Abstract
Consumption of the trichothecene mycotoxin deoxynivalenol (DON) induces interleukin-6 (IL-6)-dependent IgA nephropathy (IgAN) in mice. This effect can be prevented by feeding long chain n-3 polyunsaturated fatty acids (PUFAs) found in fish oil. The purpose of this study was to identify the signal transduction pathways by which DON upregulates IL-6 in the peritoneal macrophage and how consumption of fish oil enriched with the n-3 PUFA, docosahexaenoic acid (DHA), suppresses these processes. Incubation with DON induced IL-6 expression in naïve macrophages maximally at 3 h. Knockdown of the transcription factor cAMP response element-binding protein (CREB) or pharmacologic inhibition of the CREB kinases, Akt1/2, MSK1 and RSK1, downregulated this expression. Inhibition of double-stranded RNA-activated protein kinase (PKR) suppressed not only IL-6 expression but also phosphorylation of CREB and its upstream kinases, Akt1, MSK1 and RSK1. Phosphorylations of PKR, CREB kinases and CREB were markedly impaired in peritoneal macrophages isolated from mice that consumed DHA-enriched fish oil for 6 to 8 wk. DHA’s effects were not explainable by increased activity of protein phosphatase 1 and 2A since both were suppressed in mice consuming the DHA diet. Although cells cultured directly with DHA expressed less IL-6 compared to cells cultured with arachidonic acid (AA), neither fatty acid treatment affected DON-induced protein phosphorylation. Furthermore, DHA and AA similarly inhibited cell-free protein kinase activity. These data suggest that DON-induced IL-6 expression is CREB-mediated and PKR-dependent and that requisite kinase activities for these pathways were suppressed in macrophages from mice fed DHA for an extended period.
Keywords: deoxynivalenol (DON), interleukin-6, DHA, CREB, macrophage, protein kinase
1. Introduction
Deoxynivalenol (DON) is a trichothecene mycotoxin produced by Fusarium that is frequently encountered in cereal-based foods and that potentially evoke adverse effects on human health. DON can induce both proinflammatory cytokine expression and apoptosis in mononuclear phagocytes depending on exposure frequency and dose [1]. Dietary exposure to DON selectively promote polyclonal activation and expansion of immunoglobulin A (IgA)-secreting B cells by activating macrophages and T cells. Production of autoreactive IgA and its deposition in the mouse kidney mimic the early stages of human IgA nephropathy (IgAN) [2] [3]. DON-induced interleukin-6 (IL-6) expression in macrophages plays a critical role in IgA upregulation [4] [5]. The upstream mechanisms by which DON induces IL-6 production in macrophages remain unclear but appear to be mediated both transcriptionally and post-transcriptionally [6] [7] [8].
IL-6 plays a critical role in inflammation initiation and maintenance of chronic inflammatory states. IL-6 also elicits T cell activation, end-stage B cell differentiation and immunoglobulin secretion. Notably circulating IL-6 levels are elevated in several autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, Crohn’s disease and psoriasis, and correlate with markers of disease activity [9] [10] [11]. IL-6 has also been related to the degree of IgA deposition in the kidney and disease progression in patients with IgAN [12] [13].
Consumption of the n-3 polyunsaturated fatty acids (PUFAs), docosahexaenoic acid (DHA) or eicosapentaenoic acid (EPA), suppresses DON-induced IgAN in mice [14] [15], which concurs with the proposed anti-inflammatory action of these fatty acids. These results are consistent with randomized clinical trials demonstrating that fish oil consumption retards the renal function loss in IgAN patients [16] [17] [18] [19].
Given the potential importance of IL-6 in the pathogenesis of IgAN and other autoimmune diseases, it is important to understand how DON induces IL-6 overexpression in macrophages and how n-3 PUFA consumption ameliorates these effects. DON-induced phosphorylation of cAMP response element binding protein (CREB), a transcription factor associated with IL-6 expression, and its subsequent binding to the IL-6 promoter have recently been shown to be inhibited in mice fed DHA or EPA [15][20]. The purpose of this study was to (1) verify that CREB activation is critical for DON-induced IL-6 expression and (2) identify upstream signaling pathways by which DHA suppresses DON-induced CREB activation.
2. Materials and methods
2.1. Materials
All chemicals including DON and cell culture components were purchased from Sigma-Aldrich, Inc. (St. Louis, MO) unless otherwise noted. DON contaminated labware and cell culture media were detoxified by sodium hypochlorite. All kinase and phosphatase inhibitors were purchased from Calbiochem, Inc. (San Diego, CA).
2.2. Animals and diet
Female B6C3F1 mice (5 wk old) weighing 16 to 18 g were obtained from Charles River Laboratories, Inc (Wilmington, MA) or Harlan (Indianapolis, IA). Housing, handling, and sample collection procedures conformed to the policies of the Michigan State University All-University Committee on Animal Use and Care in accordance with NIH guidelines. Mice were fed Harlan Teklad 22/5 Rodent chow or fat-amended diets prepared as described in previous studies [20] [15]. Briefly, corn oil (Dyets, Bethlehem, PA), high oleic acid safflower oil (Hain Celestial Group, Inc., Melville, NY) and MEG-3™ DHA-enriched fish oil (containing DHA 483 g/kg and 113 g/kg EPA) (Ocean Nutrition Canada, Dartmouth, Nova Scotia) were added to AIN 93G basal diet (Dyets) to generate a control diet (10 g corn oil and 60 g safflower oil/kg diet) and a DHA diet containing 30 g DHA/kg diet (10 g corn oil and 60 g DHA enriched oil/kg diet), respectively. Mice were fed one of the diets for 6 to 8 wk before peritoneal macrophage harvest. The DHA concentration was selected based on previous work [14] and the time period was chosen based on its efficacy in preliminary studies to consistently suppress DON-induced IL-6 expression.
2.3. Peritoneal macrophage cultures
Mice were injected ip with 1.5 ml of sterile 3% (w/v) thioglycollate broth. After 4 d, mice were euthanized and macrophages collected by peritoneal lavage with ice-cold Hank’s BSS (Invitrogen Corporation, Carlsbad, CA). Cells were pelleted by centrifugation at 1,100 × g for 5 min. Cells were washed with BSS once and resuspended in RPMI-1640 containing 10% (v/v) heat-inactivated fetal bovine serum (Atlanta Biologicals, Norcross, GA), 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells were cultured at 37°C under 6% CO2 in a humidified incubator for 24 h before treatment.
Macrophages were incubated with or without DON (250 mg/ml in ddH2O as stock solution) for various time periods and analyzed for mRNA expression by real-time PCR or protein phosphorylation by Western analysis. DON was dissolved in PBS first to make a 250 µg/ml stock solution and then added to cell culture media as 1:1000 (v/v) dilution to generate 250 ng/ml working solution. Two milliliters of cell suspension (1 × 106 /ml) were incubated in each well of 6-well cell culture plates (Corning Life Sciences, Lowell, MA) for experiments requiring RNA isolation. For protein collection, 10 ml cell suspensions (1 × 106 /ml) were incubated in 100 mm-diameter cell culture dishes (Corning Life Sciences).
For protein kinase studies, inhibitors of MSK1/RSK1 (Ro31–8220) and Akt1/2 (Akt inhibitor IV, V and VIII) were dissolved in DMSO and added to cultures 1 h before DON treatment. DMSO alone was used at the vehicle control PKR inhibitor C16 and its negative control were added to cultures 45 min before DON treatment.
For protein phosphatase studies, calyculin A (20 nM), an inhibitor to type 1 and 2A protein phosphatase, was added to cell cultures 1 or 2 h prior to DON treatment. None of the inhibitors at the indicated concentrations affected cell viability, as verified by trypan blue staining, or induced morphological changes, as verified by phase contrast microscopy.
2.4. Real-time PCR
RNA was extracted using RNeasy Mini (Promega, Madison, WI) and analyzed by real-time PCR for IL-6 mRNA expression [15]. TaqMan primers and probes were purchased from Applied Biosystems (Foster City, CA). β-2 microglobulin RNA expression is not affected by DON treatment and thus was used as endogenous control to normalize target gene expression.
2.5. Western analysis
For protein phosphorylation studies, macrophages were washed with ice-cold PBS, lysed in Tris buffer (10 mM, pH 7.4) containing 1% (w/v) SDS and phosphatase inhibitor cocktail (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), boiled and sonicated. After centrifugation at 18,000 × g for 15 min, extracts were subjected to Western analysis using specific antibodies to CREB, phospho-CREB, phospho-Akt1, phospho-RSK1, phospho-MSK1 (Cell Signaling Technology, Inc., Danvers, MA), PKR (Millipore, Billerica, MA), phospho-PKR (Calbiochem) and β-actin (Sigma-Aldrich). Alexa Fluor 680 goat-anti rabbit and IRDye® 800 goat-anti mouse secondary antibodies were purchased from Invitrogen Corporation and Rockland Immunochemicals, Inc. (Gilbertsville, PA) respectively. Infrared fluorescence was directly detected by using an Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE).
