Abstract
Background:
Postoperative bile leak secondary to a fistula is a known complication of hepatic surgery. Four different biliary fistula sub-types have been described: type A refers to minor leakage from the bile duct stump; type B to major leakage caused by insufficient closure of the bile duct stump; type C to major leakage caused by injury to the bile duct, and type D (the rarest) to the division and exclusion of a bile duct. This complication results from functional liver parenchyma in which bile drainage is excluded from the main duct.
Methods:
A retrospective review of the database for 163 patients diagnosed with post-hepatic surgery bile leak from April 1992 to June 2007 was performed.
Results:
Three patients were found to have type D biliary fistula, with durations of 3–21 months. The bile leak developed after a right hepatectomy in two patients and a right hepatectomy extending to segment IV in one patient. All three patients were rescheduled for surgical exploration, following failure of medical treatment. The procedure consisted of repeat resection of the independent liver parenchyma containing the fistula. One patient developed a postoperative leak from a hepaticojejunal anastomosis (treated conservatively) and the other two patients had an uneventful recovery. No recurrence of bile leak was encountered during their follow-up.
Conclusions:
Our experience indicates that conservative treatment is deceptive and not efficacious. For this condition, surgical intervention is the treatment of choice because it is very effective and is associated with a low morbidity.
Keywords: excluded bile duct, bile leak, liver resection
Introduction
Postoperative morbidity following liver surgery has decreased substantially in the last decade.1–3 However, because surgical procedures have become increasingly aggressive, complications such as biliary leaks continue to be reported with incidences in the range of 2.6–15.6%.4–7 Indeed, such complications carry higher rates of morbidity (in terms of sepsis, liver failure, longer hospital stay), as well as postoperative mortality.7
To date, the best classification of biliary fistula derives from Nagano et al.,5 who described four types of fistula as follows: type A refers to minor leakage from the bile duct stump; type B to major leakage caused by insufficient closure of the bile duct stump; type C to major leakage resulting from injury to the bile duct, and type D to the division and exclusion of a bile duct. Type D, or excluded segmental bile duct leakage (ESBDL), results from functional liver parenchyma in which the bile duct is divided and excluded from the main biliary tree. This is the rarest type and is difficult to diagnose, as well as to treat. In thispaper, we report our treatment strategy as well as our results in this rare situation.
Materials and methods
Definition
The Nagano type D fistula is a bile leak resulting from a small, functional segregated segment of liver in which the individual bile duct is divided and excluded from the main biliary tree.5 The nomenclature of segments and types of liver resection follow the Brisbane 2000 terminology.8
Diagnosis requires the presence of:
bile drainage from abdominal wound and/or drain, with a total bilirubin level of >5 mg/ml or three times the serum level;
intra-abdominal collection of bile confirmed by percutaneous drainage, and
cholangiographic evidence of bile leakage.
Patients
From April 1992 to June 2007, 163 of 2409 (6.7%) patients submitted for liver resections were diagnosed with postoperative bile leak.
Based on the classification by Nagano et al.,5 of the initial group of 163 patients treated for postoperative bile leak in our department, three (1.8%) were diagnosed with type D biliary fistula. Epidemiology and treatment indications are summarized in Fig. 1.
Figure 1.
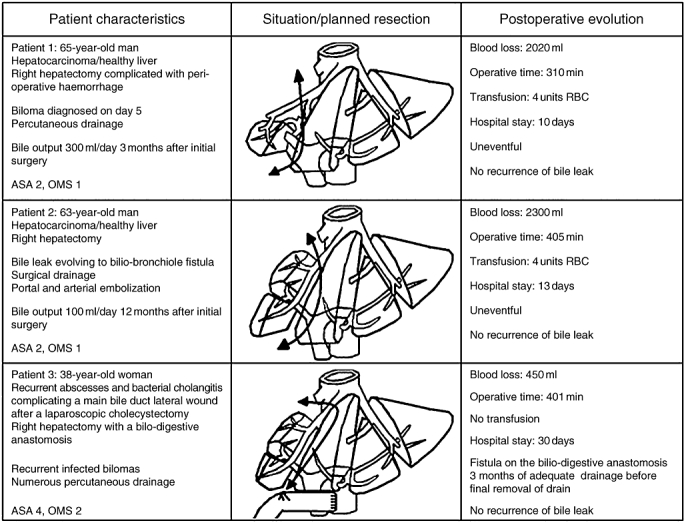
Illustration of patients' clinical histories, planned resections and postoperative courses
Patient 1
The first patient underwent a hepatectomy (in a different surgical centre) for a 10-cm non-cirrhotic hepatocellular carcinoma (HCC) involving segments V, VI, VII and VIII and part of segment IV. The resection started with extrahepatic ligation of the right portal structures (bile duct, artery and vein). During the procedure considerable bleeding was encountered as a result of accidental tumour rupture, resulting in a quick resection because no good vascular control could be achieved. On postoperative day 5, this patient developed a 12-cm biloma. A percutaneous drain was inserted and retained for 3 months, during which the patient was regularly monitored by ultrasound imaging. During this period, the fistula output remained high (∼300 ml/day); however, there were no other associated symptoms. The patient was referred to our department for further management of this complication.
