Abstract
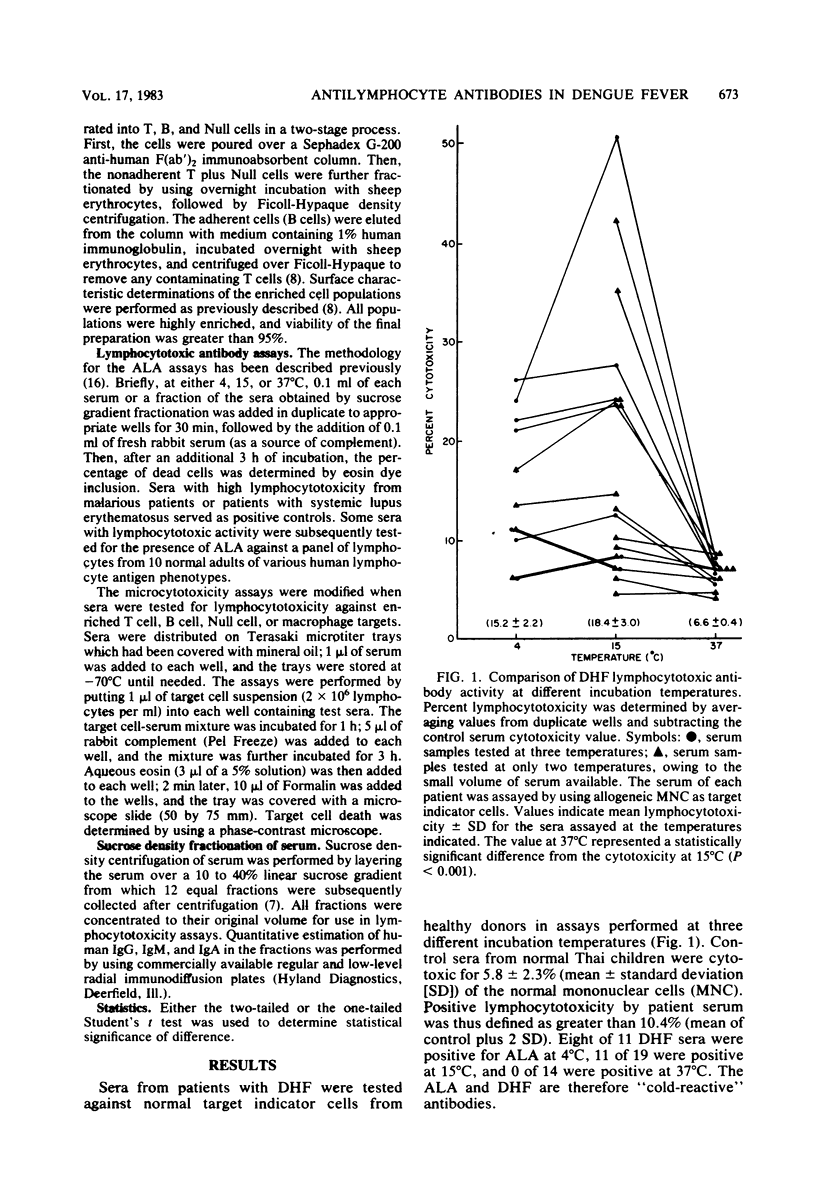
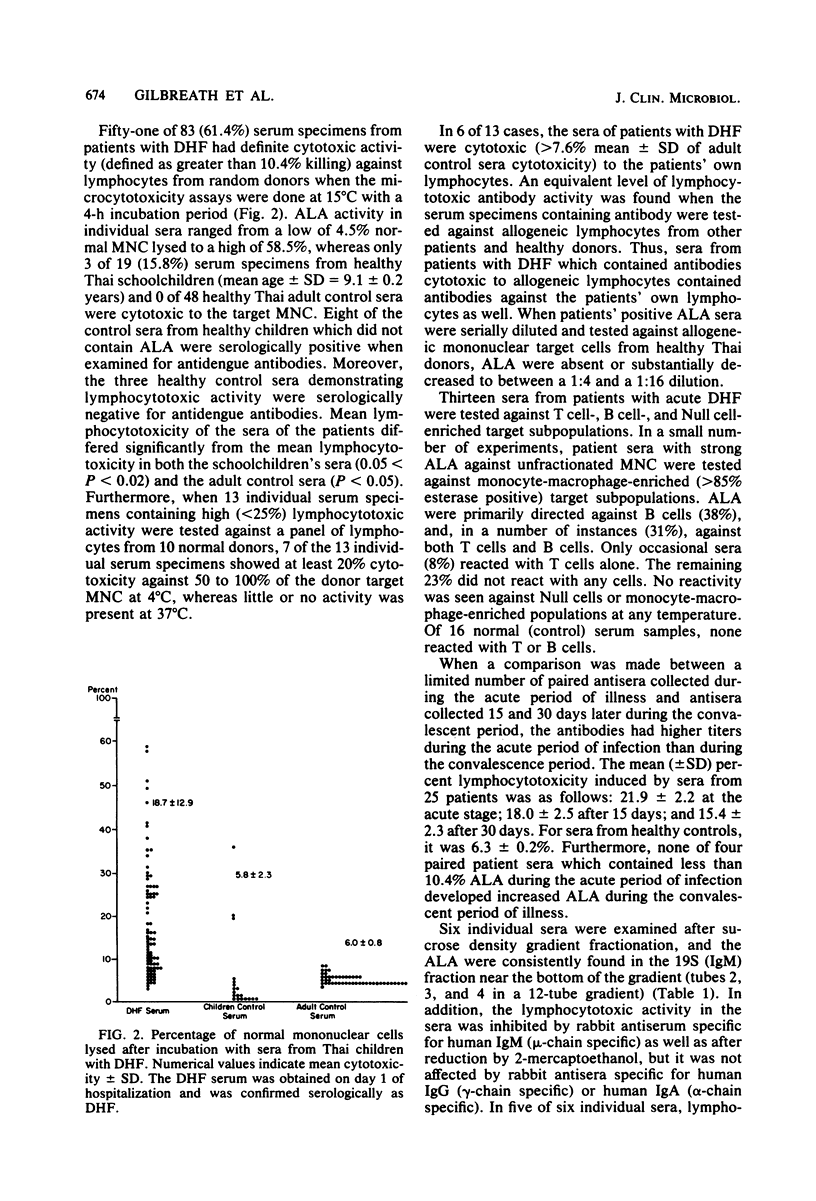
Antilymphocyte antibodies were found in 51 of 83 serum specimens from Thai children with dengue hemorrhagic fever (DHF). The lymphocytotoxic activity was complement dependent, and cytotoxicity was detected in the 19S immunoglobulin M-associated serum fractions at a temperature optimum of 15 degrees C. Sera with lymphocytotoxic activity were cytotoxic to autologous as well as allogeneic lymphocytes from patients and healthy adult donors and were directed primarily against B cells, with some T cell cross-reactivity. This study suggests that infection with DHF induces predominately cold-reactive antilymphocyte antibodies in DHF patients that could potentially interact with peripheral blood cells of patients and modulate the humoral immune responses of patients during infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. G., Britton S. A new approach to the study of human B lymphocyte function using an indirect plaque assay and a direct B cell activator. Immunol Rev. 1979;45:41–67. doi: 10.1111/j.1600-065x.1979.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Boonpucknavig S., Lohachitranond C., Nimmanitya S. The pattern and nature of the lymphocyte population response in dengue hemorrhagic fever. Am J Trop Med Hyg. 1979 Sep;28(5):885–889. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cicciarelli J. C., Chia D., Terasaki P. I., Barnett E. V., Shirahama S. Human IgM anti-IgM cytotoxin for B lymphocytes. Tissue Antigens. 1980 Mar;15(3):275–282. doi: 10.1111/j.1399-0039.1980.tb00918.x. [DOI] [PubMed] [Google Scholar]
