Abstract
Objective
To examine if early weight gain in depot medroxyprogesterone acetate (DMPA) users predicts continued excessive weight gain and to identify risk factors of early weight gain in DMPA users.
Methods
DMPA users (N=240) were assessed prior to initiating contraception and every 3 months for 36 months. Early weight gain was defined as over 5% baseline weight gain within 6 months of DMPA use. Mean weight gain at 6-month intervals was estimated based on early weight gain status (at or below 5% gain vs. above 5% gain). Multiple logistic and mixed-model regression analyses were used.
Results
About one-fourth of DMPA users had early weight gain. The mean weight gain of the at or below 5% group and above 5% group was 0.63 kg and 8.04 kg, 1.48 kg and 10.86 kg, and 2.49 kg and 11.08 kg after 12, 24, and 36 months (P < 0.001 at all observations), respectively. Early weight gainers also had a much steeper slope of weight gain over time than the regular weight gainers (0.35 kg/month vs. 0.08 kg/month, P < 0.001). Risk factors for early weight gain were: BMI less than 30 (OR 4.00, 95% confidence interval (CI): 1.513 – 10.455), parity (OR 2.23, 95% CI: 1.040 – 4.761), and self-reported increased appetite after 6 months of DMPA use (OR 3.06, 95% CI: 1.505 – 6.214).
Conclusion
Most DMPA users who gain excessive weight experience more than a 5% weight increase within 6 months. These data help physicians predict who is at risk of excessive gain and counsel them appropriately.
Introduction
Many studies have investigated the beneficial and adverse effects of depot medroxyprogesterone acetate (DMPA) as an injectable contraceptive. Beneficial effects of DMPA use include an efficacy rate of 99%, relatively low cost, and convenience of administration once every three months. However, studies have demonstrated that significant weight gain often occurs as a result of DMPA use (1–6). The amount of weight gain associated with this method is unclear as mean weight gains reported have varied from 3.0 kg after 12 months’ use (5) to 9.4 kg after 18 months’ use (3). Furthermore, most studies have focused on mean weight gain and not patterns of weight change to determine who is at risk of gaining weight on DMPA. The few studies that have reported range of weight change showed much variability: −5 kg to +12 kg at 12 months (5) and −25 lbs to + 69 lbs at 6 months (7). Given the range of weight change associated with DMPA use, we wanted to examine if stratifying DMPA users by weight gain group would be a clinically useful measure for predicting continued weight gain and who is at risk for weight gain.
We focused our investigation on detecting which DMPA users are at risk of gaining excessive weight because large amounts of weight gain can lead to other health problems. Studies have defined excessive weight gain as gaining more than 10 pounds over a 6-month period (7) or as >5% baseline weight gain at 3 months (5). We used >5% baseline weight gain as our measure of excessive weight gain because our participants started at differing weights and body mass index (BMI). This prospective, longitudinal study examined the hypothesis that early weight gain, as defined as >5% increase in body weight within 6 months of DMPA use, is predictive of continued weight gain. First, we identified the earliest time point at which weight gain would be predictive over time. Then, we examined if DMPA users’ weight gain status was predictive of continued weight gain over the next 30 months. Finally, we explored risk and protective factors associated with early weight gain at 6 months’ DMPA use.
Materials and Methods
As part of a larger study, 805 non-Hispanic Black, non-Hispanic White, and Hispanic women between 16 and 33 years of age were recruited between October 9, 2001, and September 14, 2004. The methods for the larger study are reported in detail elsewhere (8). Briefly, recruitment was conducted to achieve a sample that was balanced by age group (16–24 years and 25–33 years), race (Black, White, Hispanic) and contraceptive method: nonhormonal (NH), oral contraceptives (OCPs), and injections (DMPA). All women underwent eligibility screening including a medical interview, anthropometry, bone density scan, and fasting phlebotomy during the follicular phase of their menstrual cycle. Criteria for exclusion included current pregnancy or breastfeeding; pregnancy planned within the next 3 years; use of DMPA within the past 6 months; use of OCPs within the past 3 months; current use of hormonal intrauterine device; contraindication to hormonal contraception; lack of menses for >3 months within the past year; bilateral oopherectomy; use of over-the-counter phytoestrogens (e.g., Estroven, Promensil); dietary isoflavone intake exceeding 84 mg per day; and eating disorder or strict vegetarian diet. Of the 805 women, five withdrew before completing the first visit, 92 had abnormal laboratory results, and five had T-scores < −2.5 on their bone scans. Thus, baseline data for the larger study were analyzed for 703 women. Those excluded (n=102) did not differ from women included in the analyses (N=703) on age, marital status, parity, or education (all P > .05).
