Although the association of partnership concurrency and HIV prevalence has been studied in sub-Saharan settings, the impact of concurrency on HIV transmission has not. We investigated the association between concurrency and HIV serodiscordance in 142 ongoing marital and nonmarital relationships in which both partners were traced and tested for HIV. Our results suggest that multiple concurrent partnerships significantly increase exposure to HIV infection in the population of Likoma (Malawi). We highlight the potential role of behavioral interventions addressing partnership concurrency for HIV prevention.
Mathematical models suggest that partnership concurrency may significantly increase rates of HIV transmission within a population [1]. Transmission is amplified because concurrent partnerships immediately expose multiple partners to the risk of HIV infection, thereby creating potentially large sexual networks through which HIV can circulate extensively. Shelton [2] has recently suggested that such multiple concurrent partnerships (MCPs) may thus be the ‘key driver’ of generalized HIV epidemics affecting southern African countries. Large-scale behavioral interventions addressing this pattern of sexual mixing are currently being advocated and rolled-out [3].
In US settings, researchers have used partner tracing designs (i.e. designs in which the partners of an index case are traced and their disease status is ascertained) to show that the odds of transmitting sexually transmitting infections (STIs) were significantly higher among index cases with MCP (e.g. [4,5]). In sub-Saharan settings, several studies have established the high prevalence of MCP in countries affected by generalized HIV epidemics [6]. However, the role of MCP in the transmission of HIV has not yet been examined with empiric data from HIV-infected individuals. This is the case because sexual behavior surveys conducted in sub-Saharan populations have not been based on partner-tracing designs (e.g. DHS). Such individual-centered studies only allow ascertaining the association between concurrency and HIV acquisition, not transmission [7]. In this study, we use unique data from a sexual network study [8] to test whether MCPs are more likely to be HIV-serodiscordant than serially monogamous partnerships.
The Likoma Network Study (LNS) conducted a sexual network survey with 923 respondents in seven villages of Likoma Island, in the northern region of Lake Malawi [8,9]. Respondents were asked to identify up to five of their most recent sexual partners during an audio computer-assisted self-interview (e.g. [10]). The sexual partners identified were then traced in rosters of island residents and all data were linked. The HIV status of consenting respondents was ascertained using two rapid tests (Determine and Unigold) by a team of local health workers. In total, we tested 597 of the 923 survey respondents, and we identified 48 cases of HIV infection (HIV prevalence = 8%). As a result of this design, we can establish the HIV status of both sexual partners in a sub-sample of the relationships identified during the survey. We define as a concurrent any sexual relationship in which at least one of the two partners had another partner at the time of the survey. We define as serial any sexual relationship in which neither partners had any other partner at the time of the survey. Our analyses first examine the characteristics of sexual relationships in which both partners were tested for HIV infection. We then describe the association between partnership concurrency and HIV serodiscordance among this sub-sample of sexual relationships.
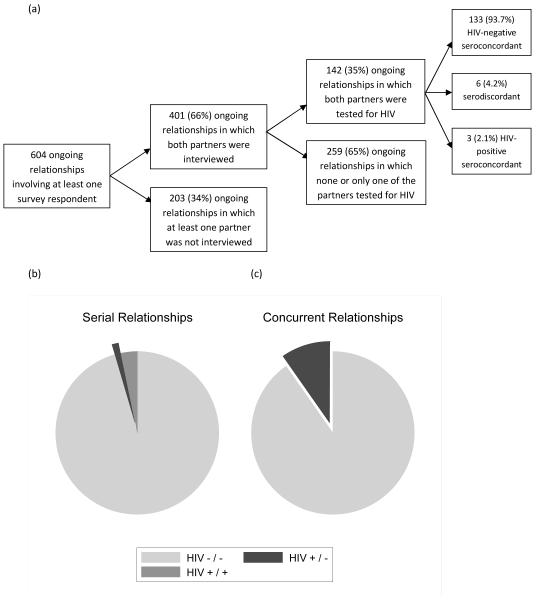
During the survey, respondents reported 776 sexual relationships defining 604 unique ongoing sexual relationships (some relationships were reported by both partners). Figure 1 Figure 1a describes attrition at different stages of the linking process. We ascertained the sexual behaviors of both partners in 401 relationships (66.4%). Two hundred and fifty-one (63%) relationships in which both partners were interviewed were concurrent. One hundred and forty-two relationships involved partners who were both tested for HIV infection during the survey (35%). Concurrent relationships were significantly less likely to involve two partners who were both tested during the LNS. Fifty-seven of the 251 concurrent relationships (22.7%) involved two partners who were both tested during the survey. On the contrary, 85 of the 150 serial relationships (56.7%) involved partners who were both tested during the survey (P < 0.001).
Fig. 1.
HIV-serodiscordance in ongoing sexual relationships identified during the Likoma Network Study, by concurrency status of the relationship. gr1
Panel (a) describes the attrition rates at various stages of the Likoma Network Study. Panels (b) and (c) describe patterns of HIV serodiscordance in serial (b) and concurrent (c) relationships. The differences between serial and concurrent relationships were significant at the P = 0.02 level (according to Fisher’s exact test). Source: Likoma Network Study.