2.6. siRNA transfection
An siRNA cocktail targeting mouse CREB and a comparable scrambled siRNA were purchased from Dharmacon (Lafayette, CO). siRNA transfection was performed by electroporation using an Amaxa Nucleofector (Gaithersburg, MD). Briefly, 2 ×106 cells were suspended in 100 µl electroporation buffer (mixture of 40 µl of buffer 1 [20% ATP-disodium and 12% MgCl2−6H2O] and 2 ml of buffer 2 [1.2% K2HPO4, 0.12% NaHCO3 and 0.04% glucose]) and mixed with 10 µM siRNA. Electroporation was performed using program D023 for macrophages according to the manufacturer’s protocol. Transfection efficacy was verified by assessing loss of CREB protein by Western blot 48 h after transfection. IL-6 expression induced by DON after transfection was analyzed by real-time PCR.
2.7. Akt1 assay
Akt1 activity in immunoprecipitates was measured by Western analysis. For immunoprecipitation, media were removed by centrifugation and adherent cells were washed twice with ice-cold PBS. After PBS was aspirated, 0.5 ml lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% [v/v] Triton X-100, 2.5 mM sodium pyrophosphate, phosphatase inhibitor cocktail and protease inhibitor cocktail [Roche Diagnostics, Indianapolis, IN]) were added. Following incubation on ice for 5 min, cells were scraped off the plates and transferred to microcentrifuge tubes, sonicated 4 × 5 sec and clarified by centrifugation at 18,000 × g for 10 min. The supernatants (40 µl) were incubated with 1 µg anti-Akt1 antibody with gentle rocking for 2 h at 4 °C. Protein A-Sepharose beads (20 µl of 50% slurry) were then added and incubated for 30 min. Beads were pelleted at 18,000 × g for 30 sec, washed twice with lysis buffer and kinase assay buffer (25 mM Tris-HCl [pH 7.5], 1 mM dithiothreitol [DTT], 10 mM MgCl2, phosphatase inhibitor cocktail and protease inhibitor cocktail) respectively. Akt1-specific CREB kinase activity in the immunoprecipitate was assessed at 30°C for 30 min using 10 µM glutathione S-transferase (GST)-CREB (Upstate, Lake Placid, NY) and 30 µM ATP as substrate. Assays were terminated by adding 2% (w/v) SDS buffer, and CREB phosphorylation was detected by Western analysis.
2.8. Protein phosphatase assay
Phosphatase activity of protein phosphatase 1 (PP1) and protein phosphatase 2 (PP2) in immunoprecipitates was determined using para-nitrophenyl phosphate (pNPP) as substrate. For immunoprecipitation, cells were rinsed twice with ice-cold Hank’s BSS and incubated with lysis buffer (50 mM HEPES [pH 7.4], 0.1 mM EDTA, 0.1 mM EGTA, 0.5% Triton X-100, 1 mM DTT and protease inhibitor cocktail) on ice. After 5 min, cells were scraped off the plates and transferred to microcentrifuge tubes, sonicated and clarified by centrifugation at 18,000 × g for 10 min. PP1 and PP2A were immunoprecipitated using mouse IgG2b antibodies specific to C subunit of PP1α or PP2A respectively (Upstate). Briefly, supernatant containing 500 µg protein was incubated with 4 µg specific antibody or mouse IgG2b and 40 µl protein A-Sepharose beads (50% slurry) at 4°C with gentle rocking for 2 h. The beads were pelleted at 18,000 × g for 30 sec and washed 3 times with lysis buffer and phosphatase assay buffer (50 mM HEPES [pH 7.2], 10 mM MnCl2, 2 mM MgCl2, 1 mM DTT and protease inhibitor cocktail) respectively. The pellet was reconstituted in phosphatase assay buffer, and pNPP was added to make a final concentration of 20 mM. The reaction mixture was incubated with agitation at 30°C for 1 h. Phosphatase activity was measured by reading the absorbance of supernatant at 405 nm. Phosphatase activity was expressed as relative values compared to control group at time 0.
2.9. Fatty acid treatment of cell cultures
DHA, arachidonic acid (AA) and oleic acid (OA) were prepared as 200 mM stock solutions in ethanol and stored under nitrogen in the dark at −20 °C until needed. Fatty acid-bovine serum albumin (BSA) complexes were made based on published methods [21] with some modifications. Briefly, fatty acids in ethanol and BSA (fatty acid free) (Serologicals proteins Inc., Kankakee, IL) were mixed in PBS at a 3:1 molar ratio under nitrogen on a rocking shaker at 37 °C for 24 h. This ratio was previously shown to suppress DON-induced IL-6 in the RAW 264.7 macrophage cell line[21]. These mixtures were then diluted with RPMI-1640 which was supplemented with 0.25% (v/v) FBS. Media were prepared freshly for each experiment. Prior to adding fatty acid-amended media, naïve peritoneal macrophages were incubated in RPMI-1640 medium with 0.25% (v/v) FBS for 18 h to elicit fatty acid deprivation. Cells were then cultured with media amended with 50 µM fatty acids for 24 h before DON (250 ng/ml) was added. Total RNA was collected after 3 h and IL-6 mRNA was detected by real-time PCR. To measure phosphorylation of CREB and its upstream kinases, cells were incubated with DON for 0, 15, 30 or 60 min after fatty acid incubation. Protein was extracted and analyzed by Western blot analysis.
2.10. Fatty acid treatment of CREB kinases
The effects of fatty acids on CREB kinases in a cell-free system were tested over a range of concentrations (0, 12.5, 25, 50 and 100 µM) using a constant ethanol concentration. Active Akt1, MSK1 or RSK1 (1 ng) (Upstate) was incubated with or without free fatty acids for 1 min in kinase assay buffer according to the protocol from Upstate. CREB (30 µM) and ATP (100 µM) were then added. The reaction was incubated at 30°C for 10 min and then was terminated with 2% SDS buffer. CREB phosphorylation was analyzed by Western analysis.
2.11. Statistics
All data were analyzed with SigmaStat v 3.1 (Jandel Scientific, San Rafael, CA) with the criterion for significance set at p<0.05. Student’s t-test was used for comparison of two groups of data. One-way ANOVA was performed for comparison of multiple groups. Holm-Sidak (if normality test passed) or Dunnett’s (ANOVA on ranks if normality test failed) tests were used as post-hoc analysis.
3. Results
3.1. DON induces IL-6 expression in peritoneal macrophages
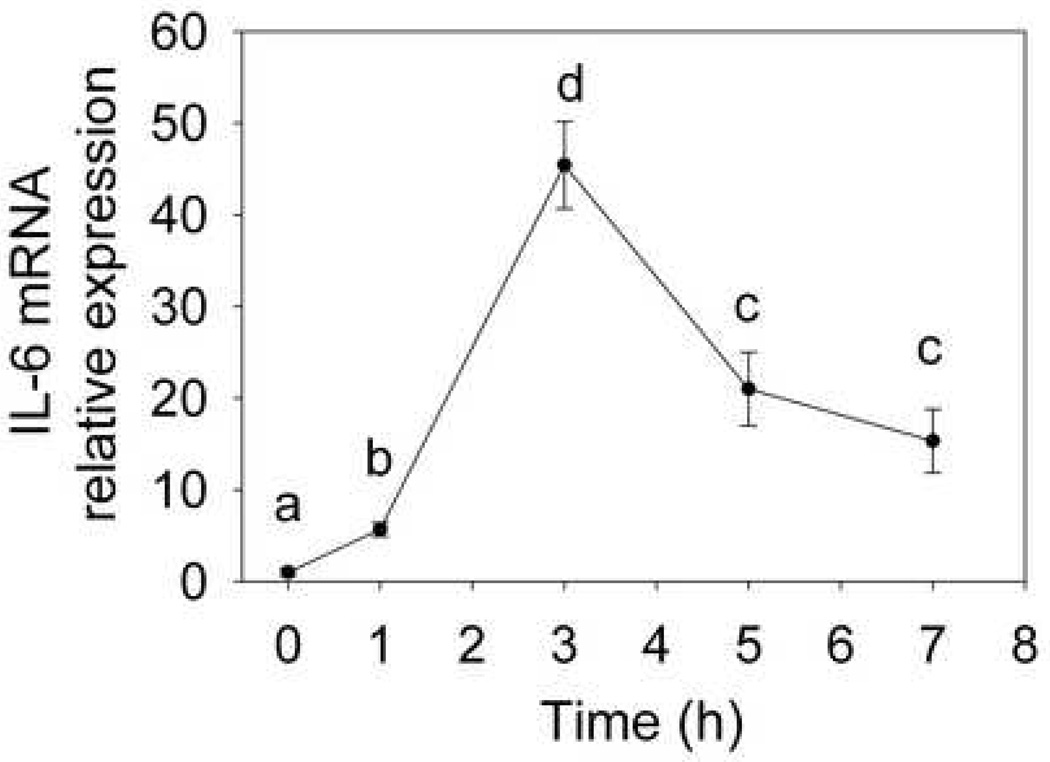
Incubation of naïve peritoneal macrophages with DON (250 ng/ml) induced IL-6 mRNA expression within 1 h (Fig. 1). Increased expression was detectable up to 7 h with maximum induction being observed at 3 h. Based on this finding, a 3 h incubation was chosen to study mechanisms for DON-induced IL-6 expression and how these are affected by DHA.