At the first visit, standardized counseling was provided regarding the risks and benefits of contraceptives. After this discussion, women chose one of the following methods for contraception during the following 36 months: OCPs (0.15 mg desogestrel+20 micrograms ethinyl E2 for 21 days followed by 2 days of placebo and 5 days of 10 micrograms ethinyl E2), DMPA, or nonhormonal contraceptives. A total of 245 women selected OCPs, 240 chose DMPA, and 218 chose nonhormonal contraceptives. Comprehensive follow-up visits (e.g., phlebotomy, urine pregnancy testing, written questionnaires including measures for eating behaviors, calcium checklist and bone densitomery) were conducted every 6 months following the initial interview while contraceptives were dispensed every 3 months and minor follow-up visits (i.e., anthropometry) were conducted every 3 months up to 36 months after the baseline visit. To obtain estimates on daily calorie intake along with amount of protein, fat, and carbohydrate consumed, a registered dietician conducted a 24-hour dietary recall interview with each participant annually.
Our analyses focused only on the DMPA users (N=240). Of the 240 initial users, 182 discontinued DMPA use sometime during the next three years, with 58 completing the study. There were no differences in baseline characteristics between DMPA users who remained in the study (n=58) and those who did not complete the study (n=182) with regard to age, race, BMI, and lifestyle variables (all P > .05). The DMPA users were classified into two groups based on percentage of body weight gained at 3 or 6 months (≤ 5% and > 5%). Those who gained more than 5% of their body weight were classified as early weight gainers. Other participants were classified as regular gainers. All procedures were approved by the Institutional Review Board of the University of Texas Medical Branch.
Student’s t tests for continuous variables and chi-square tests for categorical variables were performed to compare the two groups at baseline. Preliminary analyses and multiple logistic regression analyses were conducted using SPSS (SPSS 16.0, SPSS Inc., Chicago, IL) to identify the correlates of early weight gain. Variables were screened for inclusion in an initial multivariable logistic regression model. Candidate variables with P < 0.20 were retained. The Hosmer-Lemeshow test (9) and area under the receiver operating characteristics (ROC) curve were used to assess the fit and predictive ability of the final model. We also used longitudinal analysis to examine the predictive ability of early weight gain status for later weight gain over the course of the study. To accommodate the repeated measures, the data were modeled using Stata’s mixed effects regression procedure (xtmixed module, STATA10, Stata Corporation, College Station, TX) which allowed us to obtain modeled weight estimates for the predictor while adjusted for the estimated errors for the repeated measurements. This class of model allows inclusion of time-dependent covariates and accommodates participants with incomplete data owing to variation in number and spacing in observations over the period of follow-up, which frequently occurs in longitudinal studies. The outcome variable used in this longitudinal model was changes in body weight as measured every 6 months for 36 months. We modeled weight change at each 6-month increment across 36 months by weight gain status (≤5% gain vs. >5% gain), after adjusting for baseline weight, race, age, parity, and physical activity. To assess the consistency of the trends across time for the two groups, an interaction term between time and the categories of weight gain status (>5% gain vs. ≤5% gain) was also included. A separate mixed model was also constructed to examine the association of total calorie intake with weight gain over 36 months (based on 12-month follow-up data).
A post-hoc power analysis using observed mean differences and standard deviations (SD) demonstrated that this study had greater than 95% power to detect differences between the >5% weight gain and ≤ 5% weight gain group after 36 months of DMPA use. Power calculations were based on the two-sample t test for unequal n using a two-sided significance level of 0.05. The estimates for the means and SDs of the two groups were based on the difference of body weight between baseline and 36 months.