- Dehoratius R. J., Henderson C., Strickland R. G. Lymphocytotoxins in acute and chronic hepatitis. Characterization and relationship to changes in circulating T lymphocytes. Clin Exp Immunol. 1976 Oct;26(1):21–27. [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., Chow J. S., Marchette N. J. Immunological enhancement of dengue virus replication. Nat New Biol. 1973 May 2;243(122):24–26. [PubMed] [Google Scholar]
- MacDermott R. P., Stacey M. C. Further characterization of the human autologous mixed leukocyte reaction (MLR). J Immunol. 1981 Feb;126(2):729–734. [PubMed] [Google Scholar]
- Marchette N. J., Halstead S. B. Phytohemagglutinin enhancement of dengue-2 virus replication in nonimmune rhesus monkey peripheral blood leukocytes. Infect Immun. 1978 Jan;19(1):40–45. doi: 10.1128/iai.19.1.40-45.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K. K., Mickey M. R., Singal D. P., Terasaki P. I. Serotyping for homotransplantation. 18. Refinement of microdroplet lymphocyte cytotoxicity test. Transplantation. 1968 Nov;6(8):913–927. doi: 10.1097/00007890-196811000-00006. [DOI] [PubMed] [Google Scholar]
- Notkins A. L., Mergenhagen S. E., Howard R. J. Effect of virus infections on the function of the immune system. Annu Rev Microbiol. 1970;24:525–538. doi: 10.1146/annurev.mi.24.100170.002521. [DOI] [PubMed] [Google Scholar]
- Terasaki P. I., Mottironi V. D., Barnett E. V. Cytotoxins in disease. Autocytotoxins in lupus. N Engl J Med. 1970 Oct 1;283(14):724–728. doi: 10.1056/NEJM197010012831403. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Brandt W. E., Russell P. K., Dixon F. T. Replication of dengue-2 virus in cultured human lymphoblastoid cells and subpopulations of human peripheral leukocytes. J Immunol. 1976 Sep;117(3):953–961. [PubMed] [Google Scholar]
