Abstract
Background
Previous studies have demonstrated that nerve growth factor (NGF) is an important mediator of pathologic pain. Many studies have focused on cutaneous mechanisms for NGF-induced hyperalgesia; few have examined its contribution in deeper tissues like muscle. This study examined pain behaviors and the expression of NGF in incised hind paw flexor digitorum brevis muscle.
Methods
Adult Sprague-Dawley rats were pretreated with anti-NGF peptibody and underwent skin or skin plus deep fascia and muscle incision. Guarding pain behaviors were measured. Muscle NGF messenger RNA (mRNA) was measured by real time polymerase chain reaction. Changes in NGF protein expression were measured using western blot, enzyme-linked immunoabsorbent assay and immunohistochemistry. In situ hybridization for NGF mRNA was also performed.
Results
Pretreatment with anti-NGF peptibody (100 mg/kg) decreased the guarding behavior caused by deep fascia and muscle incision. Muscle NGF mRNA increased abruptly 2 h after incision and was the same as control by postoperative day 1. NGF protein increased from 4 h after incision, and was sustained for several days. NGF was localized in many calcitonin gene related peptide positive axons, few N52 positive axons, but not isolectin B4 positive axons in incised muscle. The sources of NGF mRNA included keratinocytes in epidermis and fibroblasts in deeper tissues.
Conclusion
Fibroblasts adjacent to the injury are sources of NGF in incised muscle. NGF is upregulated by incision of muscle and contributes to guarding pain behavior.
Introduction
Nerve growth factor (NGF) promotes survival of nociceptors and the sympathetic nervous system during late embryonic development.1 In the adult, NGF is essential for the sprouting of nerve fibers and induction of inflammatory pain; from this, NGF has garnered considerable interest in pain research.2
Administration of exogenous NGF has been shown to produce hyperalgesia in rats3 and humans,4,5 and neutralization of NGF by trkA-IgG fusion molecule prevents the sensitization of nociceptors in some pain models.6 The most direct evidence for sensitization of cutaneous afferents by NGF was demonstrated in electrophysiological recordings of nocicetors using the in vitro skin–nerve preparation.7 Application of NGF to the receptive fields of cutaneous C-mechanoheat nociceptors resulted in sensitization to noxious heat.
Interest in postoperative pain management has prompted further investigation for novel therapies that will facilitate better pain control and limit side effects. NGF is one mediator which likely contributes to nociceptor sensitization, hyperalgesia and clinical pain after surgery.8 Previous studies on skin demonstrated upregulation of NGF mRNA immediately after skin incision; increased NGF protein is sustained for several days.9 The increase in NGF occurs at times that are coincident with its role in pain-related behaviors,10 indicating a relationship between NGF, cutaneous incision and pain.
In contrast to skin, deep tissue like muscle also contributes to postoperative pain. For example, after abdominal surgery, patients have pain with deep breathing, during cough and with rising from a supine position. Muscle pain is often dull and aching, and difficult to localize. Indeed, muscle afferents form fewer synapses on spinal cord neurons than cutaneous afferents, and descending inhibition of nociceptive spinal cord neurons is stronger for muscle input than for cutaneous input, suggesting different nociceptive processes from muscle compared to skin.11 Muscle incisional pain is likely unique and different than pain from skin incision. Nothing is known about the expression pattern of NGF in incised muscle.
In this study, we compared the effect of NGF blockade on pain-related behaviors after skin incision and after skin and deep incision of the hind paw fascia and flexor digitorum brevis muscle. NGF expression in the hind paw flexor digitorum brevis muscle after plantar incision was measured using quantitative reverse-transcriptase polymerase chain reaction (PCR), in situ hybridization, enzyme-linked immunosorbent assay, Western blot and immunohistochemistry. The source of NGF mRNA in normal and incised muscle, changes in NGF protein and mRNA at different times following muscle incision, and distribution of muscle NGF immunoreactivity after incision were determined.
Materials and methods
This study was approved by the institutional Animal Care and Use Committee at the University of Iowa (Iowa City, Iowa). Adult male Sprague-Dawley rats, 225–275 g, were used for all the experiments. Postoperatively, the rats were housed in groups of two or three in clear plastic cages with food and water available ad libitum.
Incision, Anti-NGF Peptibody and Guarding Pain Scores
Rats were acclimated to the testing environment. After acclimation, 100 mg/kg anti-NGF peptibody12 or vehicle was administered subcutaneously at the neck. The next day the baseline pain score was measured and the rat underwent plantar incision.13 Under isoflurane anesthesia (1.5–2.0%), using aseptic conditions, a 1 cm longitudinal incision was made 0.5 cm from the heel on the plantar aspect of the right hind paw. The underlying flexor muscle was dissected, longitudinally incised, and the wound was closed with 5−0 nylon suture. Immediately following surgery, antibiotic ointment was applied to the incision. Sutures were removed on postoperative day (POD) 2. A separate group of rats underwent incision of skin only; no fascia or muscle was incised.
A cumulative pain score was used to assess non-evoked pain behaviors as described previously.14 Unrestrained rats were placed on a plastic mesh floor (8 × 8 mm). The incised and non-incised paws were viewed. Both paws of each animal were closely observed during a 1minute period repeated every 5 minutes for 1 hour. Depending on the position in which each paw was found during the majority of the 1 minute scoring period, a 0, 1, or 2 is given. Full weight bearing of the paw (score = 0) was present if the wound is blanched or distorted by the mesh. If the paw is completely off the mesh, a score of 2 was recorded. If the area of the wound touched the mesh gently without any blanching or distorting, a 1 was given. The sum of the 12 scores (0 – 24) obtained during the 1 hour session for each paw was obtained. The difference between the scores from the incised paw and non-incised paw is the cumulative pain score for that 1 hour period. The person performing the behavioral experiments was blinded to the type of incision and the drug (or vehicle) administered.
Quantitative Reverse Transcriptase-PCR analysis
Total ribonucleic acid (RNA) from incised rat flexor digitorum brevis muscle tissue was prepared according to manufacturer's instructions using the RNeasy mini kit (Qiagen, Valencia, CA). Some muscle samples from sham-incised rats were used as control. The RNA was quantified using Ribogreen reagent (Molecular Probes, Eugene, OR). Two-step Reverse Transcriptase-PCR was performed on 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) by using the TaqMan Gold Reverse Transcriptase-PCR kit (Applied Biosystems). The RNA was reverse transcribed using random hexamers, and the complementary deoxyribonucleic acid (cDNA) was amplified using a primer/probe set specific for NGF (RatNGF-fwd:TGACTCCAAGCACTGGAACTCAT, complementary to 693–715 nucleotides in the rat NGF mRNA. RatNGF-rev:GTTTGTCGTCTGTTGTCAACGC, complementary to 947–926 nucleotides in the rat NGF mRNA. RatNGF-Tamra: TGCACCACGACTCACACCTTTGTCA, complementary to 718–742 nucleotides in the rat NGF mRNA). The resulting PCR product was 309 bp in length. Thermal cycling was initiated with an initial incubation at 95°C for 10 min. After that, 40 cycles of PCR were carried out; each PCR cycle consisted of heating at 95°C for 15 sec for melting and at 60°C for 1 min for annealing and extension. The samples were analyzed in triplicate from the reverse-transcription step and normalized to the internal control, a housekeeping gene, β-actin. No template samples were used as controls. PCR data was analyzed by ΔΔCt method described in detail by Livak et al.15
Enzyme-linked Immunosorbent Assay
Flexor digitorum brevis muscle samples were taken from control rats and injured rats at 4-h, 1, 2, 5, and 10 days following plantar incision. Muscle tissue from origin to insertion was harvested from each animal after euthanizing them with carbon dioxide. Immediately following extraction, the tissue samples were homogenized and lysed in ice-cold Radio-Immunoprecipitation Assay buffer containing protease inhibitors. The homogenates were centrifuged and the supernatant was taken. Total protein concentration in each sample was determined using a modified Lowry protein assay. The NGF concentration was measured using a commercially available assay per manufacturer’s instructions (NGF Emax Immunoassay, Promega, Madison, WI). This assay exhibits very low cross reactivity with structurally related growth factors and a minimum of 7.8 pg/mL NGF can be quantified.