Results
At baseline, the sample (N = 240) included 72 Blacks, 82 Whites, and 86 Hispanics with a mean age of 23.8 years. Two multiple regression models were constructed to identify predictors of future weight gain (i.e., weight gain at 12 months). The predictors were: age, race, marital status, baseline obesity, prior DMPA use, lifestyle variables, and weight gain at 3 months or 6 months. Our first overall model using weight gain at 3 months as a predictor was significant, F (9, 133) = 3.91, P < 0.001, adjusted R2 = .16. Weight gain at 3 months was the only significant predictor, the regression coefficient was 0.92, 95% CI: 0.580 – 1.252, P < 0.001. The second overall model using weight gain at 6 months was also significant, F (9, 133) = 26.12, P < 0.001. However, this model accounted for 64% of the variance in future weight gain. Weight gain at 6 months was the only significant predictor with a regression coefficient of 1.22, 95% CI: 1.049 – 1.397, P < 0.001. Thus, we used weight gain at 6 months as both a predictor and outcome variable in our subsequent analyses. At 6 months, 45 participants dropped out leaving a sample of 195. The 45 participants who dropped out did not differ from the remaining 195 participants with regard to age, race, baseline weight, BMI, or lifestyle variables (all P > .05).
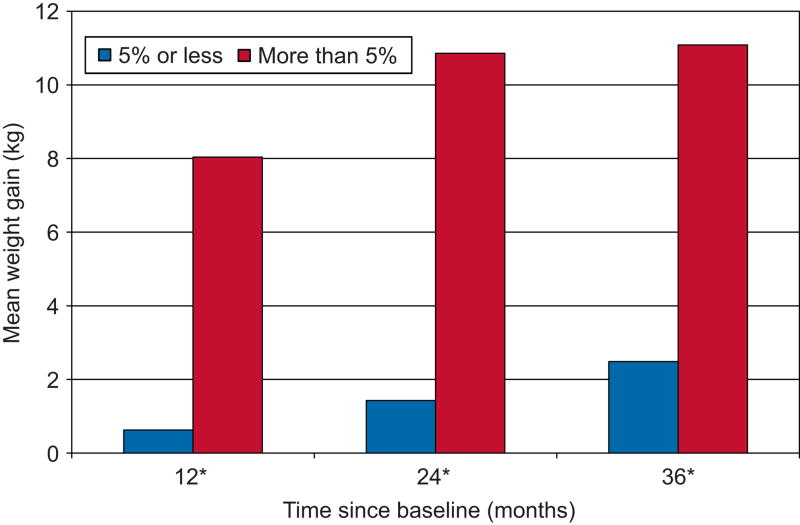
A little more than one-fourth of DMPA users (51 of 195) increased their weight by > 5% at 6 months. There were baseline differences between the ≤ 5% group (i.e., regular gainers) and > 5% group (i.e., early gainers) (Table 1). Early gainers were similar to regular gainers in terms of age, race, previous DMPA use, marital status, alcohol use, smoking, and exercise. However, early gainers were more likely to weigh less and have a BMI < 30 than regular gainers. The mean weight gain of the ≤ 5% group and > 5% group was 0.63 kg and 8.04 kg, 1.48 kg and 10.86 kg, and 2.49 kg and 11.08 kg after 12, 24, and 36 months, respectively (Figure 1). At all three time points, early gainers’ mean weight gain was significantly higher than regular gainers (P < 0.001).
Table 1.