- Wells R. A., Pavanand K., Zolyomi S., Permpanich B., Macdermott R. P. Anti-lymphocytotoxic antibodies in sera of Thai adults infected with Plasmodium falciparum or Plasmodium vivax. Clin Exp Immunol. 1980 Mar;39(3):663–667. [PMC free article] [PubMed] [Google Scholar]
- Wells R. A., Scott R. M., Pavanand K., Sathitsathein V., Cheamudon U., Macdermott R. P. Kinetics of peripheral blood leukocyte alterations in Thai children with dengue hemorrhagic fever. Infect Immun. 1980 May;28(2):428–433. doi: 10.1128/iai.28.2.428-433.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield J. B., Winchester R. J., Kunkel H. G. Association of cold-reactive antilymphocyte antibodies with lymphopenia in systemic lupus erythematosus. Arthritis Rheum. 1975 Nov-Dec;18(6):587–594. doi: 10.1002/art.1780180609. [DOI] [PubMed] [Google Scholar]
- Winfield J. B., Winchester R. J., Wernet P., Fu S. M., Kunkel H. G. Nature of cold-reactive antibodies to lymphocyte surface determinants in systemic lupus erythematosus. Arthritis Rheum. 1975 Jan-Feb;18(1):1–8. doi: 10.1002/art.1780180101. [DOI] [PubMed] [Google Scholar]
- Winter P. E., Nantapanich S., Nisalak A., Udomsakdi S., Dewey R. W., Russell P. K. Recurrence of epidemic dengue hemorrhagic fever in an insular setting. Am J Trop Med Hyg. 1969 Jul;18(4):573–579. doi: 10.4269/ajtmh.1969.18.573. [DOI] [PubMed] [Google Scholar]
- Wood T. A., Frenkel E. P. The atypical lymphocyte. Am J Med. 1967 Jun;42(6):923–936. doi: 10.1016/0002-9343(67)90073-3. [DOI] [PubMed] [Google Scholar]