Western Blot Analysis
Following assay of total protein, 20 µg of protein in 20 µl of volume from each sample was electrophoresed on 12% sodium dodecyl sulfate-polyacrylaminde gels and then transferred to polyvinylidene difluoride membranes (Bio-Rad Lab, Hercules, CA). Overnight incubation of the membranes in rabbit anti-NGF polyclonal antibody (1:1000, Cat # sc-548, Santa Cruz Biotechnology Inc., Santa Cruz, CA) at 4°C was followed by incubation in peroxidase-conjugated donkey anti-rabbit IgG secondary antibody (1:10,000; Jackson ImmunoRes, West Grove, PA) for 4 hours. Membranes were then developed with SuperSignal West Femto Maximum Sensitivity Substrate (Pierce Biotechnology Inc., Rockford, IL) and scanned by Kodak Image Station 440 scanner (Eastman Kodak Company, Rochester, NY). After the membranes were scanned, they were incubated in stripping buffer at 50°C for 30 min and probed for actin as a loading control. Immunoreactive NGF band intensity was quantified and normalized with actin bands by Image J software (NIH, Bethesda, MD). In a previous study, we demonstrated the antibody was immunoreactive mature to rat NGF (N2513; Sigma, St. Louis, MO) no immunoreactive bands were noted when the blocking hapten peptide (cat. No. sc-548 P; Santa Cruz Biotechnology Inc.), containing the antigen (100 µg/0.5 ml) used to raise the antibody was added.9
Immunohistochemistry
Briefly, rats were anesthetized with sodium pentobarbital (150 mg/kg) and transcardiacally perfused with 0.1 M phosphate buffered saline, followed by 4% paraformaldehyde. The glabrous plantar skin samples together with deep tissue and flexor muscle were removed from sham and incised hindpaws, and post-fixed in the same fixative for 4 hours. After washing in gradually increasing concentrations of sucrose, samples were rapidly frozen in 2-methylbutane chilled at −80°C. Consecutive sections (10 µm) were prepared and frozen at −80°C until usage. NGF expression was visualized by NGF immunohistochemisty using the ABC method. The following antibodies were used: rabbit anti-NGF antibody (1:2000, Cat # sc-548, Santa Cruz Biotechnology Inc) and biotinylated anti-rabbit IgG (1:500, Vector Laboratories, Burlingame, CA). For controls, some sections were processed after preabsorption of the anti-NGF antibody with the blocking peptide (4pg/µl). Other sections were processed without the primary or the secondary antibody as controls.
Immunofluorescence
Double-labeling con-focal microscopy and immunofluorescent staining were performed for NGF and protein gene product 9.5 (PGP 9.5, a panaxonal marker), calcitonin gene related peptide (CGRP, a peptidergic C-fiber marker), isolectin B4 (IB4, a non peptidergic C-fiber marker) or neurofilament 200 (N52, a myelinated A-fiber marker) to examine the colocalization of NGF and sensory fibers. To block nonspecific reactions, sections were incubated in 0.1 mol/L phosphate buffered saline containing 1.5% bovine serum albumin, and 0.1% Tween-20 for 1 hour. Sections were processed for NGF and PGP9.5, CGRP, IB4 or N52 double-labeling. The following primary antibodies were used: rabbit anti-NGF polyclonal antibody (1:1000, Cat # sc-548, Santa Cruz Biotechnology Inc), guinea pig anti-PGP9.5 polyclonal antibody (1:1000, Cat # AB5898, Chemicon, Temecula, CA), rabbit anti-CGRP polyclonal antibody (1:1000, Cat # T-4032, Peninsula Laboratories Inc, San Carlos, CA), biotinylated griffonia simplicifolia lectin I (IB4, 1:8000, Cat # B-1105, Vector Laboratories), mouse anti-neurofilament 200 monoclonal antibody (1:1000, Cat # N0142, Sigma). Sections were then incubated in the following secondary antibodies, Cy3-conjugated goat anti-rabbit IgG, Cy2-conjugated donkey anti-guinea pig IgG, Cy2-conjugated donkey anti-rabbit IgG, Cy2-conjugated streptavidin and Cy2-conjugated goat anti-mouse IgG. All the secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA) and applied to sections with a concentration of 1:200. Sections were mounted and observed under Zeiss 510 con-focal microscopy (Carl Zeiss MicroImaging, Inc., Thornwood, NY). Digital images were collected in the LSM 510 software (Carl Zeiss MicroImaging, Inc). For controls, sections were stained without primary or secondary antibodies.
In situ Hybridization
Rat hindpaw flexor digitorum brevis muscle was taken 1 h after incision. Total RNA was isolated using RNeasy mini kit (Qiagen). One microgram of RNA was used for reverse-transcriptase PCR to amplify a 416 bp band of NGF using primers, forward: 5'-CCATGGTACAATCTCCTTCA-3' reverse: 5'-TGCAGGCAAGTCAGCCTCTT-3'. The product was purified using QIAquick PCR purification kit (Qiagen) and then subcloned into T-easy vector (Promega). After sequencing, the template was linerized with SalI digestion and gel purified using QIAquick Gel Extraction kit (Qiagen). Digoxigenin-labeled probes were made using DIG RNA Labeling Kit (Roche, Mannheim, Germany) and the labeling efficiency was quantified using DIG Luminescent Detection Kit (Roche). All procedures were performed following the manufacturers’ instruction.
Sections were postfixed in 4% paraformaldehyde for 10 min and permeabilized by treatment with 0.002% proteinase K for 5 min. Fixation in 4% paraformaldehyde again for 5 min and underwent acetylation (0.5% acetic anhydride in 0.1 M triethanolamine) for 10 min. Probes were diluted to 10 ng/ml in hybridization buffer (50% formamide, 4 × standard saline citrate, 0.5 × Denhardt’s solution, 0.025% yeast tRNA, 0.025% PolyA, 0.025% salmon sperm DNA, 20% dextran sulfate, and 0.1M dithiothreitol). The sections were hybridized in a humidified chamber at 55°C overnight with either sense, antisense or internal control (actin) probes. Some sections were also incubated in a hybridization solution containing no probe. Adult rat cortex sections were hybridized with NGF sense or antisense probes as positive control because it is well accepted that NGF is produced by neurons in cortex.
After hybridization, sections were subjected to post hybridization washes to remove unbound probes. Endogenous alkaline phosphatase was blocked with blocking reagent (Roche) dissolved in 1 × MABT (0.88% NaCl, 1.16% maleic acid, 0.72% NaOH) containing 0.01% Tween-20 and 20% goat serum for 60 min. Signal was detected by incubating sections with sheep anti-digoxigenin-AP, Fab fragments antibody (1:2000, Cat# 11093274910, Roche) at 4°C overnight, visualized with alkaline phosphatase color substrates, nitroblue tetrazolium/5-Bromo-4-Chloro-Indolyl-Phosphatase for 2 hours. The color reaction was stopped by immersing sections in 0.1M phosphate-buffered saline. Sections were then counterstained in nuclear fast red for 1 min, dehydrated and mounted for microscopic examination.
Statistical analyses
All statistical analyses were performed using Prism 4.0 (GraphPad Software, Inc., San Diego, CA). Data was expressed as mean ± SEM. Differences between means were determined by 1-way ANOVA followed by Dunnett’s post hoc test for comparisons of incision versus sham. Guarding pain scores were analyzed by ANOVA. Subsequent 1-way ANOVA for repeated measures and post hoc t-tests compared the effect of drug and Dunnett’s test vs baseline examined the effect of incision. P<0.05 was considered statistically significant.