Baseline characteristics of study population according to the weight gain status at 6 months (n=195)
| Characteristic | ≤ 5% Gain (n=144) |
>5% Gain (n=51) |
P |
|---|---|---|---|
| Age (y) | .537 | ||
| 16–24 | 79 (54.9) | 31 (60.7) | |
| 25–33 | 65 (46.1) | 20 (39.3) | |
| Race/ethnicity | .298 | ||
| Black | 40 (27.7) | 20 (39.2) | |
| White | 54 (37.5) | 15 (29.4) | |
| Hispanic | 40 (27.8) | 16 (31.4) | |
| Marital Status | .935 | ||
| Not married | 115 (80.0) | 41 (80.4) | |
| Married | 29 (20.0) | 10 (19.6) | |
| Weight (kg) | 73.99 (±19.31) | 66.58 (± 13.69) | < .05 |
| BMI | 27.97 (± 6.99) | 25.17 (± 5.40) | < .05 |
| Parity | < .05 | ||
| 0 | 64 (44.4) | 14 (27.5) | |
| 1 or more | 80 (56.6) | 37 (72.5) | |
| Initial obesity | < .001 | ||
| No | 90 (62.5) | 45 (88.2) | |
| Yes | 54 (38.5) | 6 (12.8) | |
| Previous DMPA use (mos) | 10.38 (± 6.99) | 8.47 (± 11.71) | .551 |
| Alcohol use | .079 | ||
| No | 116 (80.5) | 45 (88.2) | |
| Yes | 28 (19.5) | 6 (12.8) | |
| Current Smoker | .314 | ||
| No | 96 (66.7) | 30 (58.8) | |
| Yes | 48 (34.3) | 21 (41.2) | |
| Exercise (min/week) | 86 (± 115) | 88 (± 86) | .924 |
Data are n (%) or mean (± standard deviation).
Student’s t tests were used for continuous variables and χ2 tests were used for categorical variables.
Figure 1.
Mean weight gain at 12, 24, and 36 months by weight gain status at 6 months. Differences* were significant at the P < .001 level using Mann-Whitney U test.
Difference in the weight gain between early weight gainers and regular weight gainers based on mixed model regression analysis after adjusting for baseline weight, age, race/ethnicity, previous use of DMPA, parity and physical activity is shown in Table 2. Early weight gainers gained an average of 7.03 kg more body weight over 36 months (95% CI: 5.519–8.541, P < 0.001) compared to that of regular weight gainers. When an interaction term between the weight gain status (early vs. regular) and duration of DMPA use was included, the model showed that early weight gainers had a much steeper slope of weight gain over time than the regular weight gainers (0.35 kg/month vs. 0.08 kg/month, P < 0.001). A separate mixed model based on 12-month follow-up data that included total calorie intake in addition to other covariates, did not show any association between total caloric intake and weight gain over time.
Table 2.
Association of weight gain status with changes in body weight over 36 months based on mixed model regression analysis
| Coefficient | 95% Confidence Interval | P | |
|---|---|---|---|
| Baseline weight | −0.018 | −0.054 to 0.019 | <.339 |
| Weight gain status (1, >5% ; 0, ≤ 5%) | 7.030 | 5.513 to 8.541 | <.001 |
| Age (1, 16–24 y; 2, 25–33 y)1 | −0.129 | −1.806 to 1.547 | .880 |
| Race2 | |||
| Black | 0.565 | −1.078 to 2.207 | .500 |
| Hispanic | 0.194 | −1.345 to 1.733 | .805 |
| Duration of DMPA use (mo) | 0.135 | 0.106 to 0.164 | <.001 |
| Weight bearing exercise (min/week) | −0.001 | −0.004 to 0.002 | .495 |
| Parity | −0.239 | −0.966 to 0.487 | .519 |
| Previous use of DMPA (mo) | −0.002 | −0.037 to 0.032 | .891 |
| Constant | 0.256 | −3.045 to 3.557 | .879 |
Dependent variable: change in body weight over 36 months
Reference group: 16–24-year-olds
Reference category: White
Univariate logistic regression analyses were conducted between each predictor and the outcome measure, early weight gain. Predictors were: age, race, marital status, parity, baseline obesity, prior DMPA use, alcohol use, smoking, exercise, and self-reported increase of appetite. Variables that met the screening criteria for inclusion (P < .20) in the multivariable logistic regression model were race, parity, initial obesity, and increase of appetite. The final logistic model yielded a P-value for the Hosmer-Lemeshow test of 0.81 and the estimated area under the ROC curve was 0.76. As shown in Table 3, DMPA users with a BMI < 30 (OR 4.00, 95% confidence interval (CI): 1.513 – 10.455), those who had given birth at least once (OR 2.23, 95% CI: 1.040 – 4.761), and those who reported to have increased appetite after 6 months of DMPA use (OR 3.06, 95% CI: 1.505 – 6.214) were more likely to have early weight gain.