Fig. 1.
Kinetics of DON-induced IL-6 mRNA expression in peritoneal macrophages. Naïve peritoneal macrophages were cultured with DON (250 ng/ml) for different time periods. Total RNA was extracted and IL-6 mRNA was analyzed by real-time PCR. Data are means ± SEM. Data points with different letters differ (p<0.05). All data were normalized against β2-microglobulin and expressed relative to the value at time 0. Results are representative of two independent experiments.
3.2. DON-induced IL-6 expression is CREB-mediated
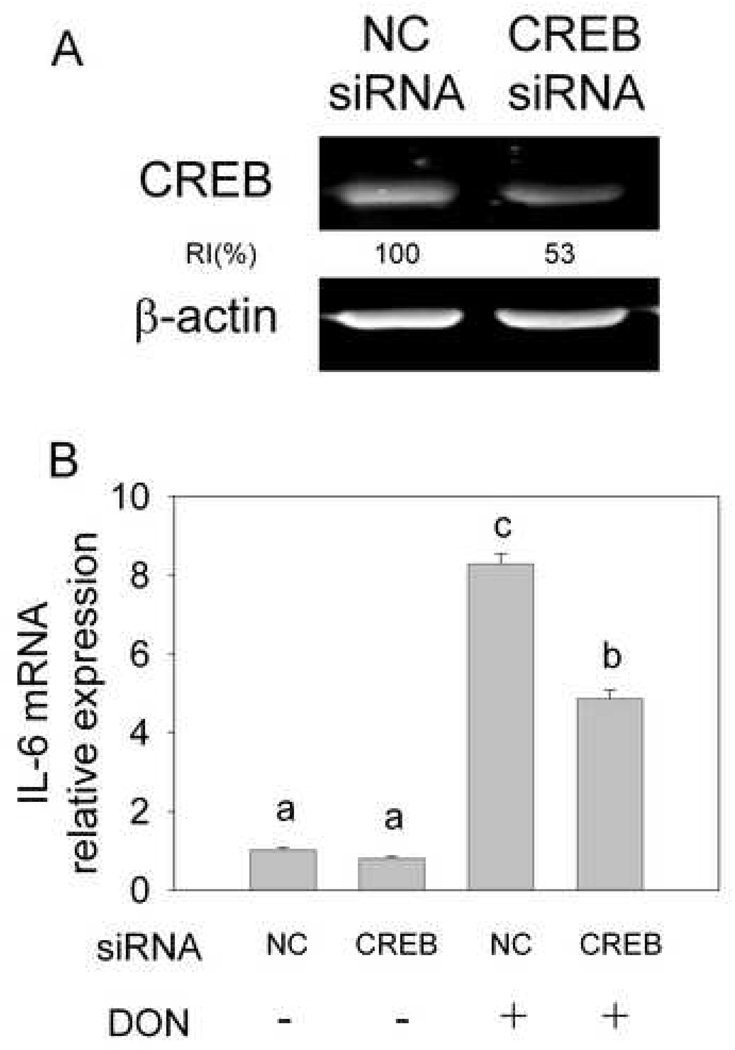
To verify the role played by CREB in DON-induced IL-6 expression, this transcription factor was knocked down by electroporating with a specific siRNA cocktail. As revealed by Western blotting, CREB protein was knocked down by 47% at 48 h after transfection. (Fig. 2A) Correspondingly, DON-induced IL-6 mRNA expression was decreased by 42%. (Fig. 2B) These data confirm that CREB is likely to be a critical transcription factor in DON-induced IL-6 expression in the macrophage.
Fig. 2.
Transcription factor CREB knockdown inhibits IL-6 mRNA expression induced by DON. siRNA specific to CREB or scrambled siRNA was transfected by electroporation into naïve peritoneal macrophages. (A) To evaluate CREB knockdown efficiency, total protein was collected after 48 h and CREB measured by Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane. (B) To detect the role of CREB on IL-6 mRNA expression, cells were treated with DON 48 h after transfection. Total RNA was collected and IL-6 mRNA was analyzed by real-time PCR. Data are means ± SEM. Bars with different letters differ (p<0.05). Results are representative of two independent experiments.
3.3. DON-induced IL-6 expression is mediated by MSK1, RSK1 and Akt1/2
DON induces phosphorylation of several protein kinases that are capable of phosphorylating and activating CREB [20]. Specific inhibitors were thus employed to investigate the relationship between IL-6 mRNA expression and CREB kinases. When naïve macrophages were preincubated with the MSK1/RSK1 inhibitor Ro31–8220 at 200 and 1000 nM (Fig 3A), DON-induced IL-6 expression was markedly inhibited. The role of another CREB kinase family, Akt 1 and 2, in DON-induced IL-6 was also assessed with three inhibitors. Akt Inhibitor IV is an ATP-competitive inhibitor of a kinase upstream of Akt, but downstream of PI-3 K while Akt Inhibitor V targets an Akt effector molecule other than PI-3 K or PDK1. Akt Inhibitor VIII selectively inhibits Akt1 and Akt 2 and appears to be pleckstrin homology (PH) domain-dependent. This inhibitor has no activity against PH domain-lacking Akts, or other closely related AGC family kinases, PKA, PKC, and SG Incubation of naïve macrophages with each of these inhibitors for 1 h prior toxin treatment, suppressed DON-induced IL-6 mRNA expression (Fig 3B–D) suggesting the involvement of Akt 1 and 2.
Fig. 3.
Inhibition of CREB kinases suppresses DON-induced IL-6 expression. Naïve peritoneal macrophages were cultured for 1 h with CREB kinase inhibitors dissolved in DMSO or the DMSO vehicle, incubated with DON (250 ng/ml) for 3 h and then IL-6 mRNA measured by real-time PCR‥ Compounds used were (A) MSK1/RSK1 inhibitor Ro 31–8220, (B ) Akt inhibitor IV, (C) Akt inhibitor V (25 µM) and (D) Akt inhibitor VIII (25 µM). Data are means ± SEM. Bar with asterisk differs from that of DON treatment without inhibitor (p<0.05). Results are representative of at least two independent experiments.
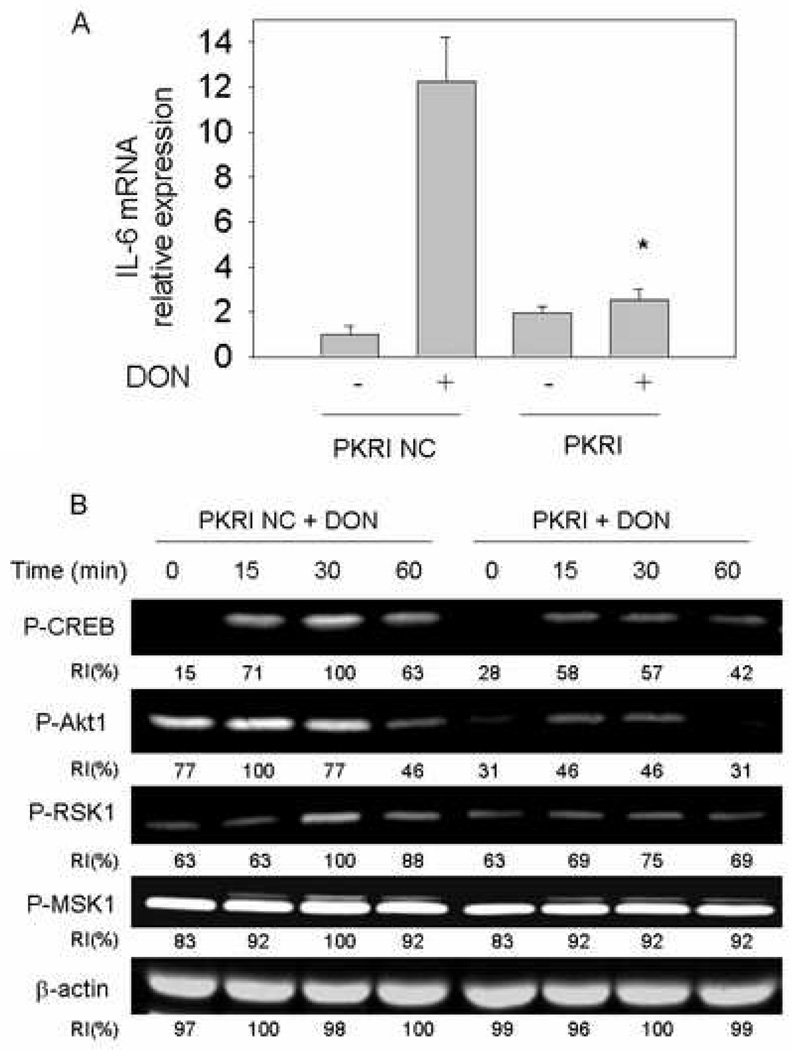
3.4. DON–induced IL-6 expression is PKR-dependent
Double-stranded RNA-activated protein kinase (PKR) has been previously shown to be a critical upstream mediator of DON-induced ribotoxic stress response [22] [23]. Incubation with a specific PKR inhibitor was found to markedly inhibit DON-induced IL-6 mRNA expression (Fig. 4A). Suppressed IL-6 expression appeared to correlate with impaired phosphorylation of CREB, Akt1, RSK1 and to a lesser extent, MSK1 by PKR inhibitor (Fig. 4B).