Results
Anti-NGF and Guarding Behavior
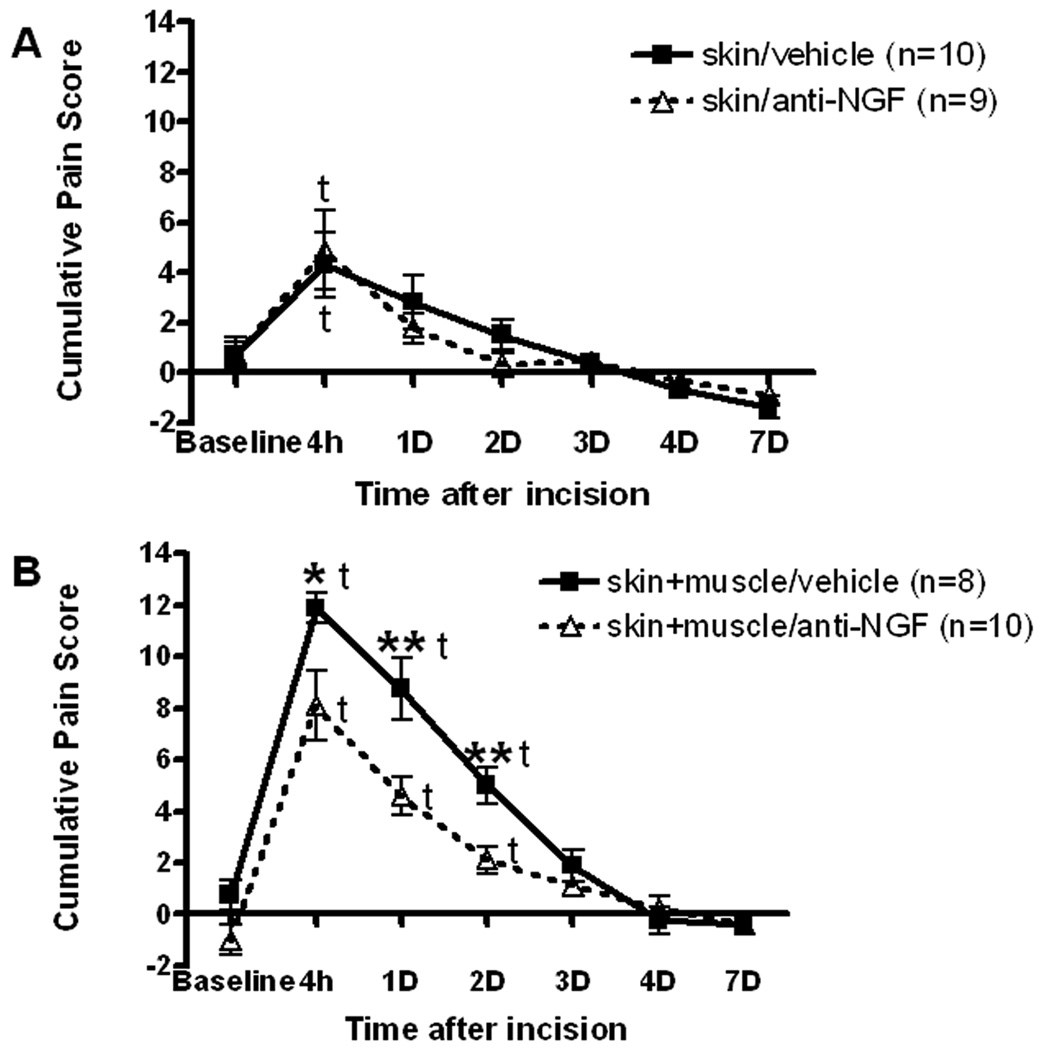
Incision of skin and treatment with vehicle resulted in a cumulative pain score of 4.3±1.3 (fig. 1A, P<0.05 vs baseline) 4 hours after incision. There was no significant guarding thereafter. Treatment with the antiNGF peptibody (100 mg/kg) and skin incision were not different than vehicle and skin incision.
Fig. 1.
Effect of anti-nerve growth factor (NGF) peptibody on guarding pain ehavior caused by plantar incision. A: Cumulative pain score after anti-NGF treatment in rats that underwent only skin incision. B: Cumulative pain score after anti-NGF treatment in rats that underwent skin, fascia and muscle incisions. t, indicates P<0.05 vs baseline. Asterisks indicate *P<0.05 and **P<0.01 vs vehicle, respectively.
In rats that underwent incision of skin, fascia and muscle and were treated with vehicle, guarding was increased at 4 hours, 1 day and 2 days to 11.9±0.6, 8.8±1.2, 5.0±0.7, respectively (fig. 1B, P<0.05 vs baseline). Guarding after pretreatment with anti-NGF peptibody (100 mg/kg) and skin, fascia and muscle incision was 8.1±1.4, 4.6±0.7, 2.1±0.5 at 4 hours, 1 day and 2 days, respectively. The antiNGF peptibody decreased guarding by skin fascia and muscle incision at 4 hours, 1 day and 2 days (P<0.05 vs vehicle).
Reverse-transcriptase PCR of mRNA Levels for NGF in Incised Hind Paw Muscle
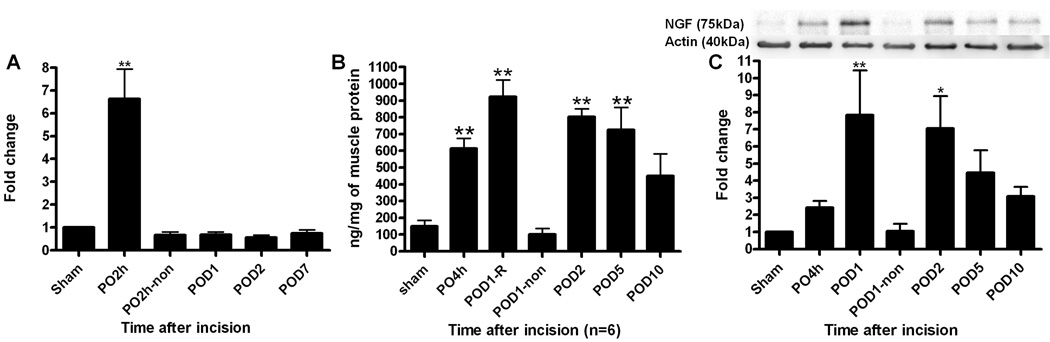
Compared to the β-actin control, NGF mRNA increased 6.6 ± 1.3 fold (P<0.01 vs sham) in incised flexor muscle at 2 h after incision compared with sham control skin (fig. 2A, n = 6 per group). The level of NGF mRNA was not significantly increased on POD 1 and thereafter and was similar to the contralateral, nonincised hind paw flexor muscle.
Fig. 2.
Nerve growth factor (NGF) expression in incised or sham operated plantar flexor digitorum brevis muscle. A: Changes in NGF mRNA relative to β-actin mRNA examined by real time polymerase chain reaction. Data are expressed as mean ± SEM of ratio of incised flexor muscle to sham controls. B: NGF protein determined by enzyme-linked immunosorbent assay. Data are shown as mean ± SEM of nanogram of NGF protein per milligram protein. C: Western blots for NGF and actin immunoreactivity. Top: Example of western blot showing immunoreactive NGF and actin control bands in detailed time course after plantar incision. Bottom: Summary of relative changes in NGF protein expression after plantar incision. Data are expressed as mean ± SEM of ratio of incised flexor muscle to sham controls. Non, contralateral non-incised muscle. PO, post operative. POD, post operative day. * P<0.05, **P<0.01 vs sham. N = 6 per group.
NGF Protein Expression in Incised Hind Paw Muscle
As shown in figure 2B, basal NGF expression was 149.3 ± 35.9 ng/mg protein in sham operated muscle. NGF levels increased to 614.3 ± 61.3 ng/mg (P<0.01 vs. sham) by 4h and peaked at 923.4 ± 101.3 ng/mg on POD 1 in incised muscle. Following POD 1, levels of NGF expression were 803.3 ± 47.3 ng/mg, and 725.1 ± 135.5 ng/mg on POD 2 (P<0.01 vs. sham, n = 6 per group), and POD 5 (P<0.01 vs sham, n = 6 per group), respectively. NGF was not different than control on POD 10 (449.8 ± 131.6 ng/mg) and at the contralateral hind paw muscle (100.1 ± 35.1 ng/mg).