Table 3.
Predictors of excessive weight gain at 6 months (total n=195, early gainers n=51)
| Predictor | Odds Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| African American1 | 2.291 | .964 – 5.447 | .061 |
| Hispanic1 | 1.198 | .500 – 2.870 | .685 |
| Parity = 1 or more2 | 2.225 | 1.040 – 4.761 | < .05 |
| BMI < 303 | 3.997 | 1.513 – 10.455 | < .01 |
| Increase of Appetite | 3.058 | 1.505 – 6.214 | < .01 |
| Constant | 0.033 | < .001 |
Overall model χ2 = 32.49, P < .001. Model R2 = 0.15
Dependent variable: early weight gain (binary: ≤5%, >5%). Independent variables: race (categorical: White, African American, Hispanic), parity (binary: 0, ≥1), BMI < 30 (binary: BMI < 30, BMI ≥ 30), self-reported increase of appetite (binary: no, yes)
Reference group: White
Reference group: Parity = 0
Reference group: BMI ≥ 30
Discussion
This study provides two levels of predictors of excessive weight gain in DMPA users. It first examined if early weight gain status predicts continued weight gain with DMPA use and then used a multiple predictor model to identify baseline risk factors for early weight gain associated with DMPA. Consistent with previous studies, DMPA use was associated with significant weight gain over time (10;11), although intensity of weight gain was significantly different between the early weight gainers and regular weight gainers. Early weight gainers gained an average of 7.03 kg more body weight over 36 months compared to regular weight gainers.
By using a longitudinal, prospective design, this study adds to the previous literature by identifying the earliest point at which weight gain associated with DMPA use would be excessive and have predictable long-term consequences. The earliest time point at which we were able to statistically predict a trend was 6 months. This 6 month time point is clinically relevant because excessive weight gain can be predicted after only two injections of DMPA, according to our study. Women who had gained > 5% of their baseline weight at 6 months were likely to continue gaining weight with continued use over the next 30 months. This finding is similar to a study conducted by Risser and colleagues (5) that reported female adolescents (aged 13–19) who gained > 5% of baseline weight at 3 months were at high risk at gaining even more weight at 12 months. Our study extends previous findings by using a larger, slighter older sample of women (aged 16–33) and by being able to predict weight gain up to 36 months.
In addition, we found that early weight gainers gained an average of 7.03 kg more body weight over 36 months than regular gainers. This provides support that DMPA users do not all follow the same trajectory of weight gain. Although some will gain minimal amounts of weight, about 25% will gain excessive weight with continued DMPA use. Therefore, clinicians may want to extend contraceptive counseling beyond discussing mean weight gain as a side effect of DMPA. If women choose DMPA, clinicians may want to tailor counseling to each individual user and her risk factors in addition to closely monitoring weight gain at every follow-up visit.
We identified three baseline risk factors for early weight gain: BMI < 30, parity of 1 or more, and self-reported increase of appetite. First, women with BMI < 30 were more likely than obese women (BMI ≥ 30) to experience early weight gain. This contrasts previous reports that overweight young women aged 12–19 were more likely to gain >5 lbs than those of normal weight (3;12). However, these studies were based upon adolescent populations whereas our study used a late adolescent-adult population of women, aged 16–33. This suggests that age may mediate the relationship between initial BMI and excessive weight gain. But we are unable to test this empirically because our sample did not include the younger women (ages, 12–15) represented in the previous reports.
Second, the risk factor of parity of 1 or more is associated with excessive weight gain since number of children has been positively associated with obesity in women (13). Given that women who gain excessive pregnancy weight or who fail to lose pregnancy weight by 6 months post partum are at increased risk of continued, excessive weight gain (14;15), future studies should examine if postpartum status is an additional risk factor of weight gain, in addition to parity.