Fig. 4.
PKR inhibition blocks DON-induced IL-6 expression and protein phosphorylation. (A) Naïve peritoneal macrophages were cultured for 45 min with PKR inhibitor (PKRI) or PKR inhibitor negative control (PKRI NC) and then with DON 250 ng/ml for 3 h. IL-6 mRNA was measured by real-time PCR. Data are means ± SEM. Bar with asterisk differs from that of DON treatment without inhibitor (p<0.05). (B) To detect the role of PKR activation in CREB phosphorylation, naïve macrophages were cultured for 45 min with PKR inhibitor (PKRI) or PKR inhibitor negative control (PKRI NC) and then with DON (250 ng/ml) for different periods. Phosphorylation of CREB and its upstream kinases was measured by Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane. Results are representative of two independent experiments.
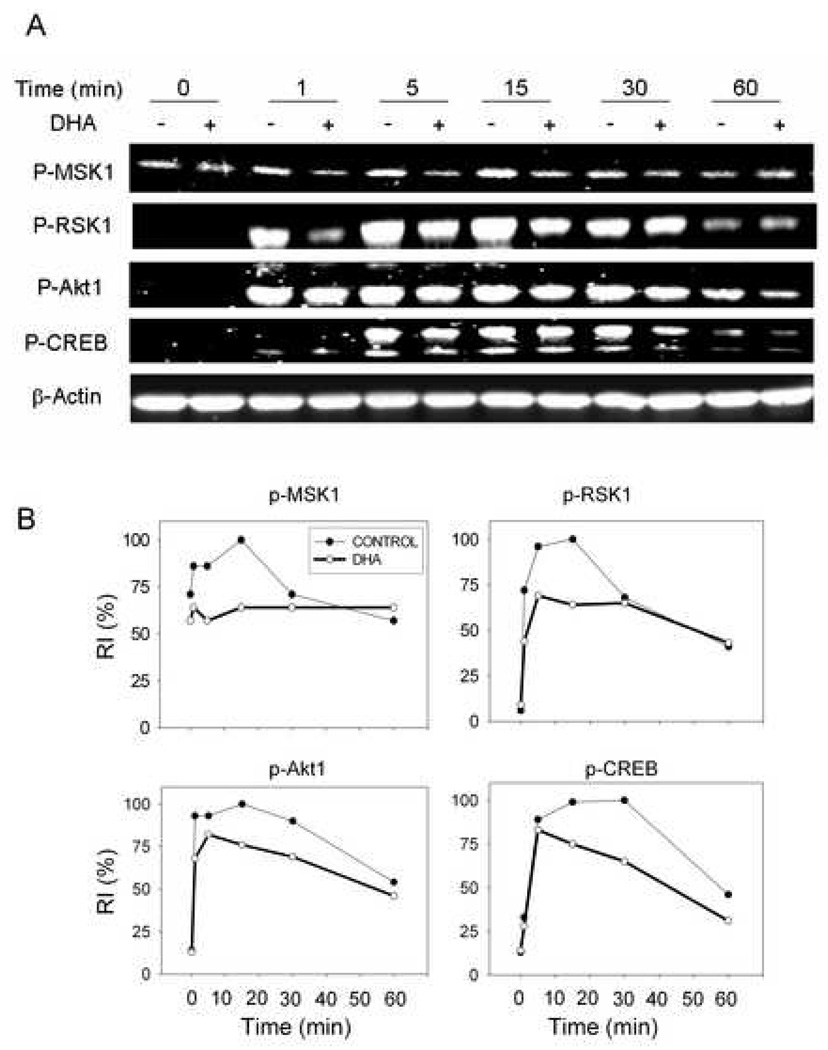
3.5. DON-induced CREB, Akt1, MSK1 and RSK1 phosphorylation is suppressed in peritoneal macrophages from DHA-fed mice
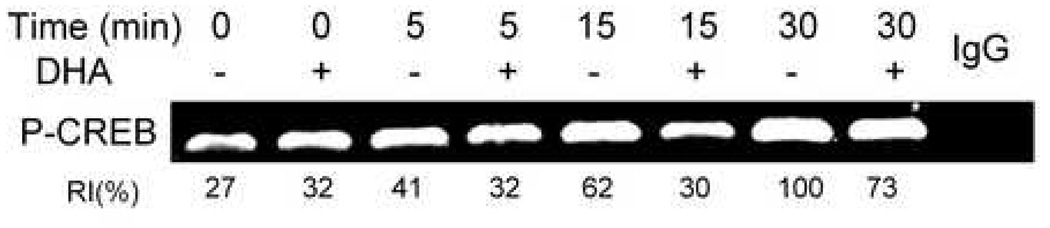
Peritoneal macrophages from mice fed control or DHA diet were compared relative to their ability to phosphorylate CREB kinases and CREB following DON exposure. DON induced Akt1, MSK1 and RSK1 phosphorylation as early as 1 min after treatment and these effects were maximal between 5 to 15 min (Fig. 5A,B). CREB was phosphorylation was maximal at 30 min but decreased dramatically after 60 min. DHA consumption suppressed DON-induced phosphorylation of CREB kinases and CREB at most of these time points.
Fig. 5.
DON-induced phosphorylation of CREB, Akt1, MSK1 and RSK1 is suppressed in macrophages from DHA-fed mice. Peritoneal macrophages from mice fed control or DHA diet were incubated with DON (250 ng/ml) for indicated time periods. (A) Cell lysates were subjected to Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane. (B) RI and DON exposure time were plotted. Results are representative of three independent experiments.
3.6. DON-induced Akt1 activation is inhibited by DHA consumption
The capacity of DHA feeding to modify DON-induced CREB kinase activity was assessed in peritoneal macrophages using Akt1 as a model. Specifically, immunoprecipitated Akt1 was pulled down from extract of DON-treated macrophages from mice fed DHA or control diets and then assessed for its ability to phosphorylate CREB (Fig. 6). CREB kinase activity was highest at 30 min after DON treatment. DHA consumption suppressed Akt1 kinase activity at all the time points.
Fig. 6.
DON-induced Akt1activation is suppressed in macrophages from DHA-fed mice. Peritoneal macrophages from mice fed control or DHA diet for were incubated with DON (250 ng/ml) for indicated time points. Following cell lysis, Akt1 was immunoprecipitated and incubated with CREB and ATP. Phosphorylation of CREB was analyzed by Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane.
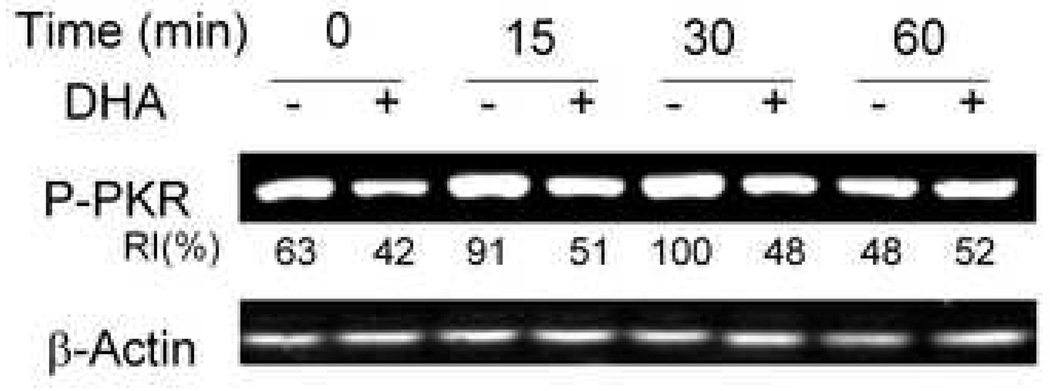
3.7. Phosphorylation of PKR in peritoneal macrophages is inhibited by DHA consumption
Peritoneal macrophages from mice fed control or DHA diet were treated with DON for 0, 5, 15, 30 or 60 min and PCR phosphorylation measured. DON treatment moderately upregulated PKR phosphorylation at 15 and 30 min (Fig. 7). In contrast, PKR phosphorylation appeared to be suppressed in macrophages from DHA-fed mice at the initiation of the experiment as well as at 15 and 30 min.
Fig. 7.
Phosphorylation of PKR in the peritoneal macrophage is inhibited by DHA consumption. Peritoneal macrophages from mice fed control or DHA diet were treated with DON (250 ng/ml) for indicated time periods. Cell lysates were subjected to Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane.