Western blot analysis revealed a single high molecular weight (MW) NGF increased by incision. Representative blots were shown in fig. 2C top. This large MW form of NGF protein, approximately 75 kDa, was increased in incised muscle. A summary quantifying the NGF immunoreactive bands for different times after incision is shown in fig. 2C bottom. The data were normalized to actin (n = 6 per group). In comparison to sham, NGF expression in the incised hind paw increased initially 2.4 ± 0.4 fold by 4h to 7.8 ± 2.6 fold (P<0.01 vs sham) on POD 1. Intensity of NGF expression was increased 7.0 ± 1.9 fold (P<0.05 vs sham) on POD 2, 4.5 ± 1.3 fold on POD 5, and on POD 10 (3.1 ± 0.5 fold). NGF expression in the contralateral, unincised hind paw flexor muscle (1.0 ± 0.4 fold) was the same as sham.
Immunohistochemistry of Incised Hind Paw Muscle for NGF
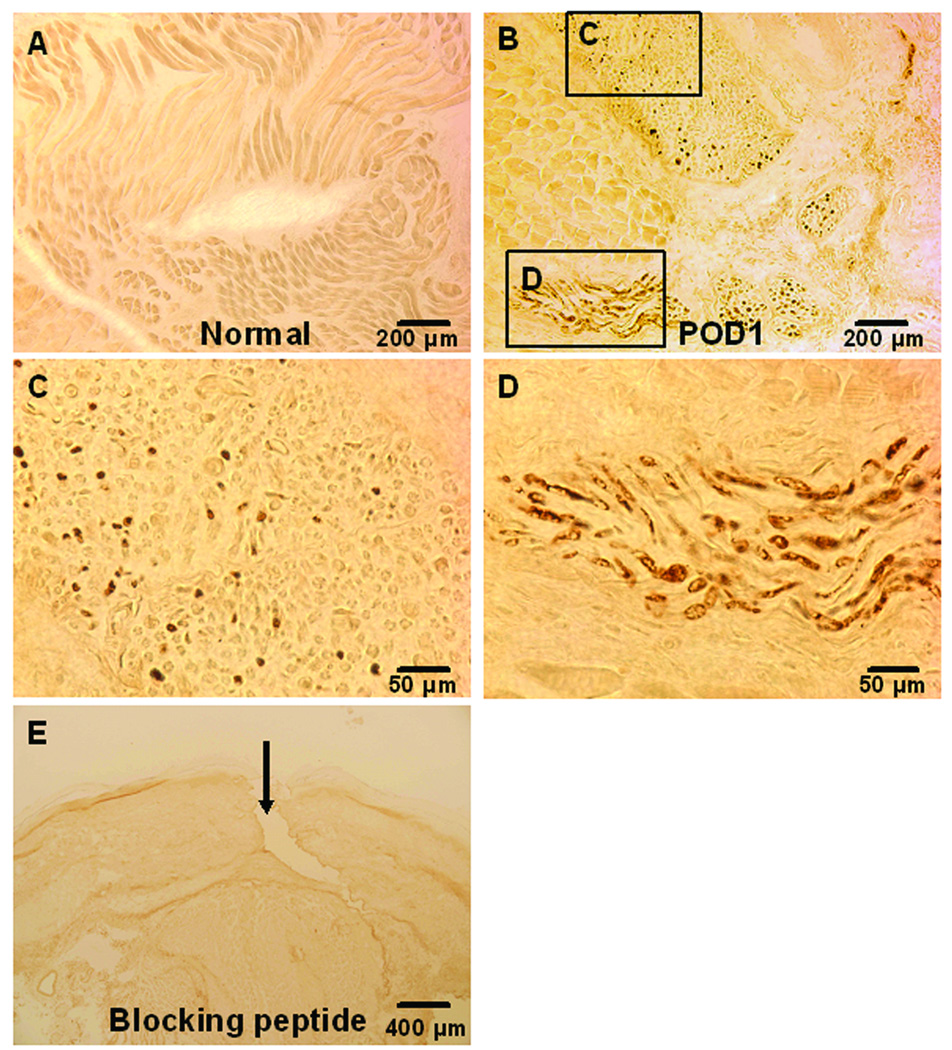
There was no NGF immunoreactivity detected in sham-operated, control flexor muscle (fig. 3A). NGF immunoreactivity was found in apparent nerve-like structures in incised muscle on POD 1 (fig. 3B). Higher magnifications of the rectangular areas marked in figure 3B were shown in figure 3C, and D. NGF immunoreactive elements were localized within transverse and longitudinal sections of nerve-like structures (fig. 3C and D). After preabsorption of the anti-NGF antibody with the hapten blocking peptide, there was no NGF immunoreactivity detected in skin, fascia and muscle (fig. 3E).
Fig. 3.
3’3-diaminobenzidine immunohistochemistry for nerve growth factor (NGF) in plantar flexor digitorum brevis muscle. A: NGF immunohistochemistry in sham operated flexor muscle. B: NGF in nerve-like structures from incised muscle on POD (post operative day) 1. C: A higher magnification of the rectangular area C in Fig. B, showing immunoreactivity for NGF within a transverse sectioned nerve-like structure. D: A higher magnification of the rectangular area D in B, showing immunoreactivity for NGF within a longitudinal sectioned nerve-like structure. E: NGF immunohistochemistry in skin, fascia and muscle after preabsorption with blocking peptide. The arrow indicates the incision.
Immunofluorescent Double Labeling for NGF in Incised Hind Paw Muscle
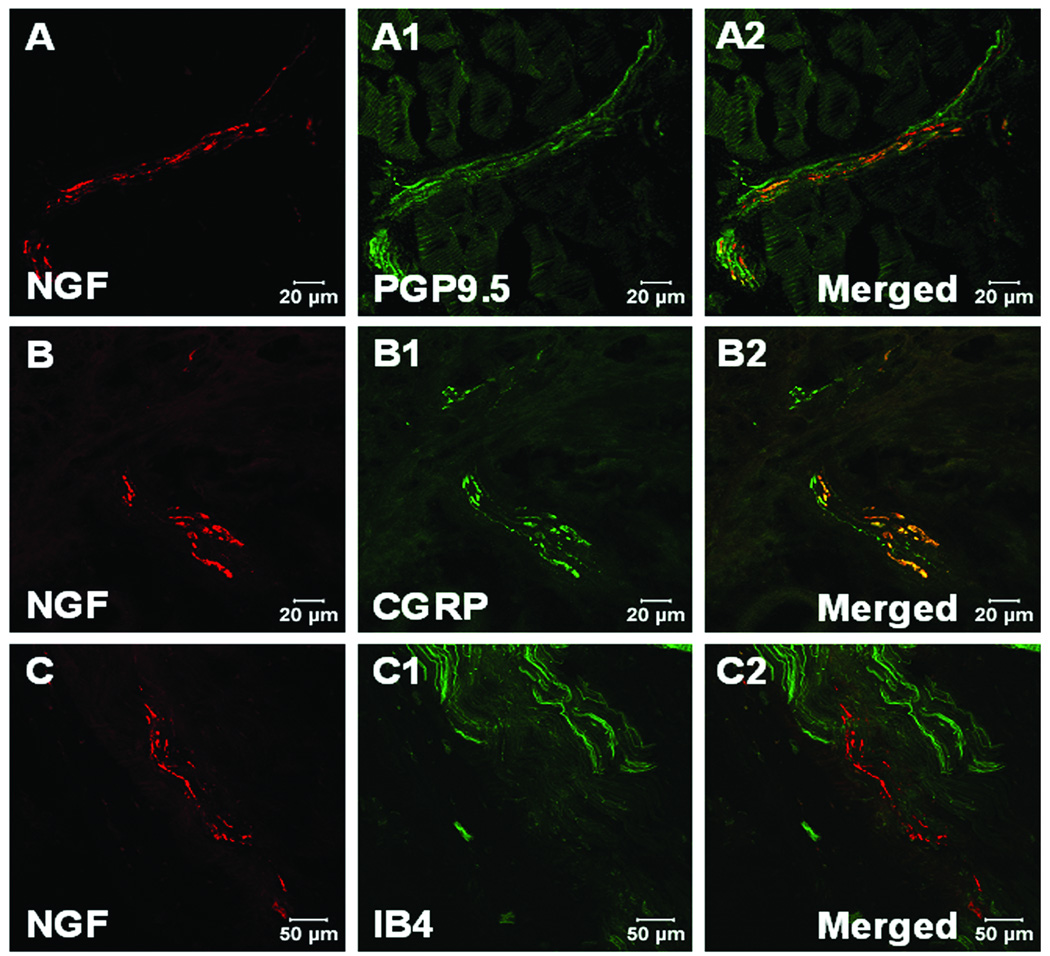
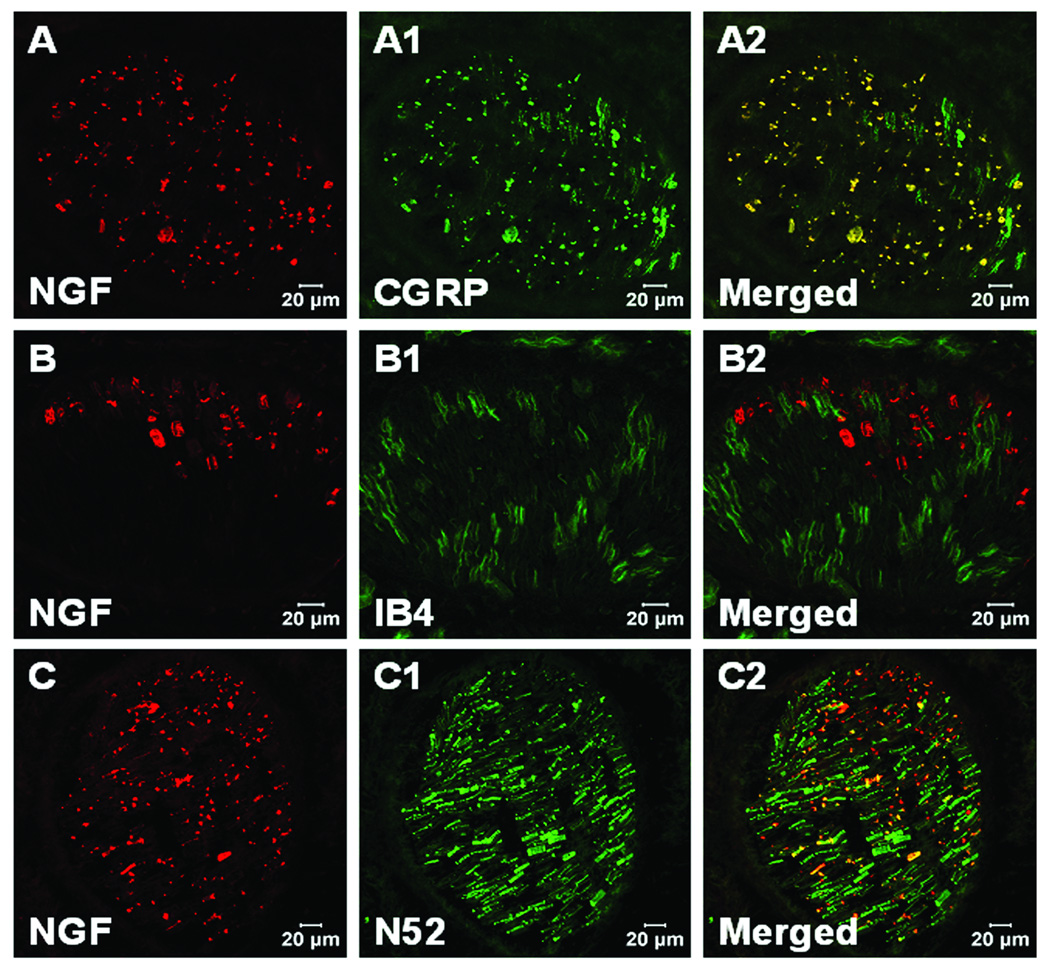