The third risk factor identified in our study is self-reported increase of appetite after 6 months of DMPA use. This contrasts a previous report which found a self-reported decrease of appetite for Black adolescent females using DMPA, but not for White adolescent females (16). However, these differences in findings may be driven by how self-reported appetite was measured in each study. We simply asked participants whether they had a change in appetite (yes/no) over the last 6 months while Bonny and colleagues (16) used a subscale assessing both cognitive and behavioral components of subjective hunger from the Three-Factor Eating Questionnaire (17). Future studies that assess appetite change as a risk factor of DMPA-related weight gain may want to use multiple measures for eating behaviors.
Given our data, we were unable to shed any light on the mechanism of DMPA-related weight gain. The existing literature does not provide a clear-cut picture either. Our observation that increased appetite was predictive of more weight gain after 6 months DMPA use did not follow increased caloric intake. Other possible mechanisms for DMPA-associated weight gain is its glucocorticoid-like activity (18) or DMPA associated interference with insulin action (19).
This study also examined whether ethnicity is associated with weight gain. Our study sample consisted of balanced groups of Black, White, and Hispanic women. Our findings provide marginal support (P = .06) that Black women were more likely to gain excessive weight over time with continued DMPA use than do White women which is similar to a previous study done using adolescents (16). Future studies should continue to examine this issue as obesity is a significant problem among Black women and places them at higher risk of hypertension and diabetes.
This study has several limitations. First, DMPA users who gained weight at the initial follow-up visit were more likely to drop out at the following visit (2). Thus the amount of weight gain associated with DMPA use may be even greater than we were able to detect in this study. However, we were able to compensate for the differences in number of observations through mixed-model regression analysis. Second, we were not able to include women over 300 pounds, due to the weight limitations of the DXA equipment. Together, these limitations could impact the overall generalizability of our findings and selection bias cannot be ruled out.
In conclusion, the potential for excessive weight gain with DMPA use is probable for some women, but weight gain is not a certainty for all users. For those women who experience ≤ 5% body weight gain with DMPA use in the first 6 months, excessive future weight gain is unlikely and continued DMPA use provides a convenient, highly effective, and relatively inexpensive method of birth control. However, for DMPA users who do experience an early weight gain, continued weight gain may lead to a number of chronic health problems such as diabetes, hypertension, and heart disease. Therefore, clinicians should advise women who gain excessive amounts of weight during the first 6 months of DMPA use of other contraceptive options. With close monitoring and counseling from clinicians, women who choose DMPA can avoid long-term weight changes that may result in obesity-related health problems.
Acknowledgments
Dr. Le is a Kirschstein-NRSA postdoctoral fellow supported by an institutional training grant (T32HD055163) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. Dr. Berenson is supported by R01HD39883 and K24HD043659 the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Presented at the 2009 National Clinical & Translational Research Meeting, April 14–15, 2009, Washington, DC.