Among relationships in which both partners were tested for HIV infection, 133 (93.7%) involved two partners who were HIV-negative according to our testing protocol, and 3 (2.1%) relationships involved two partners who were HIV-positive. Six (4.2%) relationships were serodiscordant, that is involved one partner infected with HIV and one susceptible partner. Patterns of HIV serodiscordance, however, varied between concurrent and serially monogamous relationships (Fig. 1b and c). Whereas only one serial relationship was serodiscordant (1.2%), this was the case for five (8.8%) concurrent relationships. Three (3.5%) serial relationships were HIV-positive seroconcordant, whereas no concurrent relationships were found to be so. These differences were significant at the P = 0.02 level according to Fisher’s exact test.
Our findings thus support previous suggestions derived from mathematical models that partnership concurrency may significantly increase the risk of HIV transmission in sub-Saharan populations. Our study, however, has important limitations. First of all, the small sample sizes limit inferences about the association of partnership concurrency and HIV serodiscordance in sexual partnerships. For example, our data do not allow identifying whether the relative risk of transmission associated with concurrent partnerships varies by sex or age of the index case. Second, a high proportion of concurrent partnerships traced during our study involved partners whose HIV status was not ascertained by the research team. This sample selection bias may limit the validity of our inferences regarding the association of partnership concurrency and HIV serodiscordance [7]. Third, our data do not allow classifying individuals as ‘transmitters’ or ‘receivers’ of infection. This limitation stems from the fact that our data are cross-sectional and the biomarkers of HIV infection we used were limited to the detection of HIV antibodies (and thus did not allow identifying recent acute HIV infection). Studies that have estimated precisely the impact of concurrency on the transmission of a pathogen in a population were either based on prospective research designs (e.g. [4]) or were able to classify connected cases of a disease by stage of disease progression (e.g. [5]).
Despite these limitations, however, the design of this study (based on partner tracing) enables us for the first time to identify the increased risk of HIV transmission that may be associated with concurrent relationships. Building on this unique feature, our study indicates that behavioral change interventions addressing patterns of partnership concurrency may have a significant impact on the incidence of HIV in sub-Saharan populations affected by generalized HIV epidemics. Further studies integrating partner tracing and longitudinal follow-up are needed to adequately design and target such interventions.
Acknowledgements
S.H. and H.P.K. designed the Likoma Network Study. S.H. conducted the analysis. S.H. and LKP drafted the manuscript. All authors reviewed and approved the manuscript.
References
- 1.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11:641–648. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Shelton J. Ten myths and one truth about generalised HIV epidemics. Lancet. 2007;370:1809–1811. doi: 10.1016/S0140-6736(07)61755-3. [DOI] [PubMed] [Google Scholar]
- 3.Southern African Development Community . Expert think-tank meeting on HIV Prevention in High-Prevalence Countries in Southern Africa. Maseru, Lesotho: 2006. [10–12 May 2006]. Report available at: http://www.healthdev.org/eforums/Editor/assets/accelerating-prevention/SADC_Think%20tank%20HIV%20meeting_Report_May2006.pdf. [Google Scholar]
- 4.Potterat JJ, Zimmerman-Rogers H, Muth SQ, Rothenberg RB, Green DL, Taylor JE, et al. Chlamydia transmission: concurrency, reproduction number, and the epidemic trajectory. Am J Epidemiol. 1999;150:1331–1339. doi: 10.1093/oxfordjournals.aje.a009965. [DOI] [PubMed] [Google Scholar]
- 5.Koumans E, Farley T, Gibson J, Langley C, Ross M, McFarlane M, et al. Characteristics of persons with syphilis in areas of persisting syphilis in the United States: sustained transmission associated with concurrent partnerships. Sex Transm Dis. 2001;28:497–503. doi: 10.1097/00007435-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Halperin D, Epstein H. Concurrent sexual partnerships help to explain Africa’s high HIV prevalence: implications for prevention. Lancet. 2004;364:4–6. doi: 10.1016/S0140-6736(04)16606-3. [DOI] [PubMed] [Google Scholar]
- 7.Morris M. Concurrent partnerships and syphilis persistence: new thoughts on an old puzzle. Sex Transm Dis. 2001;28:504–507. doi: 10.1097/00007435-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Helleringer S, Kohler H-P. Sexual network structure and the spread of HIV in Africa: evidence from Likoma Island, Malawi. AIDS. 2007;21:2323–2332. doi: 10.1097/QAD.0b013e328285df98. [DOI] [PubMed] [Google Scholar]
- 9.Helleringer S, Kohler H-P, Chimbiri A, Chatonda P, Mkandawire J. The Likoma Network Study: context, data collection and initial results. University of Pennsylvania, Population Studies Center; 2007. PSC Working Paper Series PSC 07-05. http://repository.upenn.edu/pscworkingpapers/9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mensch BS, Hewett PC, Erulkar A. The reporting of sensitive behavior among adolescents: a methodological experiment in Kenya. Demography. 2003;40:247–268. doi: 10.1353/dem.2003.0017. [DOI] [PubMed] [Google Scholar]