NGF and PGP9.5, CGRP or IB4 double labeling was performed to confirm the immunoreactive NGF on nerve structures. NGF positive staining was found in nerve axons labeled with PGP9.5 in incised muscle one day after incision (fig. 4A–A2). These NGF positive axons were colocalized with CGRP positive fibers (fig. 4B–B2), but not IB4 positive non peptidergic C-fibers (fig. 4C–C2). NGF positive staining could also be found in large nerve bundles in deep tissue, which innervates the flexor digitorum brevis muscle (fig. 5) and possibly other deep structures. In these bundles NGF immunoreactivity was colocalized with CGRP positive fibers (fig. 5A–A2). There was no co-labeling of NGF with IB4 positive C-fibers (fig. 5B–B2), but co-labeling was present on some N52 labelled myelinated axons (fig. 5C–C2).
Fig. 4.
Confocal image of nerve fibers in muscle tissue for nerve growth factor (NGF) and protein gene product 9.5 (PGP 9.5), calcitonin gene related peptide (CGRP), isolectin B4 (IB4) fluorescent immunohistochemistry. A, B and C: NGF fluorescent immunohistochemistry (red) within incised flexor muscle on POD (post operative day) 1. A1: PGP 9.5 immunohistochemistry for nerve axons (green) in incised flexor muscle. A2: Merged image shows NGF colocalizes with PGP9.5 immunoreactive axons (yellow). B1: CGRP immunohistochemistry for axons (green) within incised flexor muscle. B2: Merged image shows NGF colocalizes with CGRP immunoreactive axons (yellow). C1: IB4 labeling (green) in incised flexor muscle. C2: Merged image shows NGF does not colocalize with IB4 axons (yellow).
Fig. 5.
Confocal image for nerve growth factor (NGF) and calcitonin gene related peptide (CGRP), isolectin B4 (IB4), neurofilament 200 (N52) fluorescent double labeling immunohistochemistry in large nerve in deep tissue. A, B and C: NGF fluorescent immunohistochemistry one day after plantar incision. A1: CGRP immunohistochemistry for axons (green) in large nerve. A2: Merged image shows NGF colocalizes with CGRP immunoreactive axons (yellow). B1: IB4 labelling for axons (green) in large nerve. B2: Merged image shows NGF does not colocalize with IB4 axons (yellow). C1: N52 labelling for axons (green) in large nerve. C2: Merged image shows NGF colocalizes with some N52 axons (yellow).
In situ Hybridization for NGF mRNA Expression in Incised Hind Paw
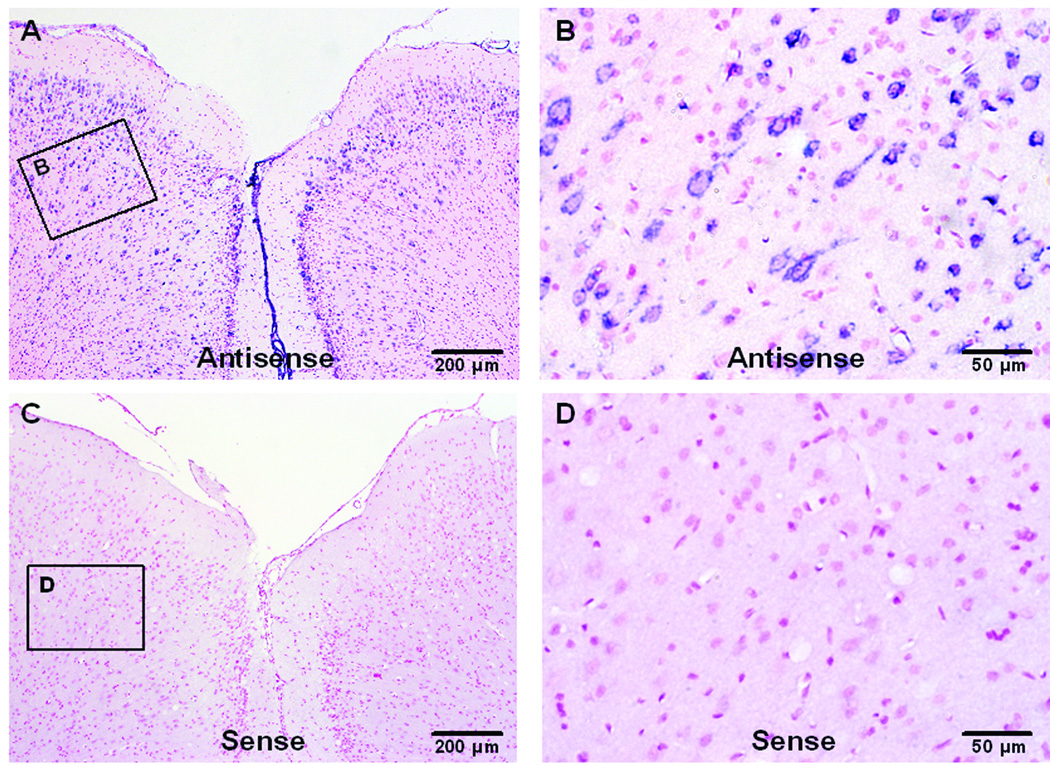
Adult rat brain cortex sections were subjected to in situ hybridization with NGF antisense and sense probes to examine the specificity of the NGF probes (fig. 6). NGF mRNA signals (blue) could be observed in cortex (fig. 6A) by antisense probe hybridization, most of the signals were localized in cytoplasm surrounding the nucleus (red) of neurons (fig. 6B). There was no NGF mRNA staining in cortex when hybridized with the sense probe (fig. 6C), only nuclear staining (red) was observed (fig. 6D).