References
- 1.Bahamondes L, Del CS, Tabares G, Arce XE, Perrotti M, Petta C. Comparison of weight increase in users of depot medroxyprogesterone acetate and copper IUD up to 5 years. Contraception. 2001 Oct;64(4):223–5. doi: 10.1016/s0010-7824(01)00255-4. [DOI] [PubMed] [Google Scholar]
- 2.Berenson AB, Rahman M. Changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio associated with injectable and oral contraceptive use. Am J Obstet Gynecol. 2009 Mar;200(3):329.e1–8. doi: 10.1016/j.ajog.2008.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonny AE, Ziegler J, Harvey R, Debanne SM, Secic M, Cromer BA. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med. 2006 Jan;160(1):40–5. doi: 10.1001/archpedi.160.1.40. [DOI] [PubMed] [Google Scholar]
- 4.Espey E, Steinhart J, Ogburn T, Qualls C. Depo-provera associated with weight gain in Navajo women. Contraception. 2000 Aug;62(2):55–8. doi: 10.1016/s0010-7824(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 5.Risser WL, Gefter LR, Barratt MS, Risser JM. Weight change in adolescents who used hormonal contraception. J Adolesc Health. 1999 Jun;24(6):433–6. doi: 10.1016/s1054-139x(98)00151-7. [DOI] [PubMed] [Google Scholar]
- 6.Templeman CL, Cook V, Goldsmith LJ, Powell J, Hertweck SP. Postpartum contraceptive use among adolescent mothers. Obstet Gynecol. 2000 May;95(5):770–6. doi: 10.1016/s0029-7844(00)00787-0. [DOI] [PubMed] [Google Scholar]
- 7.Leiman G. Depo-medroxyprogesterone acetate as a contraceptive agent: its effect on weight and blood pressure. Am J Obstet Gynecol. 1972 Sep 1;114(1):97–102. doi: 10.1016/0002-9378(72)90296-7. [DOI] [PubMed] [Google Scholar]
- 8.Berenson AB, Rahman M, Breitkopf CR, Bi LX. Effects of depot medroxyprogesterone acetate and 20-microgram oral contraceptives on bone mineral density. Obstet Gynecol. 2008 Oct;112(4):788–99. doi: 10.1097/AOG.0b013e3181875b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosmer DW, Jr, Lemeshow S. Applied Logistic Regression. 2. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 10.Kaunitz AM, Miller PD, Rice VM, Ross D, McClung MR. Bone mineral density in women aged 25–35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception. 2006 Aug;74(2):90–9. doi: 10.1016/j.contraception.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Schwallie PC, Assenzo JR. Contraceptive use--efficacy study utilizing medroxyprogesterone acetate administered as an intramuscular injection once every 90 days. Fertil Steril. 1973 May;24(5):331–9. doi: 10.1016/s0015-0282(16)39669-8. [DOI] [PubMed] [Google Scholar]
- 12.Mangan SA, Larsen PG, Hudson S. Overweight teens at increased risk for weight gain while using depot medroxyprogesterone acetate. J Pediatr Adolesc Gynecol. 2002 Apr;15(2):79–82. doi: 10.1016/s1083-3188(01)00147-4. [DOI] [PubMed] [Google Scholar]
- 13.Weng HH, Bastian LA, Taylor DH, Jr, Moser BK, Ostbye T. Number of children associated with obesity in middle-aged women and men: results from the health and retirement study. J Womens Health (Larchmt ) 2004 Jan;13(1):85–91. doi: 10.1089/154099904322836492. [DOI] [PubMed] [Google Scholar]
- 14.Linne Y, Dye L, Barkeling B, Rossner S. Long-term weight development in women: a 15-year follow-up of the effects of pregnancy. Obes Res. 2004 Jul;12(7):1166–78. doi: 10.1038/oby.2004.146. [DOI] [PubMed] [Google Scholar]
- 15.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol. 2005 Dec;106(6):1349–56. doi: 10.1097/01.AOG.0000185480.09068.4a. [DOI] [PubMed] [Google Scholar]
- 16.Bonny AE, Britto MT, Huang B, Succop P, Slap GB. Weight gain, adiposity, and eating behaviors among adolescent females on depot medroxyprogesterone acetate (DMPA) J Pediatr Adolesc Gynecol. 2004 Apr;17(2):109–15. doi: 10.1016/j.jpag.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Stunkard A, Messick S. The Three-Factor Eating Questionnaire to measure dietary restraint, disinihibition, and hunger. J Psychosom Res. 1985;29:71–8. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 18.Guthrie GP, Jr, John WJ. The in vivo glucocorticoid and antiglucocorticoid actions of medroxyprogesterone acetate. Endocrinology. 1980 Nov;107(5):1393–6. doi: 10.1210/endo-107-5-1393. [DOI] [PubMed] [Google Scholar]
- 19.Fahmy K, bdel-Razik M, Shaaraway M, al-Kholy G, Saad S, Wagdi A, et al. Effect of long-acting progestagen-only injectable contraceptives on carbohydrate metabolism and its hormonal profile. Contraception. 1991 Oct;44(4):419–30. doi: 10.1016/0010-7824(91)90032-b. [DOI] [PubMed] [Google Scholar]