Patient 2
The second patient underwent a hepatectomy (in a different surgical centre) for a 7-cm non-cirrhotic HCC, involving the posterior part of the right liver and infiltrating the diaphragm. From the initial surgical report it was apparent that the right hepatectomy had been preceded by an unusual ligation of two right bile ducts that joined the main bile duct in its extrahepatic portion. This procedure was performed before transecting the liver parenchyma. During the intervention the diaphragmatic part infiltrated by the tumour was resected together with the right lobe of the liver. The diaphragmatic defect was then primarily repaired. On postoperative day 10, this patient presented with pulmonary effusion and sepsis, which did not respond to antibiotic treatment. A subsequent development of bilioptysis indicated that a biliary pleural fistula had developed. As a result of sepsis aggravation, the patient was reoperated (21 days after the first surgical intervention) with the intention of identifying and draining the fistula on the remaining liver parenchyma. During the intervention (exploratory laparotomy), the site of the fistula was drained after attempting a closure, and the abdomen was washed and closed up. By contrast, the collection in the chest (probably facilitated by the resection of the diaphragm) was simply drained via a large-bore chest tube. This was decided on the basis that if the biliary fistula was controlled, the biliary pleural fistula would also settle.
One month later, because of recurrence of bilioptysis, a third surgical intervention was performed, this time involving a thoraco-laparotomy (thoraco-abdominal incision) in an attempt to close the biliary pleural fistula. Intraoperatively, it was possible to localize and reclose the biliary fistula. An abdominal drain was placed between the liver and the diaphragm. The thoracotomy instead revealed a collection with a very inflamed and atelectatic lower lobe of the right lung. After washing, the chest tube was replaced, the earlier diaphragmatic repair reinforced, and the thorax was closed. No pulmonary resection was attempted.
However, 2 months after the third procedure, the patient was referred to our department for further management of a persistent biliary leak (∼200 ml/day) from the abdominal drain.
Patient 3
The third patient underwent a hepatectomy in our department for the treatment of a hepatic abscess and right portal pylephlebitis secondary to recurrent cholangitis as a consequence of a stenotic hepaticojejunal anastomosis. The initial bilioenteric anastomosis had been performed 3 years earlier (in another department), following an iatrogenic injury (during laparoscopic cholecystectomy) to the main bile duct as well as the right hepatic artery.
A right liver resection was performed in this patient because of right lobe atrophy, local sepsis and the fact that it was impossible to utilize the damaged bile duct for a new hepaticojejunal anastomosis. At the end of the hepatectomy, the new hepaticojejunal anastomosis was created using the left bile duct. The postoperative period was marked by development of a biliary leak, which, in turn, was complicated by biliopleural fistula following the removal of the abdominal drain 3 months after the hepatectomy. This complication was treated by the percutaneous abdominal drainage of bilomas formed as a consequence of the fistula. Over the following 18 months, this patient required several hospital admissions for recurrent bilomas, for which repeated percutaneous drainage was necessary.
Diagnostic investigations
All patients underwent a number of investigations including computed tomography (CT) scan, magnetic resonance imaging (MRI), percutaneous transhepatic cholangiography (PTC) and endoscopic retrograde cholangiography pancreatography (ERCP).
Results
Diagnosis of ESBDL
All three patients had positive bile cultures, but only the third patient developed septicaemia which required antibiotic treatment prior to surgery. This patient also developed severe malnutrition. None of the patients had preoperative jaundice. All patients had drains in situ at the time of the final treatment, with output in the range of 100–300 ml/day.
In the first patient, preoperative CT scan demonstrated a remnant of segment VI of the liver. An ERCP did not show any leak from the left biliary system or any communication between the left biliary system and the drain.