Fig. 6.
In situ hybridization for nerve growth factor (NGF) mRNA in cortex of adult rat brain. Sections are counter stained with fast red for nuclei. A: In situ hybridization (ISH) for NGF mRNA with antisense probe. B: A higher magnification of the rectangular area in A, showing NGF expression (blue) in neuron cytoplasm. C: ISH for NGF mRNA with sense probe. D: A higher magnification of the rectangular area in C.
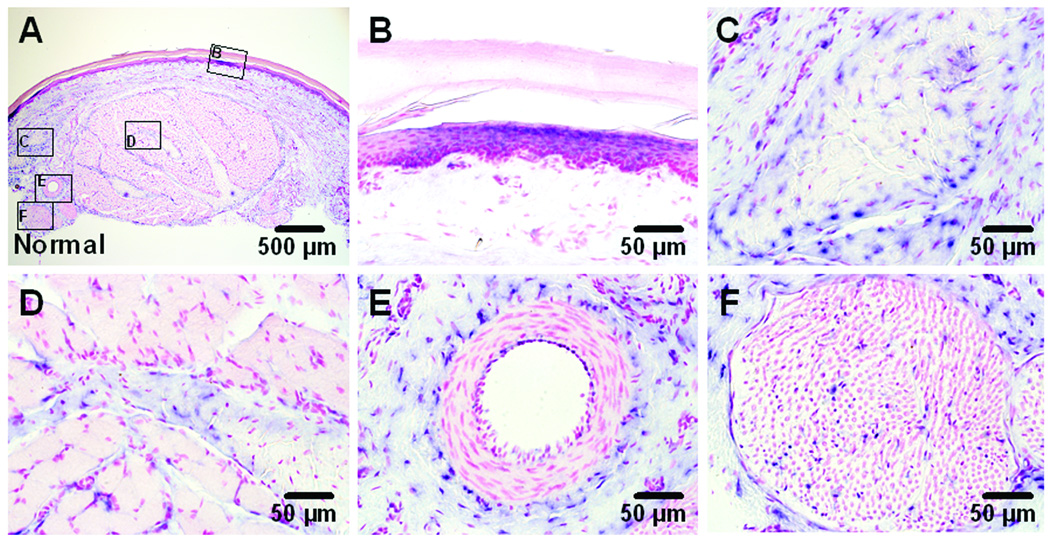
NGF mRNA expression could be found in sections obtained from sham incised hindpaw (fig. 7A). The sources of NGF mRNA included keratinocytes in the epidermis and fibroblasts in dermis (fig. 7B). In deeper tissue, fibroblasts in dense connective tissue (fig. 7C), perimysium of muscle (fig. 7D), peri-vascular connective tissue (fig. 7E) and a large nerve bundle (fig. 7F) innervating the muscle were positive for NGF mRNA.
Fig. 7.
In situ hybridization (ISH) for nerve growth factor (NGF) mRNA in sham operated plantar skin and flexor digitorum brevis muscle. Sections are counter stained with fast red for nuclei. A: ISH for NGF mRNA with antisense probe. B–F: Higher magnification photographs show NGF mRNA expression (blue) within epidermis and dermis (B), dense connective tissue in subcutaneous tissue (C), perimysium which separates muscle fibers (D), peri-vascular connective tissue (E) and large nerve bundle (F) in deep layer which innervates plantar skin and flexor muscle.
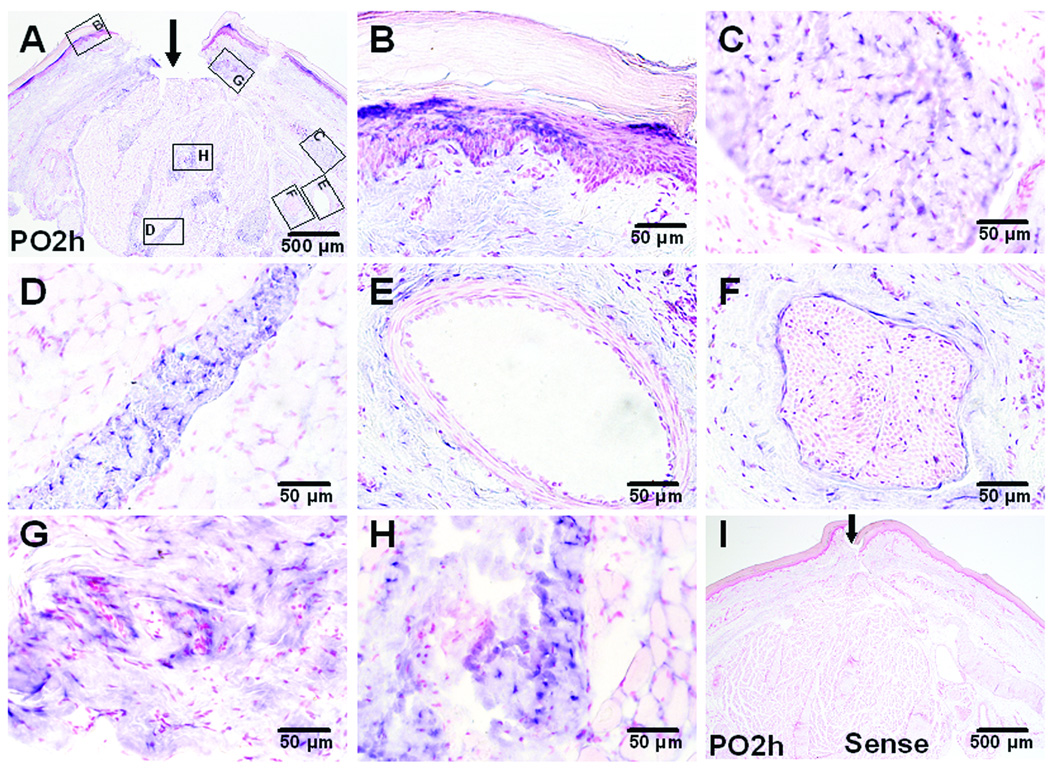
NGF mRNA labeling was observed in sections obtained at 2 h after plantar incision (fig. 8A) when NGF mRNA by real time PCR was increased. In addition to structures labeled in the unincised tissue (fig. 8B–F), fibroblasts in the subcutaneous region adjacent to the dissected tissue and the incision (fig. 8G), and fibroblasts adjacent to the muscle incision (fig. 8H) produced NGF mRNA. In situ hybridization with NGF sense probe at 2 hours after incision did not show NGF mRNA positive signals (fig. 8I).
Fig. 8.
