |
| Distal | |
| Pancreatic cancer | 39 |
| Cystadenocarcinoma | 1 |
| Chronic pancreatitis and lung cancer | 1 |
| Duodenal cancer | 1 |
| Colon cancer metastasis | 1 |
| Cancer of the papilla of Vater | 4 |
| Small bowel cancer | 1 |
Figure 1.
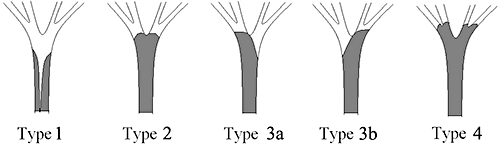
The Bismuth–Corlette classification of hilar tumours10
Median overall survival was estimated to be 3.8 months; the first quartile amounted to 1.5 months and the third quartile to 9.7 months. Six months after stent insertion, 16 patients remained alive with a patent stent. Twelve months after stent insertion, this number had decreased to six. Stent failure was seen in 19 of 64 patients (30%), the causes of which are presented in Table 2. Only four patients survived >30 days (range 3.7–10.0 months) after stent failure. Failure within 30 days occurred in six patients (9%). Five of these patients had a distal tumour and one had a hilar tumour type 4. The diameter of the stent was 10 mm in four, and 9 mm and 8 mm in one patient each. The reason for failure within 30 days was related to stent fracture in one case and stent occlusion in one. Despite adequate drainage in three of the patients with both stents and PTBD, liver function deteriorated and the subjects died. Late stent failures (>30 days; range 1.0–21.1 months) was seen in 16 of 64 patients (25%). A pancreatic tumour treated with a stent is shown in Fig. 2 and a hilar tumour type 4 is shown in Fig. 3.
Table 2.
Stent types and causes of failure
| n | % | |
|---|---|---|
| Stent type | ||
| Wallstent™ | 4 | 6 |
| ZA™ | 8 | 12 |
| Luminexx™ | 48 | 75 |
| Sinus Flex™ | 4 | 6 |
| Causes of failure | ||
| Stent fracture | 4 | 6 |
| Tumour overgrowth | 13 | 20 |
| Clotting | 1 | 2 |
| Miscellaneous causes | 1 | 2 |
Figure 2.
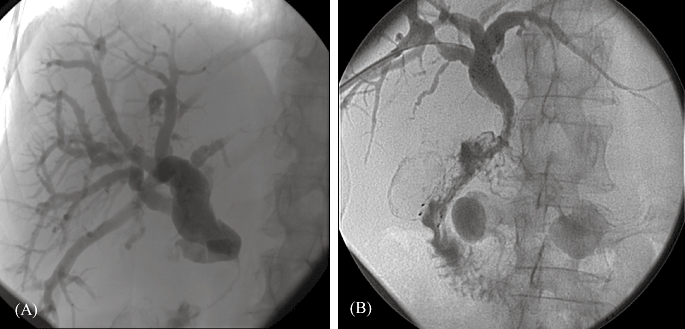
A 63-year-old woman diagnosed with a carcinoma of the head of the pancreas. (A) Contrast medium injection after percutaneous transhepatic placement of a drainage catheter into the duodenum. The distal common bile duct is obstructed. (B) Six weeks later a Luminexx(tm) stent (diameter 10 mm, length 8 cm) was placed in the common bile duct with the distal part of the stent protruding into the duodenum
Figure 3.
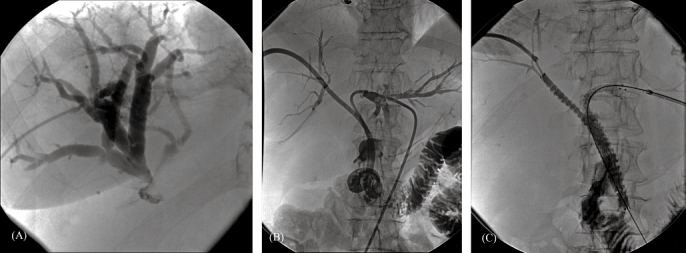
A 71-year-old woman diagnosed with a cholangiocarcinoma. (A) Percutaneous transhepatic cholangiogram demonstrating dilated bile ducts of the right liver lobe with a stricture at the level of the hilum and no filling of the left bile duct or common bile duct. (B) Contrast medium injection after percutaneous transhepatic placement of drainage catheters through both the right and left liver lobes into the duodenum, showing a stricture at the level of the hilum involving the main left and right bile ducts. (C) Contrast filling of the bile ducts after placing Luminexx(tm) stents from the right and left sides. One of the stents protrudes into the duodenum
In a Kaplan–Meier analysis in which death but patent stent was considered a censored event, median patency was found to be longer than overall survival (Fig. 4).