In the second patient, a fistulogram proved to be the diagnostic investigation. It clearly demonstrated that the fistula resulted from a distal leak of the bile duct draining the anterior sector of the right liver. This large bile duct was closed proximally during the first hepatectomy. Percutaneous drainage of the involved bile duct was performed, with the aim of preventing intra-abdominal bile collection formation (Figs 2 and 3). A subsequent ERCP indirectly supported the diagnosis as it failed to demonstrate a communication between this duct and the remaining biliary tree.
Figure 2.
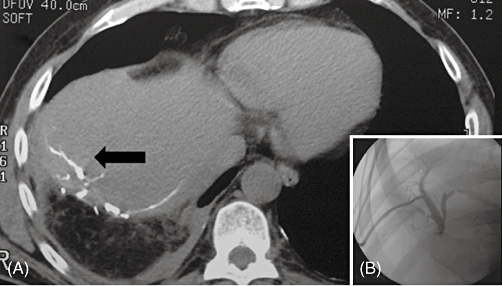
(A) Plain computed tomography performed after percutaneous cholangiography shows a communicating biliary fistula adjacent to the resected surface of the liver (black arrow). (B) Percutaneous cholangiography demonstrating no communication between a dilated intrahepatic bile duct and the principal bile duct
Figure 3.
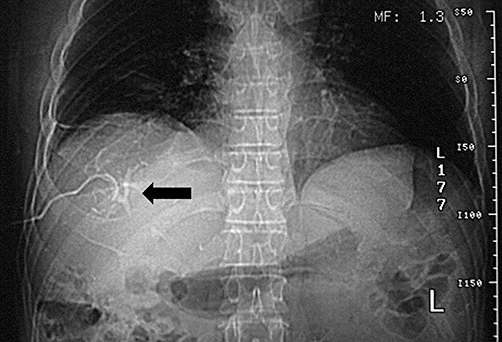
Demonstration of the right anterior branch of the biliary system by fistulography. A distal injury to the bile duct was responsible for the non-communicant biliary fistula. The bile duct which was ligated proximally during the initial hepatectomy was mistakenly preserved as part of the anterior segment of the right liver
Lastly, in the third patient, who had recurrent bilomas, a CT scan demonstrated an isolated dilatation of a segmental bile duct on the resected site of the liver. Adjacent to it, a collection extending up into the costophrenic reflexion of the parietal pleura (Fig. 4) was noted. This information, coupled with a very suggestive clinical history, gave us enough certainty to conclude that the source of the leak was a functional hepatic remnant, the isolated duct of which was completely excluded from the remaining biliary tree.
Figure 4.
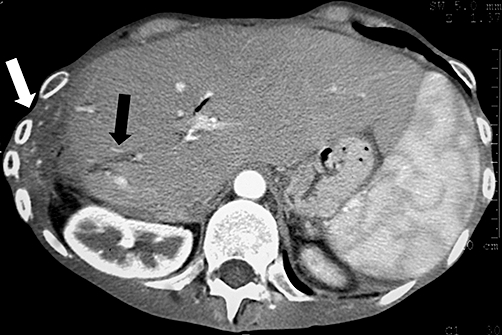
Computed tomography demonstrating a dilated duct in isolation (black arrow), from which the biliary fistula originated. The associated bile collection extended in an upward direction as far as the costophrenic reflection of the parietal pleura (the inferior part of the collection is marked by the white arrow)
Portal and arterial embolization
In the second patient, an embolization of the anterior branch of the right portal vein was performed. This procedure decreased the biliary flow from 200 ml/day to 100/ml/day. One month later, a selective arterial embolization of the anterior sector was carried out. However, despite lowering the fistula output, the patient continued to drain around 80 ml/day of bile.
Planned resection
Given the results of the investigations, all three patients were scheduled to undergo liver resection.
In the first patient, a resection of the remnant parenchyma of segments V–VIII as well as a part of segment IV was planned.
The planned procedure for the second patient involved resection of the remnants of segment VI. In the third patient, a liver resection (removal of the remains of segments V–VIII) with a new hepaticojejunostomy was planned.
The planned operations are summarized in Fig. 1.
Intraoperative course
All patients required extensive adhesiolysis as an initial step in the procedure. This was followed by hilar dissection, which was possible only in two patients. In one patient this step was not performed because of fibrosis and bleeding. Following the adhesiolysis, intraoperative ultrasonography (IOUS) was performed, with the aim of identifying the excluded segments, as well as their relationships with important vascular structures. A thorough examination was also carried out to exclude malignant recurrence; no recurrences were identified in any of the three cases. The resections were performed according to the preoperative plan using the technique described by Bismuth.9 Non-selective pedicular intermittent clamping was utilized in two patients (2 × 15 min, 6 × 10 min), but not in the third patient because hilar dissection was not possible. Average blood loss was 1590 ml (450–2300 ml) and two patients required transfusion of 4 units each.