In situ hybridization (ISH) for nerve growth factor (NGF) mRNA in incised plantar skin and flexor digitorum brevis muscle. Sections are counter stained with fast red for nuclei. A: ISH for NGF mRNA with the antisense probe 2 hours after incision. B–H: Higher magnification photographs show NGF mRNA expression within epidermis and dermis (B), dense connective tissue in subcutaneous region (C), perimysium (D), peri-vascular connective tissue (E) and large nerve bundle (F) in deep tissue layer. ISH in subcutaneous region adjacent to skin incision (G), and flexor muscle fibers adjacent to muscle incision (H). I: ISH for NGF mRNA with sense probe at 2 hours after incision showing no positive staining. The arrows indicate the incision. PO – post operative.
Discussion
In this study, incision that included hind paw fascia and flexor digitorum brevis muscle produced guarding behavior that was reduced by blockade of NGF. NGF expression was examined in normal and in incised hind paw muscle. NGF mRNA was increased at 2 h after incision and returned to normal on POD 1. NGF protein increased 4 hours after muscle incision, was increased for 5 days, and not different than control on POD 10. A large MW form of NGF was produced and increased by incision. NGF immunoreactivity was not identified in normal muscle; however, NGF immunoreactive structures were observed in nerve bundles from incised muscle. Further delineation of the immunoreactive structures revealed that NGF staining was present in CGRP and N52 positive axons. IB4 positive axons did not stain for NGF. NGF mRNA was present in normal hindpaw sections. Fibroblasts were the sources of NGF mRNA in perimysium, peri-vascular connective tissue and nerve adjacent to the incised muscle.
NGF is an important pain mediator in skin,2 however, NGF and muscle has been studied to a lesser extent. NGF is synthesized in normal skeletal muscle tissue but the cellular elements producing NGF are not known.16,17 Studies by others indicate NGF contributes to muscle healing after injury.18 NGF is also up regulated in muscle after intramuscular formalin injection.19
Previous human studies indicate the potential role of NGF in muscle pain. Local injection of NGF into the masseter muscle produces long-term local signs of mechanical allodynia and hyperalgesia and is associated with pain during jaw movement.20 In trials of parenterally administered NGF for patients with diabetic neuropathy, NGF did not improve symptoms, rather, administration of NGF elicited whole body myalgias in patients.4 The duration and severity of these myalgias were dose-dependent.
Muscle nerves contain motor axons, sensory axons and sympathetic axons; the sensory fibers are both myelinated and unmyelinated.21 Our data have shown immunocytochemical labeling for NGF in CGRP positive peptidergic axons which may also express the high affinity NGF receptor trkA.22–24 NGF labeling was not found in IB4 positive non-peptidergic axons. In incisions, NGF was co labeled with the myelinated axon marker N52 positive in a few fibers. Since there is approximately 10% overlap between CGRP and N52 positive axons, these NGF positive, N52 positive fibers may also have the trkA receptor.25
In animal studies, intramuscular injection of NGF excites primary afferent muscle nociceptors26 and dorsal horn neurons transmitting nociception from muscle.27,28 One study was not able to detect sensitization of nociceptors by NGF injected into rat masseter muscle.29 Muscle afferents transmit both mechanosensitivity and chemosensitivity.30–33 There is also evidence that muscle afferents have thermosensitivity.34
NGF released by muscle incision likely influences the sensitivity of nociceptors to a variety of mediators although no systematic studies of NGF and muscle sensory ion channels and receptors have been described. In other tissues, NGF regulates a number of ligand-gated ion channels including purinergic receptor of ATP (P2X3), acid sensing ion channel 3, transient receptor potential vanilloid 1 and G protein–coupled receptors including bradykinin B2 receptors.35 NGF also increases voltage-gated sodium channels, including tetradodotoxin-sensitive and tetradodotoxin-resistant sodium channels (Nav 1.3, 1.8, and 1.9). Whether these mechanisms through which NGF contributes to nociception in other tissues also influence nociception from muscle is not known. We speculate NGF influences chemosensitivity of muscle afferents after incision, potentiating its responses to mediators like prostaglandins, acid, and lactate.
NGF is produced in several forms by a variety of tissues and cells depending upon the conditions. A large MW form of NGF, approximately 75 kDa, was shown after muscle incision in the present study and skin incision in a previous study.9 Since mature NGF is approximately 16 kDa, this large MW form is likely a glycosylated precursor, like preproNGF, or a dimer of a precursor form.36,37 Isaacson et al37,38 demonstrated that ‘mature’ forms of the lower MW NGF proteins are uncommon in tissues and each tissue exhibits a characteristic NGF expression pattern. In adults, muscle and skin have the same large MW form and both increase in response to incision.
In vitro studies of primary cell cultures indicate that NGF is produced by fibroblasts39, keratinocytes40 and T cells.41 In inflammatory pain models, NGF is likely produced by macrophages42 and mast cells,43, whereas after nerve injury, Schwann cells are a source of NGF.44
In the present study, NGF mRNA was identified using in situ hybridization in normal plantar skin and flexor muscle as well as in incised tissue. Fibroblasts from fascia and connective tissues surrounding muscle express NGF in the area injured by the incision. NGF mRNA was found in non-neuronal cells, while NGF protein was localized in nerve fibers. NGF expression by inflammatory cells was not prominent. The different NGF source in our incisional pain model compared with other inflammatory pain models supports the unique characteristics for incisional pain mechanisms versus inflammatory pain. Our data are supported by another study of cutaneous wound healing45, which demonstrates NGF is produced by keratinocytes and fibroblasts and contributes to cutaneous wound healing.
In previous studies in skin 9, we demonstrated that NGF protein is present in the area surrounding incised skin. In transverse hindpaw skin incisions, immunoreactive NGF was prominent in distal axons in skin but not found in axons proximal to the incision. Based on these previous results, the present study and work by others 46,47, we propose the following model. NGF is synthesized in fibroblasts and connective tissue in incisions, is retrogradely transported to the dorsal root ganglia in intact axons and sensitizes these nociceptors. The distal portions of cut axons take up NGF but do not transport it retrogradely.46 These distal portions of the cut axons are immunoreactive for NGF. The co-labeling of NGF with CGRP, indicates these are Trk A positive axons. In incised skin, NGF produces heat sensitization.10 In deep muscle incision, we propose NGF produces spontaneous activity resulting in ongoing pain.
In summary, NGF contributes to guarding pain behaviors caused by deep muscle incision. In incised muscle, NGF mRNA increases 2 hours after incision and increased protein expression is evident for several days. Immunohistochemical studies of incisions reveals NGF protein in CGRP but not IB4 positive nerve fibers. Fibroblasts adjacent to the injury are sources of NGF in incised muscle. NGF is not only important for cutaneous pain mechanism but also for pain from incised muscle.
Acknowledgement
Support: This work was supported by the Department of Anesthesia at the University of Iowa and by National Institutes of Health, Bethesda, Maryland grants GM-55831 AND GM-067752 to T.J.B.
The authors are grateful for the technical assistance of Alberto Subieta, B.S. Research Assistant, Department of Anesthesia, University of Iowa, Iowa City, Iowa
Footnotes
Conflict of Interest: Dr. Brennan has served as a consultant for Amgen Inc., Thousand Oaks, California
Brief Summary Statement: Nerve growth factor is produced by fibroblasts and contributes to muscle incisional pain.