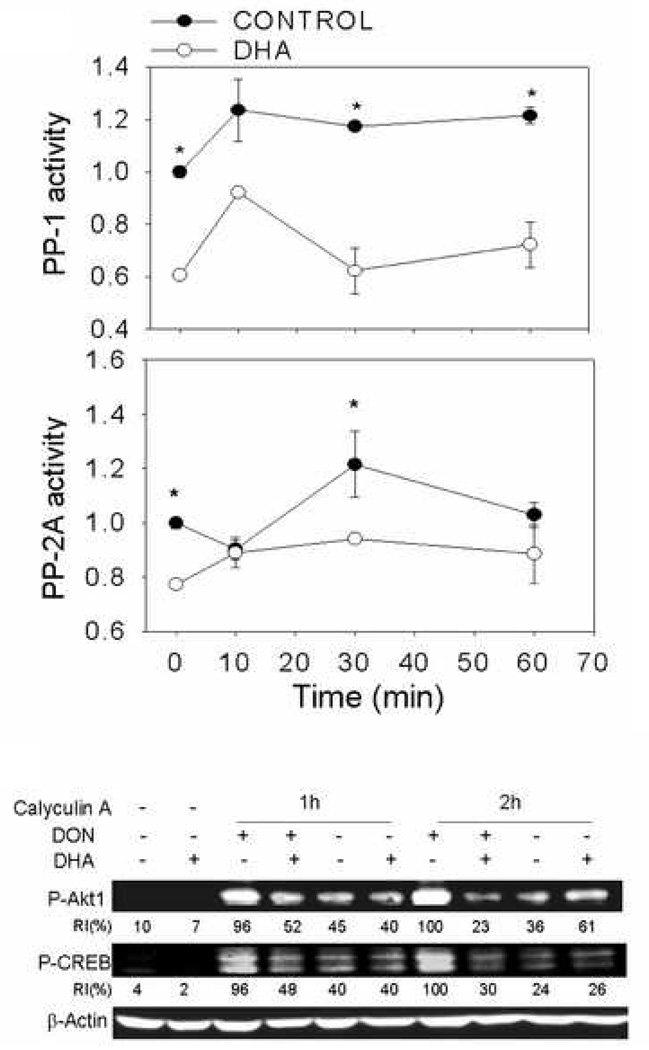
3.8. DHA consumption inhibits protein phosphatase PP1 and PP2A activities
The possibility exists that DHA consumption inhibits phosphorylation of CREB and CREB kinases by upregulating protein phosphatase activities. Therefore, the activities of PP1 and PP2A were measured. DON treatment slightly induced phosphatase activities in macrophages from mice fed control diet (Fig. 8A). However, both phosphatase activities in macrophages from DHA-fed mice were decreased as compared to control diet regardless of whether they were treated with DON or not.
Fig. 8.
PP1 and PP2A phosphatase activities are not increased in macrophages from DHA-fed mice. (A) Peritoneal macrophage from control- or DHA-fed mice were treated with DON (250 ng/ml) for 0, 10, 30, or 60 min. PP1 and PP2A in the cell lysates were immunoprecipitated and analyzed respectively for phosphatase activities. Values were expressed relative to control at time 0. Data are means ± SEM. Points with asterisk differ from those of corresponding DHA group (p<0.05). (B) To evaluate effects of PP1 and PP2A on protein phosphorylation, peritoneal macrophages from mice fed control or DHA diet were pretreated with vehicle or calyculin A (20 nM) for 1 or 2 h and then incubated with DON for 30 min. Cell lysates were subjected to Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane.
To further assess possible roles of phosphatases in DHA-suppressed protein phosphorylation, peritoneal macrophages from mice fed control or DHA diet were incubated with the general protein phosphatase inhibitor calyculin A prior to DON treatment. Calyculin A did not abolish the inhibition of CREB and Akt1 phosphorylation by DHA consumption. (Fig. 8B)
3.9. DHA effects can not be reconstituted in cell culture or cell-free models
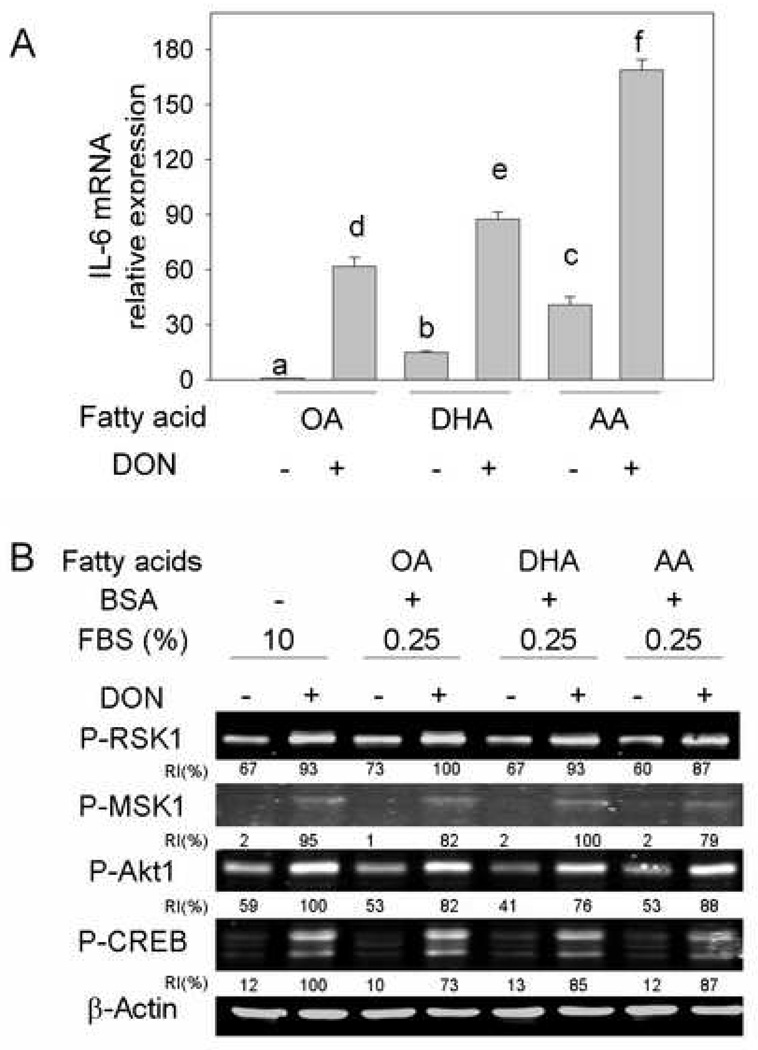
To test the direct effects of fatty acid treatments on responses of macrophages to DON, naïve peritoneal macrophages were incubated with different fatty acids complexed with BSA. Both DHA and AA increased IL-6 expression compared to monounsaturated fatty acid oleic acid (OA). DON induced IL-6 expression in all three groups according to the rank order: AA>DHA>OA. (Fig. 9A) When the effects of in vitro fatty acid treatment on IL-6 expression were related to protein phosphorylation, fatty acid treatments did not affect phosphorylation of CREB, Akt1, MSK1 or RSK1 induced by DON compared to control group. (Fig. 9B)
Fig. 9.
Fatty acids differentially affect DON-induced IL-6 mRNA expression but not protein phosphorylation in peritoneal macrophages. Serum-deprived peritoneal macrophages were treated with 50 µM fatty acid complexed with BSA for 24 h. (A) For IL-6 measurement, RNA was extracted after 3-h DON (250 ng/ml) treatment and analyzed by real-time PCR. Data are means ± SEM. Bars with different letters differ (p<0.05). (B) To detect protein phosphorylation affected by different fatty acids, cells were incubated with DON for 20 min, and total protein was analyzed by Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane.