Figure 4.
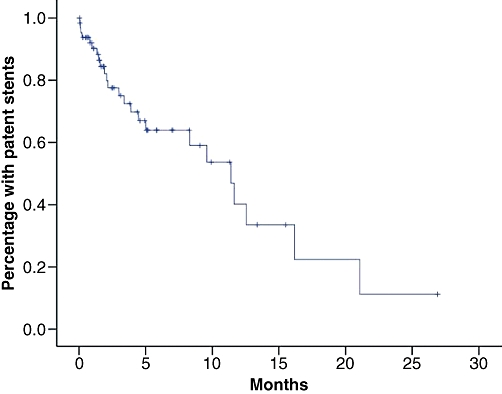
Patency time, all patients
In a Kaplan–Meier analysis based on the whole sample, median patency was estimated at 11.4 months (first quartile 3.4 months, third quartile 16.2 months). Stent diameter >10 mm and a distal location of the stricture were found to be associated with significantly longer patency time in univariate analysis (Table 3). In the multivariate analysis, only the location of the stricture was found to be independently and significantly associated with patency time. Kaplan–Meier curves stratified for stent diameter and type of stricture are shown in Figs 5 and 6.
Table 3.
Univariate and multivariate Cox proportional hazard analyses of variables predicting stent failure
| Factor | Univariate model | Final multivariate model | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| Age | ||||||
| ≤median (72 years) | 1 | Reference | ||||
| >median (72 years) | 0.48 | 0.21–1.14 | 0.096 | |||
| Gender | ||||||
| Female | 1 | Reference | ||||
| Male | 0.78 | 0.32–1.89 | 0.58 | |||
| Stent diameter | ||||||
| ≥median (10 mm) | 1 | Reference | ||||
| <median (10 mm) | 2.39 | 1.009–5.67 | 0.048 | |||
| Stent length | ||||||
| <median (80 mm) | 1 | Reference | ||||
| ≥median (80 mm) | 1.48 | 0.54–4.10 | 0.45 | |||
| Proximal stent overlap | ||||||
| <median (20 mm) | 1 | Reference | ||||
| ≥median (20 mm) | 0.50 | 0.21–1.22 | 0.13 | |||
| Distal stent overlap | ||||||
| <median (20 mm) | 1 | Reference | ||||
| ≥median (20 mm) | 1.37 | 0.57–3.29 | 0.48 | |||
| Stent protruding through papilla of Vater | ||||||
| <median (15 mm) | 1 | Reference | ||||
| ≥median (15 mm) | 0.67 | 0.26–1.71 | 0.40 | |||
| Stricture location | ||||||
| Distal | 1 | Reference | 1 | Reference | ||
| Proximal | 1.95 | 1.14–3.34 | 0.015 | 1.95 | 1.14–3.34 | 0.015 |
The multivariate analysis was constructed by stepwise selection, with entry testing based on the significance of the score statistic, and removal testing based on the probability of a likelihood ratio statistic based on the maximum partial likelihood estimates. The analysis is based on the 56 patients for whom data on all variables were available
95% CI, 95% confidence interval
Figure 5.
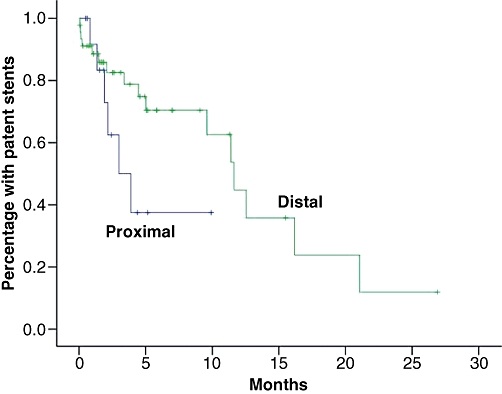
Kaplan–Meier curves stratified for location of the obstruction
Figure 6.