Median operative time was 392 min (range 310–465 min).
Postoperative course
The first two patients had an uneventful postoperative recovery. Their drains were removed on postoperative days 5 and 6, respectively, and they were discharged from hospital on postoperative days 10 and 13, respectively. No evidence of fistula recurrence was seen at the time of discharge.
The patient who underwent the repeat hepaticojejunostomy developed a postoperative anastomotic leak. This was treated conservatively by keeping the drain inserted during the intervention in situ. This patient had no further problems and was discharged on postoperative day 30. Eventually the drainage completely ceased, after which the drain was removed without any problems.
Histology
No evidence of malignancy was found in the resected specimens and sampled lymph nodes. However, all specimens had evidence of cholangitis, which, in the case of the third patient, was associated with micro-abscesses. Histological evidence of secondary biliary cirrhosis involving only the resected remnant parenchyma was also seen, confirming the diagnosis.
Discussion
Excluded segmental bile duct leakage is an exceptional complication. It is believed that this condition can result from a failure to recognize anatomical variations of the biliary system or if the anatomy of the liver is not strictly respected during the liver resection. As a consequence, part of the liver parenchyma with its biliary ducts becomes isolated from the main biliary tree, while retaining a normal intrahepatic vascularization.
This complication should be suspected when a persistent bile leak or recurrent bile collections develop in the absence of a distal obstruction of the main bile duct. An ERCP followed by a fistulography are the two most frequently used initial investigations. The ERCP helps to exclude a fistula originating from the main biliary tree. Defining the main biliary tree without contrast extravasation in the presence of a biliary leak (fistula) would indicate that the leak originates from liver parenchyma with an isolated and independent biliary duct. This aspect is important because, in such cases, there is no indication for papilotomy and stenting. By contrast, demonstration by the fistulogram of a fistula track which does not communicate with the main biliary tree will certainly confirm the diagnosis. In the event that the fistulogram fails to demonstrate the fistula, magnetic resonance cholangiography (MRC) should be performed. Recent improvements in MRC are producing impressive anatomical reconstructions which facilitate diagnosis and often negate the need for an invasive ERCP procedure. Similarly, a spiral CT scan can contribute to the diagnosis and is particularly useful in identifying the portion of the isolated liver parenchyma in which the fistula is localized and in confirming or excluding the presence of intra-abdominal collections.
In general, more than one investigation modality is required to diagnose this type of fistula. Our experience indicates that the information obtained by a fistulogram combined with that of ERCP or MRC can diagnose this condition in the majority of cases.
Treatment is not easy and a number of strategies have been proposed. The following reports (Table 1) reflect the different approaches utilized.
Table 1.
Reported cases of excluded segmental bile duct leakage
| Author | Year | Hepatectomies, n | ESBDL, n (%) | Treatment | Outcomes |
|---|---|---|---|---|---|
| Lo et al.6 | 1998 | 1222 | 2 (0.16%) | Surgery | 1 death, 1 resolution |
| Yamashita et al.4 | 2001 | 781 | 3 (0.4%) | Ethanol injection | 3 resolutions |
| Tanaka et al.10 | 2002 | 363 | 2 (0.5%) | Ethanol injection | 2 resolutions |
| Nagano et al.5 | 2003 | 313 | 1 (0.3%) | Surgery | 1 resolution |
ESBDL, excluded segmental bile duct leakage
Lo et al.6 reported two cases observed among 1222 patients with intractable postoperative biliary leak. Both cases were operated after undergoing non-therapeutic ERCP and sphincterotomy, suggesting again that ERCP in such circumstances has only a diagnostic value.
Nagano et al.5 reported only one case of ESBDL in their series of 313 patients. They performed a repeat liver resection on the patient and, indeed, considered this approach as ideal. They also stressed that caution should always be taken with repeat resection procedures and proposed that a less invasive treatment with ethanol or fibrin glue injection into the damaged bile duct should be attempted as a first-line strategy, and that surgery should be regarded as a second-line treatment (in refractory cases). A minimally invasive technique was used successfully by Tanaka et al.10 They injected fibrin glue directly into the bile duct in two patients diagnosed (by fistulography) with ESBDL. However, this treatment demands that two criteria should be fulfilled: the fistula must be free of infection and output must be <50 ml/day.