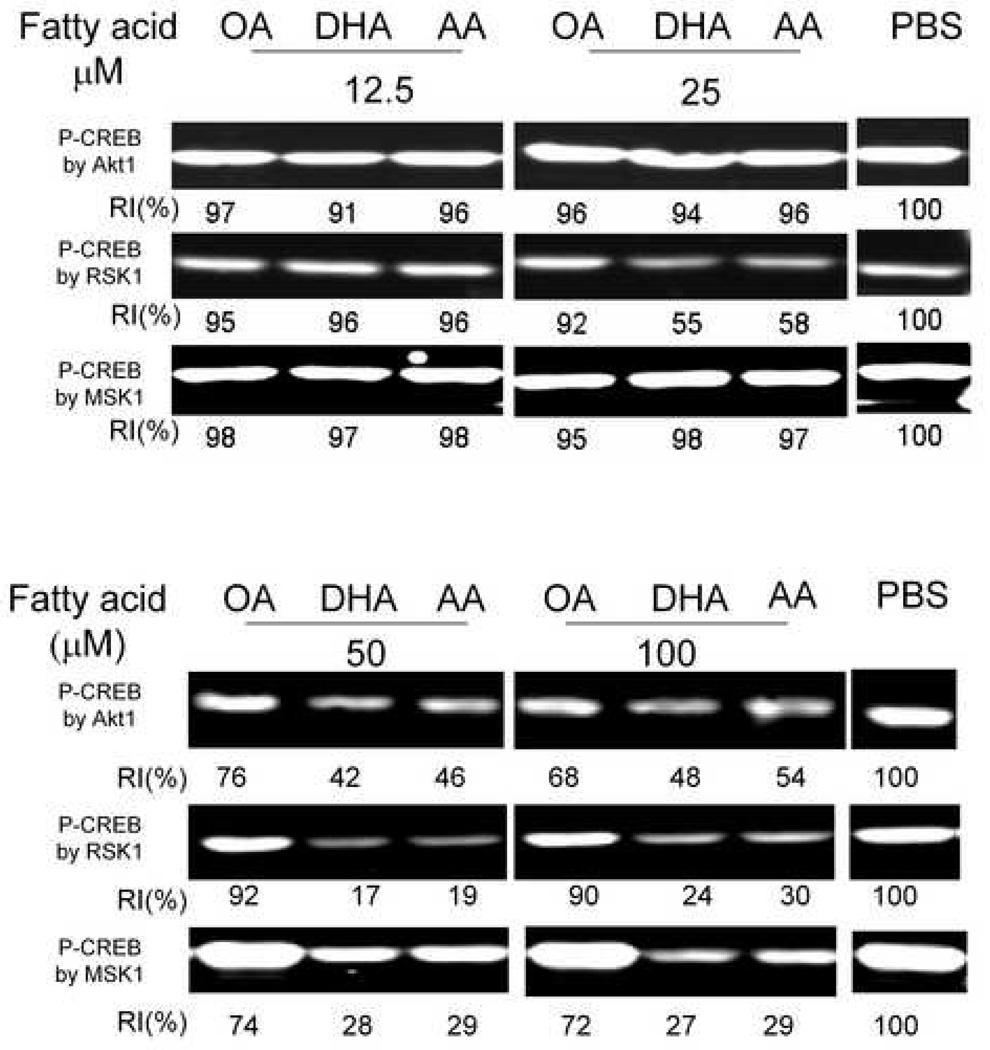
The effects of direct incubation of free fatty acids on Akt1, MSK1 and RSK1 activity was also assessed using CREB as substrate. Both DHA and AA similarly inhibited AKT1 (≥50 µM), RSK1 (≥25 µM) and MSK1 (≥50 µM) activity to a much greater extent than OA. (Fig. 10)
Fig. 10.
AA and DHA similarly decrease CREB kinase activities in the cell-free system. Protein kinases (Akt1, RSK1 and MSK1) were incubated with 12.5, 25, 50 and 100 µM fatty acids or with PBS vehicle before CREB and ATP were added. CREB phosphorylation was analyzed by Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence (for PBS vehicle control, far right) in the same row.
4. Discussion
Clinical studies suggest that consumption of n-3 PUFAs is efficacious for prophylaxis and treatment of chronic inflammatory diseases that impact millions of people in the U.S. and contribute extensively to morbidity, mortality and health care costs. Our laboratory has focused on the mechanisms by which n-3 PUFAs suppress IgAN, the most common primary glomerulonephritis worldwide, using an experimental mouse model. Consumption of n-3 PUFAs has been determined to attenuate DON-induced IgAN and that correlates with impairment of both systemic IgA hyperproduction and IL-6 gene expression [27] [15] [14].
Since IL-6 is a proinflammatory cytokine that plays a role in numerous inflammatory and autoimmune diseases [10] [12], the capacity of n-3 PUFAs to reduce its transcription is of fundamental importance. The transcription factor CREB which binds to promoter region of IL-6 gene and regulate its expression contains several functional domains. The C-terminal basic domain facilitates DNA binding (conserved sequence: TGACGTCA) and the leucine zipper domain facilitates dimerization with CREB or other members of the CREB family such as cAMP response element modulator (CREM) and activating transcription factor 1 (ATF-1). Most importantly, CREB has a kinase inducible domain (KID) that contains the critical serine-133 amino acid residue. Exposure to DON results in phosphorylation of this residue through the action of Akt1, ribosomal S6 kinase 1 (RSK1) and mitogen /stress-activated protein kinase 1 (MSK1) [20]. Since knockdown of CREB by siRNA and pharmacologic inhibition of CREB kinases suppressed IL-6 expression, we conclude that this transcription factor and its upstream kinases, Akt1, MSK1 and RSK1, are likely to be critical for DON-induced IL-6 production.
A key question relates to the molecular mechanism by which DON-induced stress upregulates IL-6 expression. The ribotoxic stress response is a mechanism by which a number of translational inhibitors, such as DON, act on cells and induce activation of mitogen-activated protein kinases (MAPK), proinflammatory cytokine production and apoptosis [24]. It has been previously shown that PKR is a critical early mediator of DON-induced ribotoxic stress response [22]. PKR is a ubiquitously expressed serine/ threonine protein kinase that is activated by double-stranded RNA, interferon, cytokines and stress signals. It is an essential signal transducer and integrator for immune cells to respond to different stresses. Upon activation, PKR inhibits translation initiation by phosphorylating eIF2α which leads to selective protein synthesis inhibition and regulates several signal transduction pathways such as activation of MAPK and NF-κB [25] [26]. The results presented here confirm that, in peritoneal macrophages, PKR is also an essential upstream regulator of DON-induced IL-6 expression and CREB activation. It should be noted that while inhibtition of PKR almost completely abolished IL-6 expression, weak CREB activation was still evident. Since DON also activates other transcription factors via PKR such as NF-κB and AP-1 that can contribute to IL-6 expression, suppression of their activation by PKR inhibition might synergisitically contribute to IL-6 suppression.
Inhibition of CREB activation by n-3 PUFAs can be caused by decreased CREB kinase activity [6] [28] [29] [30] [31]. In this study, we compared kinetic changes of protein phosphorylation and kinase activity induced by DON in macrophages from mice fed control or DHA diet. Phosphorylation of Akt1, MSK1 and RSK1 occurred earlier than that of CREB and all such phosphorylations were suppressed in macrophages from DHA-fed mice. The results presented here suggest that suppression of PKR activation contributed to reduced CREB kinase and CREB phosphosphorylation.
An alternative explanation for DHA’s inhibitory effects is that it interrupts CREB activation by increasing serine/threonine protein phosphatase activities in macrophages. Phosphorylation of serine and/or threonine is important for activation of CREB, Akt1, MSK1 and RSK1. Phosphorylation can be fine-tuned by competing dephosphorylations carried out by protein phosphatases. The primary phosphatases that dephosphorylate these residues are PP1 and PP2A [32] [33] [34] [35]. PP1 and PP2A consist of multimeric structures including a catalytic subunit complexed to a number of accessory subunits that are able to regulate the activity of catalytic subunit. Here, activities of the phosphatases were measured rather than protein amount of catalytic subunit. The results showed that prior DHA consumption decreased both basal and DON-induced PP1 and PP2A activities in peritoneal macrophages, suggesting the n-3 PUFAs do not suppress protein phosphorylation by upregulating phosphatase activities. This conclusion was further supported by studies employing calyculin A, a potent PP1 and PP2A inhibitor, which did not restore the reduced phosphorylation of CREB and Akt1 observed in macrophages from DHA-fed mice.
A further possibility was that DHA suppressed CREB phosphorylation by direct interaction with macrophages. We thus examined the direct effects of fatty acids on IL-6 expression and protein phosphorylation in naïve peritoneal macrophages. The concentrations of total non-esterified fatty acids (NEFAs) in plasma range from 0.2 to 1.7 mM and the individual concentrations of the major fatty acids can range from 30 to 130µM. Most (>99%) NEFAs bind with albumin to make complexes with the remainder exist as unbound free fatty acid [36] [37] [38] [39]. Therefore the concentrations of fatty acid-BSA complexes used in our in vitro experiments were in a physiological range.
The in vitro experiments showed that although macrophages secreted more DON-induced IL-6 following treatment with AA than with OA or DHA, there were marked differences in DON-induced phosphorylation of CREB or CREB kinases among the three different fatty acid treatments. Thus n-3 PUFA effects in the in vitro did not mimic those seen ex vivo. One explanation for these differences might relate to the use of primary macrophages which are a central to innate immunity and are crucial for initiating, maintaining and resolving an adaptive immune response. Macrophages are not a homogeneous cell population, but rather encompass different phenotypes, which exhibit a wide range of pro- and anti-inflammatory activities depending on their stage of differentiation and activation. Fatty acid consumption could suppress inflammation by differentially modulating expression of genes related to proinflammatory responses such as colony-stimulating factor-1 (CSF-1) and PU.1, or anti-inflammatory responses such as adenosine A3 receptor, CD1d, and IL-1 receptor II [40] [41] [42] [43]. It might be speculated that the DHA effects observed herein represent a cumulative change in macrophage phenotypes resulting from subchronic n-3 PUFA consumption.