References
- 1.Levi-Montalcini R, Dal Toso R, della Valle F, Skaper SD, Leon A.Update of the NGF saga J Neurol Sci 1995130119 0127 [DOI] [PubMed] [Google Scholar]
- 2.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 3.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 4.Petty BG, Cornblath DR, Adornato BT, Chaudhry V, Flexner C, Wachsman M, Sinicropi D, Burton LE, Peroutka SJ. The effect of systemically administered recombinant human nerve growth factor in healthy human subjects. Ann Neurol. 1994;36:244–246. doi: 10.1002/ana.410360221. [DOI] [PubMed] [Google Scholar]
- 5.Dyck PJ, Peroutka S, Rask C, Burton E, Baker MK, Lehman KA, Gillen DA, Hokanson JL, O'Brien PC. Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heat-pain threshold in humans. Neurology. 1997;48:501–505. doi: 10.1212/wnl.48.2.501. [DOI] [PubMed] [Google Scholar]
- 6.McMahon SB, Bennett DL, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med. 1995;1:774–780. doi: 10.1038/nm0895-774. [DOI] [PubMed] [Google Scholar]
- 7.Rueff A, Mendell LM. Nerve growth factor NT-5 induce increased thermal sensitivity of cutaneous nociceptors in vitro. J Neurophysiol. 1996;76:3593–3596. doi: 10.1152/jn.1996.76.5.3593. [DOI] [PubMed] [Google Scholar]
- 8.Zahn PK, Subieta A, Park SS, Brennan TJ. Effect of blockade of nerve growth factor and tumor necrosis factor on pain behaviors after plantar incision. J Pain. 2004;5:157–163. doi: 10.1016/j.jpain.2004.02.538. [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Boustany L, Liang H, Brennan TJ. Nerve growth factor expression after plantar incision in the rat. Anesthesiology. 2007;107:128–135. doi: 10.1097/01.anes.0000267512.08619.bd. [DOI] [PubMed] [Google Scholar]
- 10.Banik RK, Subieta AR, Wu C, Brennan TJ. Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. Pain. 2005;117:68–76. doi: 10.1016/j.pain.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Schaible H-G. In: Basic mechanisms of deep somatic pain, Textbook of Pain. 5th Edition. McMahon SB, Koltzenburg M, editors. Elsevier Publisher; 2006. pp. 621–633. [Google Scholar]
- 12.Wild KD, Bian D, Zhu D, Davis J, Bannon AW, Zhang TJ, Louis JC. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J Pharmacol Exp Ther. 2007;322:282–287. doi: 10.1124/jpet.106.116236. [DOI] [PubMed] [Google Scholar]
- 13.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 14.Zahn PK, Gysbers D, Brennan TJ. Effect of systemic and intrathecal morphine in a rat model of postoperative pain. Anesthesiology. 1997;86:1066–1077. doi: 10.1097/00000542-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Amano T, Yamakuni T, Okabe N, Sakimura K, Takahashi Y. Production of nerve growth factor in rat skeletal muscle. Neurosci Lett. 1991;132:5–7. doi: 10.1016/0304-3940(91)90418-s. [DOI] [PubMed] [Google Scholar]
- 17.Stuerenburg HJ, Kunze K. Tissue concentrations of nerve growth factor in aging rat heart and skeletal muscle. Muscle Nerve. 1998;21:404–406. doi: 10.1002/(sici)1097-4598(199803)21:3<404::aid-mus17>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Menetrey J, Kasemkijwattana C, Day CS, Bosch P, Vogt M, Fu FH, Moreland MS, Huard J. Growth factors improve muscle healing in vivo. J Bone Joint Surg Br. 2000;82:131–137. doi: 10.1302/0301-620x.82b1.8954. [DOI] [PubMed] [Google Scholar]
- 19.Schafers M, Sorkin LS, Sommer C. Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain. 2003;104:579–588. doi: 10.1016/S0304-3959(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 20.Svensson P, Cairns BE, Wang K, Arendt-Nielsen L. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain. 2003;104:241–247. doi: 10.1016/s0304-3959(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 21.Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54:241–289. doi: 10.1016/0304-3959(93)90027-M. [DOI] [PubMed] [Google Scholar]
- 22.Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996;206:33–36. doi: 10.1016/0304-3940(96)12418-6. [DOI] [PubMed] [Google Scholar]
- 24.McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 25.Meyer RA, Ringkamp M, Campbell JN, Raja SN. In: Peripherial mechanisms of cutaneous nociception, Textbook of Pain. 5th Edition. McMahon SB, Koltzenburg M, editors. Elsevier Publisher; 2006. pp. 3–34. [Google Scholar]
- 26.Hoheisel U, Unger T, Mense S. Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain. 2005;114:168–176. doi: 10.1016/j.pain.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Taguchi T, Hoheisel U, Mense S. Dorsal horn neurons having input from low back structures in rats. Pain. 2008;138:119–129. doi: 10.1016/j.pain.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Hoheisel U, Unger T, Mense S. Sensitization of rat dorsal horn neurons by NGF-induced subthreshold potentials and low-frequency activation. A study employing intracellular recordings in vivo. Brain Res. 2007;1169:34–43. doi: 10.1016/j.brainres.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 29.Mann MK, Dong XD, Svensson P, Cairns BE. Influence of intramuscular nerve growth factor injection on the response properties of rat masseter muscle afferent fibers. J Orofac Pain. 2006;20:325–336. [PubMed] [Google Scholar]
- 30.Kumazawa T, Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977;273:179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mense S, Meyer H. Different types of slowly conducting afferent units in cat skeletal muscle and tendon. J Physiol. 1985;363:403–417. doi: 10.1113/jphysiol.1985.sp015718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paintal AS. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with ending in skeletal muscle. Circ Res. 1982;50:133–139. doi: 10.1161/01.res.50.1.133. [DOI] [PubMed] [Google Scholar]
- 34.Graven-Nielsen T, Arendt-Nielsen L, Mense S. Thermosensitivity of muscle: high-intensity thermal stimulation of muscle tissue induces muscle pain in humans. J Physiol. 2002;540:647–656. doi: 10.1113/jphysiol.2001.013336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 36.Yardley G, Relf B, Lakshmanan J, Reinshagen M, Moore GP. Expression of nerve growth factor mRNA and its translation products in the anagen hair follicle. Exp Dermatol. 2000;9:283–289. doi: 10.1034/j.1600-0625.2000.009004283.x. [DOI] [PubMed] [Google Scholar]
- 37.Bierl MA, Jones EE, Crutcher KA, Isaacson LG. ‘Mature’ nerve growth factor is a minor species in most peripheral tissues. Neurosci Lett. 2005;380:133–137. doi: 10.1016/j.neulet.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Bierl MA, Isaacson LG. Increased NGF proforms in aged sympathetic neurons and their targets. Neurobiol Aging. 2007;28:122–134. doi: 10.1016/j.neurobiolaging.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda H, Kannan Y, Ushio H, Kiso Y, Kanemoto T, Suzuki H, Kitamura Y. Nerve growth factor induces development of connective tissue-type mast cells in vitro from murine bone marrow cells. J Exp Med. 1991;174:7–14. doi: 10.1084/jem.174.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tron VA, Coughlin MD, Jang DE, Stanisz J, Sauder DN. Expression and modulation of nerve growth factor in murine keratinocytes (PAM 212) J Clin Invest. 1990;85:1085–1089. doi: 10.1172/JCI114539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehrhard PB, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc Natl Acad Sci U S A. 1993;90:10984–10988. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987;330:658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- 43.Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson EM, Jr, Taniuchi M, DiStefano PS. Expression and possible function of nerve growth factor receptors on Schwann cells. Trends Neurosci. 1988;11:299–304. doi: 10.1016/0166-2236(88)90090-2. [DOI] [PubMed] [Google Scholar]
- 45.Matsuda H, Koyama H, Sato H, Sawada J, Itakura A, Tanaka A, Matsumoto M, Konno K, Ushio H, Matsuda K. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J Exp Med. 1998;187:297–306. doi: 10.1084/jem.187.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raivich G, Hellweg R, Kreutzberg GW. NGF receptor-mediated reduction in axonal NGF uptake and retrograde transport following sciatic nerve injury and during regeneration. Neuron. 1991;7:151–164. doi: 10.1016/0896-6273(91)90083-c. [DOI] [PubMed] [Google Scholar]
- 47.Curtis R, Tonra JR, Stark JL, Adryan KM, Park JS, Cliffer KD, Lindsay RM, DiStefano PS. Neuronal injury increases retrograde axonal transport of the neurotrophins to spinal sensory neurons and motor neurons via multiple receptor mechanisms. Mol Cell Neurosci. 1998;12:105–118. doi: 10.1006/mcne.1998.0704. [DOI] [PubMed] [Google Scholar]