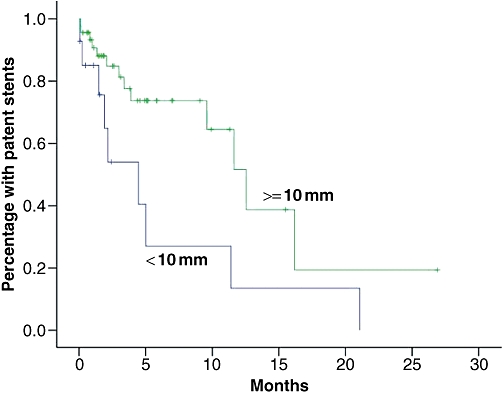
Kaplan–Meier curves stratified for stent diameter
Discussion
In the present study, the median stent patency time was found to be approximately 1 year. Given the very poor prognosis of patients with these diagnoses, 1 year is usually sufficient. The relief achieved by internal drainage may give patients a relatively good quality of life for the final period of their lives, without requiring them to rely on constant support from health care services, as is often the case with other forms of drainage.
As death with a patent stent was treated as a censored event, median patency was found to be longer than overall survival. The number of patients at risk for an event is therefore very low towards the end of the period of observation. Because the chance of being censored as a result of death with a patent stent was found to be higher than the chance of survival with a stent that had ceased to function, estimated projected stent patency time was higher than overall survival.
Until recently, there has been no way of prolonging survival for patients with tumours that most commonly cause malignant biliary obstruction, in whom surgery is not possible. Decisions on how to manage the obstruction have thus been based on the assumption of a very short life expectancy. However, studies from the last decade have shown that chemotherapy and radiotherapy may prolong the life of patients with pancreatic cancer 2 and cholangiocarcinoma11 in selected cases. This has to be taken into account before inserting an SEMS which may cease to function before the tumour has progressed. In such cases, surgical biliary anastomoses, drainage by PTBD or endoscopic stenting with plastic stents may be considered. Although plastic stents have shorter patency time than metal stents,8,12 they can be repositioned or extracted once deployed, which is much more difficult with metal stents. However, in the event of a metal stent ceasing to function, a new stent can be positioned within the first.
Metal stents are more expensive than plastic stents.6 However, the costs of health care interventions in patients with percutaneous biliary drainage, or the cost of a surgical biliary drainage procedure, far outweigh the cost of the SEMS. The development of covered stents12,13 may increase patency even more, although the advantage in terms of prolonged patency has not yet been fully proved.14
All patients were managed temporarily with PTBD for a period of ≥1 month. This may have contributed to the long patency observed in our study because patients with very rapidly progressing tumours or poor liver function had less chance of being considered for percutaneous stenting. The vast majority of patients with such poor prognoses died with a PTBD before being considered for percutaneous stenting.
The choice of management of patients with malignant biliary obstruction is largely a matter of local tradition and competence. Percutaneous transhepatic biliary stenting relies on the presence of an experienced interventional radiologist and effective co-operation between the clinician and radiologist.
Whereas relatively long patency times were seen for patients with obstructions located distally, the outcome was much worse for hilar tumours. The prognoses of patients with inoperable hilar cholangiocarcinomas are often very poor, irrespective of treatment.15 The shorter patency time of metal stents in patients with hilar tumours compared with patients with distal tumours has been observed previously 16,17 and has been attributed to tumour overgrowth of the stent. In our study, we saw a tendency towards reduced risk for stent failure when a proximal overlap ≥20 mm was applied, although the association was not significant.
Only four of 22 patients lived >30 days after the first stent failure. The poor survival following stent failure probably reflects the fact that many of the failures were caused by accelerated tumour progression. In such cases, all attempts to achieve bile drainage become futile.
Conclusions
Percutaneous stenting is a good alternative for patients with obstructive jaundice and a life expectancy of ≤1 year. It can give instant relief from the symptoms associated with jaundice and allow the patient to remain independent of support from health care services during the final months of his or her life. Patency time is, however, short for Klatskin tumours. Patency time may be prolonged by using stents with a diameter ≥10 mm and placing them with the proximal end ≥20 mm above the stricture.
Conflicts of interest
None declared.