Another explanation for the differences between ex vivo and in vivo responses is that DHA is a precursor to some more potent anti-inflammatory mediators such as resolvins and protectins[44] [45]. These mediators are produced by epithelial cells, neutrophils and glial cells in intact animals and can have anti-inflammatory effects on macrophages. Their effects might not be immediately detectable in purified macrophage cell culture treated with DHA for a short period. A further possible reason for the difference, is that arachidonic acid depletion upon DHA incorporation might result in less PGE2 production. Since DHA can be incorporated into the cell membrane relatively rapidly, the latter might be a greater factor in suppressing IL-6 expression in vitro than ex vivo [46].
After cell entry, free fatty acids bind to fatty acid binding proteins, which facilitate their transportation, storage and metabolism[47] [48]. These fatty acids can directly interact with proteins and modulate their activities. It has been reported that unsaturated fatty acids are ligands not only for nuclear [49] [50] and membrane receptors [38], but also for protein kinases [51] [52]. A cell-free system was therefore used to assess direct interactions among three molecules: kinase, substrate and fatty acid. Although AA and DHA inhibited CREB kinase activities at 100 and 50 µM compared to OA, these n-6 and n-3 PUFAs did differ in the extent of inhibition. It should be further noted that although direct effects of fatty acids on protein kinases were observed, the concentrations employed were relatively high. The total unbound intracellular fatty acids and FA-CoA levels reported previously are lower than 10 µM [53] [54]. Since no inhibition of kinase activity by fatty acids was observed at 12.5 µM, the effects of PUFA on Akt1, RSK1 and MSK1 at high concentrations in peritoneal macrophages might not be physiologically relevant.
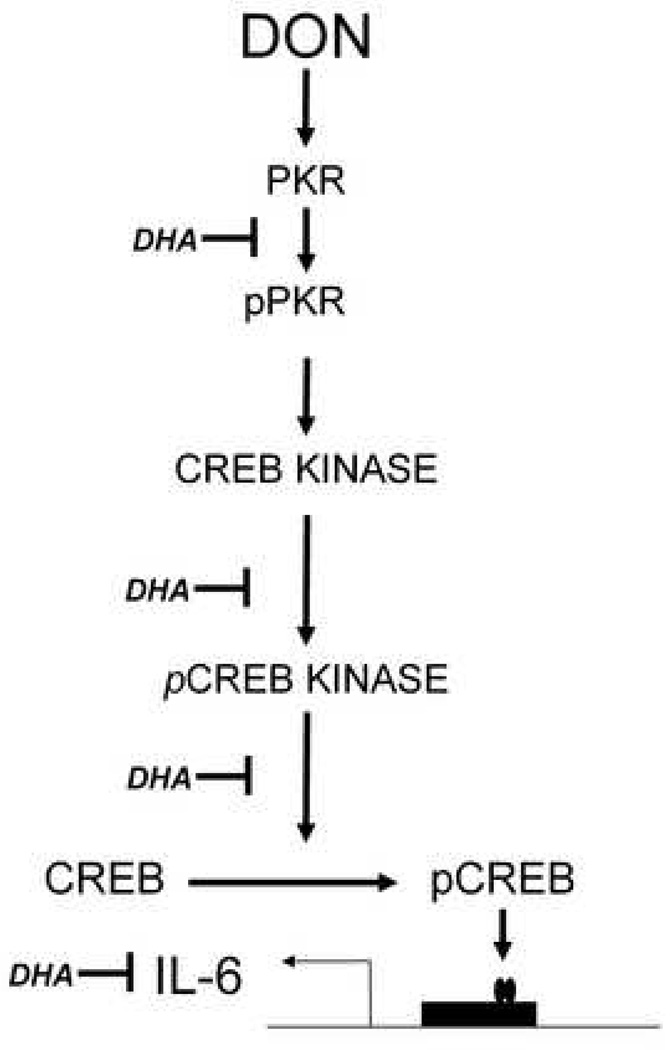
In summary, the data presented here suggest that IL-6 expression induced by DON is PKR-dependent and mediated, in part, by the transcription factor CREB. DHA consumption appears to suppress these pathways in macrophages rendering them less capable of CREB activation and thus IL-6 transcription. (Fig. 11) Suppression of IL-6 expression by DHA might have general importance to human health relative to the prevention and treatment of inflammatory and autoimmune diseases mediated by this proinflammatory cytokine.
Fig. 11.
Effects of DHA consumption on signal transduction pathways mediating DON-induced IL-6 expression in peritoneal macrophages ex vivo. Possible CREB kinases inhibited by DHA feeding include AKT, RSK1 and MSK1. The symbol ⊣ on the left indicates inhibition of the pathway step on the right.
Acknowledgements
The authors would like to acknowledge Katie Loniewski, Sonika Patial and Dr. Nara Parameswaran in the Department of Physiology at Michigan State University for their help with siRNA knockdown. We also thank Drs. Julia Busik and Donald Jump for extremely helpful insight in these studies.
Supported by Public Health Service Grants DK58833
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Pestka JJ, Zhou HR, Moon Y, Chung YJ. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: unraveling a paradox. Toxicol Lett. 2004;153:61–73. doi: 10.1016/j.toxlet.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Pestka JJ. Deoxynivalenol-induced IgA production and IgA nephropathy-aberrant mucosal immune response with systemic repercussions. Toxicol Lett. 2003;140–141:287–295. doi: 10.1016/s0378-4274(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 3.Pestka JJ, Moorman MA, Warner RL. Dysregulation of IgA production and IgA nephropathy induced by the trichothecene vomitoxin. Food Chem Toxicol. 1989;27:361–368. doi: 10.1016/0278-6915(89)90141-5. [DOI] [PubMed] [Google Scholar]
- 4.Yan D, Zhou HR, Brooks KH, Pestka JJ. Potential role for IL-5 and IL-6 in enhanced IgA secretion by Peyer's patch cells isolated from mice acutely exposed to vomitoxin. Toxicol. 1997;122:145–158. doi: 10.1016/s0300-483x(97)00087-5. [DOI] [PubMed] [Google Scholar]
- 5.Pestka JJ, Zhou HR. Interleukin-6-deficient mice refractory to IgA dysregulation but not anorexia induction by vomitoxin (deoxynivalenol) ingestion. Food Chem Toxicol. 2000;38:565–575. doi: 10.1016/s0278-6915(00)00041-7. [DOI] [PubMed] [Google Scholar]
- 6.Caravatta L, Sancilio S, di G, Rana R, Cataldi A, Di Pietro R. PI3-K/Akt-dependent activation of cAMP-response element-binding (CREB) protein in Jurkat T leukemia cells treated with TRAIL. J Cell Physiol. 2008;214:192–200. doi: 10.1002/jcp.21186. [DOI] [PubMed] [Google Scholar]
- 7.Wong S, Schwartz RC, Pestka JJ. Superinduction of TNF-alpha and IL-6 in macrophages by vomitoxin (deoxynivalenol) modulated by mRNA stabilization. Toxicol. 2001;161:139–149. doi: 10.1016/s0300-483x(01)00331-6. [DOI] [PubMed] [Google Scholar]
- 8.Wong SS, Zhou HR, Marin-Martinez ML, Brooks K, Pestka JJ. Modulation of IL-1beta, IL-6 and TNF-alpha secretion and mRNA expression by the trichothecene vomitoxin in the RAW 264.7 murine macrophage cell line. Food Chem Toxicol. 1998;36:409–419. doi: 10.1016/s0278-6915(97)00167-1. [DOI] [PubMed] [Google Scholar]
- 9.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8 Suppl 2:S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8 Suppl 2:S3. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–368. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 12.Lim CS, Yoon HJ, Kim YS, Ahn C, Han JS, Kim S, Lee JS, Lee HS, Chae DW. Clinicopathological correlation of intrarenal cytokines and chemokines in IgA nephropathy. Nephrol (Carlton) 2003;8:21–27. doi: 10.1046/j.1440-1797.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 13.Harada K, Akai Y, Kurumatani N, Iwano M, Saito Y. Prognostic value of urinary interleukin 6 in patients with IgA nephropathy: an 8-year follow-up study. Nephron. 2002;92:824–826. doi: 10.1159/000065465. [DOI] [PubMed] [Google Scholar]
- 14.Jia Q, Zhou HR, Bennink M, Peskta JJ. Docosahexaenoic acid attenuates mycotoxin-induced immunoglobulin a nephropathy, interleukin-6 transcription, and mitogen-activated protein kinase phosphorylation in mice. J Nutr. 2004;134:3343–3349. doi: 10.1093/jn/134.12.3343. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Pestka JJ. Attenuation of mycotoxin-induced IgA nephropathy by eicosapentaenoic acid in the mouse: dose response and relation to IL-6 expression. J Biochem. 2006;17:697–706. doi: 10.1016/j.jnutbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Donadio JV, Jr, Bergstralh EJ, Offord KP, Spencer DC, Holley KR. A controlled trial of fish oil in IgA nephropathy. Mayo Nephrology Collaborative Group. N Engl J Med. 1994;331:1194–1199. doi: 10.1056/NEJM199411033311804. [DOI] [PubMed] [Google Scholar]
- 17.Donadio JV, Jr, Grand JP, Bergstralh EJ, Dart RA, Larson TS, Spencer DC. The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial. Mayo Nephrology Collaborative Group. J Am Soc Nephrol. 1999;10:1772–1777. doi: 10.1681/ASN.V1081772. [DOI] [PubMed] [Google Scholar]
- 18.Donadio JV, Jr, Larson TS, Bergstralh EJ, Grande JP. A randomized trial of high-dose compared with low-dose omega-3 fatty acids in severe IgA nephropathy. J Am Soc Nephrol. 2001;12:791–799. doi: 10.1681/ASN.V124791. [DOI] [PubMed] [Google Scholar]