An alternative procedure has been reported by Yamashita et al.4 They described the use of ethanol injection therapy in three patients with postoperative bile leak, resulting in a complete resolution of fistula drainage at 3–6 weeks following the treatment. Others units have reported similar experiences and results using the ethanol injection technique to treat cases of ESBDL.11,12
Portal vein embolization is another alternative treatment, the use of which has been reported in three cases of biliary fistula.13 Although the results of this technique were reportedly good, our experience with one patient was unsatisfactory as the method failed to stop the drainage.
Liver resection, although difficult, is the definitive treatment.6 Contributing factors to the difficulties encountered include adhesions, infection or abscess formation, and anatomical distortions brought about by the regeneration of the remaining liver and the anatomical errors of the first operation. In these circumstances, any surgical intervention should strictly adhere to anatomical landmarks in order to avoid repeat complications.
In order achieve good results, such operations should be carried out in specialized units experienced in hepatobiliary surgery and in IOUS assessment.14 Like the patients reported by Nagano et al.,5 our three reoperated patients achieved very good results with complete resolution of bile leaks.
Conclusions
Diagnosis of EBDSL is difficult but the condition should be suspected in cases of intractable bile leak and/or recurring abdominal bile collections.
Extensive interventional and radiological investigations such as ERCP, fistulography, CT and ultrasound are necessary to diagnose and identify the site of the bile fistula. A number of non-surgical treatments, including fibrin glue or ethanol injection of the bile duct and portal vein embolization, have been described. However, surgical intervention remains the mainstay treatment. It is effective, is associated with low morbidity and should be considered for patients in whom non-surgical interventions are either unsuccessful or not feasible.
Conflicts of interest
None declared.
References
- 1.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. doi: 10.1001/archsurg.138.11.1198. discussion 1206. [DOI] [PubMed] [Google Scholar]
- 3.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708. doi: 10.1097/01.sla.0000141195.66155.0c. discussion 708–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamashita Y, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Shimada M, et al. Bile leakage after hepatic resection. Ann Surg. 2001;233:45–50. doi: 10.1097/00000658-200101000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagano Y, Togo S, Tanaka K, Masui H, Endo I, Sekido H, et al. Risk factors and management of bile leakage after hepatic resection. World J Surg. 2003;27:695–698. doi: 10.1007/s00268-003-6907-x. [DOI] [PubMed] [Google Scholar]
- 6.Lo CM, Fan ST, Liu CL, Lai EC, Wong J. Biliary complications after hepatic resection: risk factors, management, and outcome. Arch Surg. 1998;133:156–161. doi: 10.1001/archsurg.133.2.156. [DOI] [PubMed] [Google Scholar]
- 7.Capussotti L, Ferrero A, Vigano L, Sgotto E, Muratore A, Polastri R. Bile leakage and liver resection: where is the risk? Arch Surg. 2006;141:690–694. doi: 10.1001/archsurg.141.7.690. discussion 695. [DOI] [PubMed] [Google Scholar]
- 8.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- 9.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–9. doi: 10.1007/BF01656368. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka S, Hirohashi K, Tanaka H, Shuto T, Lee SH, Kubo S, et al. Incidence and management of bile leakage after hepatic resection for malignant hepatic tumours. J Am Coll Surg. 2002;195:484–489. doi: 10.1016/s1072-7515(02)01288-7. [DOI] [PubMed] [Google Scholar]
- 11.Kyokane T, Nagino M, Sano T, Nimura Y. Ethanol ablation for segmental bile duct leakage after hepatobiliary resection. Surgery. 2002;131:111–113. doi: 10.1067/msy.2002.118711. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto T, Iwaki K, Hagino Y, Kawano K, Kitano S, Tomonari K, et al. Ethanol injection therapy of an isolated bile duct associated with a biliary-cutaneous fistula. J Gastroenterol Hepatol. 2002;17:807–810. doi: 10.1046/j.1440-1746.2002.02661.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamakado K, Nakatsuka A, Iwata M, Kondo A, Isaji S, Uemoto S, et al. Refractory biliary leak from intrahepatic biliary-enteric anastomosis treated by selective portal vein embolization. J Vasc Interv Radiol. 2002;13:1279–1281. doi: 10.1016/s1051-0443(07)61980-0. [DOI] [PubMed] [Google Scholar]
- 14.Castaing D, Garden OJ, Bismuth H. Segmental liver resection using ultrasound-guided selective portal venous occlusion. Ann Surg. 1989;210:20–23. doi: 10.1097/00000658-198907000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]