References
- 1.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 2.Yip D, Karapetis C, Strickland A, Steer CB, Goldstein D. Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database Syst Rev. 2006;3:CD002093. doi: 10.1002/14651858.CD002093.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Molnar W, Stockum AE. Relief of obstructive jaundice through percutaneous transhepatic catheter – a new therapeutic method. AJR Am J Roentgenol. 1974;122:356–367. doi: 10.2214/ajr.122.2.356. [DOI] [PubMed] [Google Scholar]
- 4.Lammer J, Neymayer K. Biliary drainage endoprostheses: experience with 201 placements. Radiology. 1986;159:625–629. doi: 10.1148/radiology.159.3.2422677. [DOI] [PubMed] [Google Scholar]
- 5.Stain SC, Baer HU, Dennison AR, Blumgart LH. Current management of hilar cholangiocarcinoma. Surg Gynecol Obstet. 1992;175:579–588. [PubMed] [Google Scholar]
- 6.Katsinelos P, Paikos D, Kountouras J, Chatzimavroudis G, Parotoglou G, Moschos I, et al. Tannenbaum and metal stents in the palliative treatment of malignant distal bile duct obstruction: a comparative study of patency and cost-effectiveness. Surg Endosc. 2006;20:1587–1593. doi: 10.1007/s00464-005-0778-1. [DOI] [PubMed] [Google Scholar]
- 7.Brountzos EN, Ptochis N, Panagiotou I, Malagari K, Tzavara C, Kelekis D. A survival analysis of patients with malignant biliary strictures treated by percutaneous metallic stenting. Cardiovasc Intervent Radiol. 2007;30:66–73. doi: 10.1007/s00270-005-0379-3. [DOI] [PubMed] [Google Scholar]
- 8.Davids PH, Groen AK, Rauws EAJ, Tytgat GNJ, Huibregtse K. Randomized trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488–1492. doi: 10.1016/0140-6736(92)92752-2. [DOI] [PubMed] [Google Scholar]
- 9.Pseer A, Cotton PB, Russell RCG, Mason RR, Hatfield AR, Leung JW, et al. Randomized trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987;2:57–70. doi: 10.1016/s0140-6736(87)92733-4. [DOI] [PubMed] [Google Scholar]
- 10.Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg. 1988;12:39–47. doi: 10.1007/BF01658484. [DOI] [PubMed] [Google Scholar]
- 11.Ortner MA, Liebetruth J, Schreiber S, Hanft M, Wruck U, Fusco V, et al. Photodynamic therapy of non-resectable cholangiocarcinoma. Gastroenterology. 1998;114:536–552. doi: 10.1016/s0016-5085(98)70537-2. [DOI] [PubMed] [Google Scholar]
- 12.Söderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63:986–995. doi: 10.1016/j.gie.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 13.Fanelli F, Orgera G, Bezzi M, Rossi P, Allegritti M, Passariello R. Management of malignant biliary obstruction: technical and clinical results using an expanded polytetrafluoroethylene and fluorinated ethylene propylene (ePTFE/FEP)-covered metallic stent after 6-year experience. Eur Radiol. 2008;18:911–919. doi: 10.1007/s00330-008-0852-x. [DOI] [PubMed] [Google Scholar]
- 14.Yoon WJ, Lee JK, Lee KH, Lee WJ, Ryu JK, Kim YT, et al. A comparison of covered and uncovered Wallstents for the management of distal malignant biliary obstruction. Gastrointest Endosc. 2006;63:996–1000. doi: 10.1016/j.gie.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 15.Weber A, Landrock S, Schneider J, Stangl M, Neu B, Classen M, et al. Longterm outcome and prognostic factors of patients with hilar cholangiocarcinoma. World J Gastroenterol. 2007;13:1422–1426. doi: 10.3748/wjg.v13.i9.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi P, Bezzi M, Rossi M, Adam A, Chetty N, Roddie ME, et al. Metallic stents in malignant biliary obstruction: results of a multicentre European study of 240 patients. J Vasc Interv Radiol. 1994;5:279–285. doi: 10.1016/s1051-0443(94)71483-4. [DOI] [PubMed] [Google Scholar]
- 17.Laméris JS, Stoker J, Nijs HG, Zonderland HM, Terpstra OT, van Blankenstein M, et al. Malignant biliary obstruction: percutaneous use of self-expandable stents. Radiology. 1991;179:703–707. doi: 10.1148/radiology.179.3.2027978. [DOI] [PubMed] [Google Scholar]