- 19.Donadio JV, Grande JP. The role of fish oil/omega-3 fatty acids in the treatment of IgA nephropathy. Semin Nephrol. 2004;24:225–243. doi: 10.1016/j.semnephrol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Jia Q, Zhou HR, Shi Y, Pestka JJ. Docosahexaenoic acid consumption inhibits deoxynivalenol-induced CREB/ATF1 activation and IL-6 gene transcription in mouse macrophages. J Nutr. 2006;136:366–372. doi: 10.1093/jn/136.2.366. [DOI] [PubMed] [Google Scholar]
- 21.Moon Y, Pestka JJ. Deoxynivalenol-induced mitogen-activated protein kinase phosphorylation and IL-6 expression in mice suppressed by fish oil. J Nutr Biochem. 2003;14:717–726. doi: 10.1016/j.jnutbio.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Zhou HR, Lau AS, Pestka JJ. Role of double-stranded RNA-activated protein kinase R (PKR) in deoxynivalenol-induced ribotoxic stress response. Toxicol Sci. 2003;74:335–344. doi: 10.1093/toxsci/kfg148. [DOI] [PubMed] [Google Scholar]
- 23.Yang GH, Li S, Pestka JJ. Down-regulation of the endoplasmic reticulum chaperone GRP78/BiP by vomitoxin (Deoxynivalenol) Toxicol Appl Pharmocol. 2000;162:207–217. doi: 10.1006/taap.1999.8842. [DOI] [PubMed] [Google Scholar]
- 24.Zhou HR, Islam Z, Pestka JJ. Induction of competing apoptotic and survival signaling pathways in the macrophage by the ribotoxic trichothecene deoxynivalenol. Toxicol Sci. 2005;87:113–122. doi: 10.1093/toxsci/kfi234. [DOI] [PubMed] [Google Scholar]
- 25.Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev. 2006;70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor SS, Haste NM, Ghosh G. PKR and eIF2alpha: integration of kinase dimerization, activation, and substrate docking. Cell. 2005;122:823–825. doi: 10.1016/j.cell.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Jia Q, Shi Y, Bennink MB, Pestka JJ. Docosahexaenoic acid and eicosapentaenoic acid, but not alpha-linolenic acid, suppress deoxynivalenol-induced experimental IgA nephropathy in mice. Nutr. 2004;134:1353–1361. doi: 10.1093/jn/134.6.1353. [DOI] [PubMed] [Google Scholar]
- 28.Kato S, Ding J, Du K. Differential activation of CREB by Akt1 and Akt2. Biochem Biophys Res Commun. 2007;354:1061–1066. doi: 10.1016/j.bbrc.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 29.Chepurny OG, Hussain MA, Holz GG. Exendin-4 as a stimulator of rat insulin I gene promoter activity via bZIP/CRE interactions sensitive to serine/threonine protein kinase inhibitor Ro 31–8220. Endocrinol. 2002;143:2303–2313. doi: 10.1210/endo.143.6.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arthur JS, Fong AL, Dwyer JM, Davare M, Reese E, Obrietan K, Impey S. Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J Neurosci. 2004;24:4324–4332. doi: 10.1523/JNEUROSCI.5227-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Zhai Q, Luo Y, Dorf ME. RANTES-mediated chemokine transcription in astrocytes involves activation and translocation of p90 ribosomal S6 protein kinase (RSK) J Biol Chem. 2002;277:19042–19048. doi: 10.1074/jbc.M112442200. [DOI] [PubMed] [Google Scholar]
- 32.Alberts AS, Montminy M, Shenolikar S, Feramisco JR. Expression of a peptide inhibitor of protein phosphatase 1 increases phosphorylation and activity of CREB in NIH 3T3 fibroblasts. Mol Cell Biol. 1994;14:4398–4407. doi: 10.1128/mcb.14.7.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Comerford KM, Leonard MO, Cummins EP, Fitzgerald KT, Beullens M, Bollen M, Taylor CT. Regulation of protein phosphatase 1gamma activity in hypoxia through increased interaction with NIPP1: implications for cellular metabolism. J Cell Physiol. 2006;209:211–218. doi: 10.1002/jcp.20726. [DOI] [PubMed] [Google Scholar]
- 34.Wadzinski BE, Wheat WH, Jaspers S, Peruski LF, Jr, Lickteig RL, Johnson GL, Klemm DJ. Nuclear protein phosphatase 2A dephosphorylates protein kinase A-phosphorylated CREB and regulates CREB transcriptional stimulation. Mol Cell Biol. 1993;13:2822–2834. doi: 10.1128/mcb.13.5.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katsiari CG, Kyttaris VC, Juang YT, Tsokos GC. Protein phosphatase 2A is a negative regulator of IL-2 production in patients with systemic lupus erythematosus. J Clin Invest. 2005;115:3193–3204. doi: 10.1172/JCI24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi C, Simon DI. Integrin signals, transcription factors, and monocyte differentiation. Trends Cardiovasc Med. 2006;16:146–152. doi: 10.1016/j.tcm.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Lloyd EE, Gaubatz JW, Burns AR, Pownall HJ. Sustained elevations in NEFA induce cyclooxygenase-2 activity and potentiate THP-1 macrophage foam cell formation. Atherosclerosis. 2007;192:49–55. doi: 10.1016/j.atherosclerosis.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Calder PC, Bond JA, Harvey DJ, Gordon S, Newsholme EA. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem J. 1990;269:807–814. doi: 10.1042/bj2690807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Rujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohar T, Hinuma S, Fugisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 40.Ehrchen J, Steinmuller L, Barczyk K, Tenbrock K, Nacken W, Eisenacher M, Nordhues U, Sorg C, Sunderkotter C, Roth J. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood. 2007;109:1265–1274. doi: 10.1182/blood-2006-02-001115. [DOI] [PubMed] [Google Scholar]
- 41.Desnues B, Ihrig M, Raoult D, Mege JL. Whipple's disease: a macrophage disease. Clin Vaccine Immunol. 2006;13:170–178. doi: 10.1128/CVI.13.2.170-178.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Richieri GV, Kleinfeld AM. Unbound free fatty acid levels in human serum. J Lipid Res. 1995;36:229–240. [PubMed] [Google Scholar]
- 44.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 46.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 47.Rolph MS, Young TR, Shum BO, Gorgun CZ, Schmitz-Peiffer C, Ramshaw LA, Hotamisligil GS, Mackay CR. Regulation of dendritic cell function and T cell priming by the Fatty Acid-binding protein AP2. J Immunol. 2006;177:7794–7801. doi: 10.4049/jimmunol.177.11.7794. [DOI] [PubMed] [Google Scholar]
- 48.Makowski L, Hotamisligil GS. Fatty acid binding proteins--the evolutionary crossroads of inflammatory and metabolic responses. J Nutr. 2004;134:2464S–2468S. doi: 10.1093/jn/134.9.2464S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kliewer SA, Sundseth SS, Jones SA, Brown SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murakami K, Ide T, Suzuki M, Mochizuki T, Kadowaki T. Evidence for direct binding of fatty acids and eicosanoids to human peroxisome proliferators-activated receptor alpha. Biochem Biophys Res Commun. 1999;260:609–613. doi: 10.1006/bbrc.1999.0951. [DOI] [PubMed] [Google Scholar]
- 51.Lopez-Nicolas R, Lopez-Andreo MJ, Marin-Vicente C, Gomez-Fernandez JC, Corbalan-Garcia S. Molecular mechanisms of PKCalpha localization and activation by arachidonic acid. The C2 domain also plays a role. J Mol Biol. 2006;357:1105–1120. doi: 10.1016/j.jmb.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 52.Eitsuka T, Nakagawa K, Suzuki T, Miyazawa T. Polyunsaturated fatty acids inhibit telomerase activity in DLD-1 human colorectal adenocarcinoma cells: a dual mechanism approach. Biochim Biophys Acta. 2005;1737:1–10. doi: 10.1016/j.bbalip.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 53.Gossett RE, Frolov AA, Roths JB, Behnke WD, Kier AB, Schroeder F. Acyl-CoA binding proteins: multiplicity and function. Lipids. 1996;31:895–918. doi: 10.1007/BF02522684. [DOI] [PubMed] [Google Scholar]
- 54.Jump DB, Clarke SD. Regulation of gene expression by dietary fat. Annu Rev Nutr. 1999;19:63–90. doi: 10.1146/annurev.nutr.19.1.63. [DOI] [PubMed] [Google Scholar]