Abstract
Background:
Cerebrovascular disease (CVD) may contribute to mild cognitive impairment (MCI). We sought to determine the relation of white matter hyperintensity (WMH) volume and infarcts in brain MRI to MCI in a community-based sample.
Methods:
A total of 679 elderly persons without dementia underwent brain MRI. WMH and infarcts were quantified using research methods. WMH was adjusted for total cranial volume. The Petersen criteria were used to define MCI. MCI was further subclassified into amnestic and non-amnestic. We used logistic regression to relate WMH and infarcts to prevalent MCI.
Results:
WMH were associated with amnestic MCI (odds ratio [OR] = 1.9; 95% confidence interval [CI] 1.1, 3.4) but not non-amnestic MCI (OR = 1.2; 95% CI 0.4, 1.6) after adjusting for age, gender, ethnic group, education, and APOE-ɛ4. Infarcts were more strongly associated with non-amnestic MCI (OR = 2.7; 95% CI 1.5, 4.8) than amnestic MCI (OR = 1.4; 95% CI 0.9, 2.3). In secondary analyses using continuous cognitive scores as outcomes, WMH, but not infarcts, were related to memory, while infarcts were more strongly related with non-amnestic domains.
Conclusion:
White matter hyperintensity (WMH) is more strongly related to amnestic mild cognitive impairment (MCI). Infarcts are more strongly related to non-amnestic MCI. The nature of WMH in amnestic MCI requires further study.
GLOSSARY
- AD
= Alzheimer disease;
- BDAE
= Boston Diagnostic Aphasia Examination;
- BVRT
= Benton Visual Retention Test;
- CAA
= cerebral amyloid angiopathy;
- CI
= confidence interval;
- CVD
= cerebrovascular disease;
- FLAIR
= fluid-attenuated inverse recovery;
- MCI
= mild cognitive impairment;
- OR
= odds ratio;
- SRT
= Selective Reminding Test;
- WAIS-R
= Wechsler Adult Intelligence Scale–Revised;
- WHICAP
= Washington/Hamilton Heights–Inwood Columbia Aging Project;
- WMH
= white matter hyperintensity.
The contribution of cerebrovascular disease (CVD) to cognitive impairment is of increasing interest.1 Amnestic mild cognitive impairment (MCI), characterized by memory complaints, objective memory impairment, and absence of functional impairment, is associated with a high conversion rate to dementia.2 MCI is increasingly recognized as a heterogeneous disorder, and non-amnestic MCI has been characterized.3–5 Most studies relating CVD to MCI are hospital or clinic-based. Our primary objective was to explore the cross-sectional relation of CVD, measured by brain MRI as white matter hyperintensity (WMH) volume and infarcts, with MCI and its subtypes in a community-based sample. Our secondary objective was to relate CVD to scores in several cognitive domains. We hypothesized that CVD would be related to MCI, and more strongly related to non-amnestic compared to amnestic MCI.
METHODS
Subjects.
The source cohort was 2,776 Medicare-eligible individuals from northern Manhattan, age 65 years and older (Washington/Hamilton Heights–Inwood Columbia Aging Project: WHICAP II). WHICAP II is comprised of continuing participants recruited in 1992 (WHICAP I; n = 602) and a cohort recruited between 1999 and 2001 (n = 2,174). The sampling strategies have been described elsewhere.1 The cohort comprises Hispanic, African American, and non-Hispanic white individuals. Ethnic group was determined by self-report using the 2000 US Census format. Participants are followed at approximately 18-month intervals. The study was approved by the Institutional Review Boards of Columbia Presbyterian Medical Center, Columbia University Health Sciences, and the New York State Psychiatric Institute. All participants provided written consent to participate in this study.
The sample for this study was participants with MRI and MCI data. MRI was obtained in 769 participants. Participants were deemed eligible for MRI if they did not meet criteria for dementia at the visit (2002–2004) before the second follow-up (2005–2007), when brain imaging was performed. A total of 2,113 participants were eligible for MRI (figure); 2,053 of these were seen at the first follow-up, and 60 of these were not seen during the first follow-up; 269 (12.7%) participants had dementia. Of the remaining 1,841 participants, 769 (41.8%) underwent MRI; 407 (38.0%) of the 1,072 excluded refused participation, 25 (2.3%) were unable to be scheduled, 166 (15.4%) died before they were scheduled for imaging, 191 (17.8%) were lost to follow-up, and 283 (26.3%) had MRI contraindications. Of the 769 persons with MRI, 52 were excluded due to dementia at the time of MRI, and 38 were excluded due to insufficient MCI data, leaving 679 persons in the final sample. In these 679 persons, 38 persons had missing data on APOE ɛ4, 4 had missing data on infarcts, and 16 had missing data on WMH. We compared the demographic characteristics of individuals with MRI (n = 769) to those who refused participation in the MRI study, but otherwise met inclusion criteria (n = 407; table e-1 on the Neurology® Web site at www.neurology.org). Those refusing participation were older and more likely to be women. African Americans were more frequent among those with MRI. Education was similar between the 2 groups.
Figure Schematic representation of derived MRI sample
Subject evaluation.
Clinical evaluation.
At each assessment, each participant underwent an in-person interview of general health and functional ability followed by a semi-structured standardized assessment, including medical history, physical and neurologic examination, and a neuropsychological battery. The diagnosis of dementia was based on standard research criteria6 and was established using all available information (except the MRI results) gathered from the initial and follow-up assessments and medical records at a consensus conference of physicians, neurologists, neuropsychologists, and psychiatrists.
Neuropsychological test performance.
Learning and memory was assessed with the Selective Reminding Test (SRT), and recognition from the Benton Visual Retention Test (BVRT). Visuospatial ability was assessed with the Rosen Drawing Test and BVRT Matching. Language was assessed with the Boston Naming Test, the Controlled Oral Word Association Test, and Category Fluency Test. Psychomotor speed was assessed with the Color Trails 1. Executive functioning was assessed with the similarities subtest from the Wechsler Adult Intelligence Scale–Revised and the Color Trails 2.
Diagnosis of MCI.
MCI criteria were retrospectively applied among persons without dementia.3 Consistent with standard criteria,7 for all subtypes of MCI, those considered for MCI were required to have 1) cognitive complaints, 2) objective impairment in at least one cognitive domain based on the average of the scores on the neuropsychological measures within that domain and a 1.5 SD cutoff using normative corrections for age, years of education, ethnicity, and sex, 3) essentially preserved activities of daily living, and 4) no diagnosis of dementia at the consensus conference. The original Petersen criteria,7 which focus on memory impairment, were expanded to include mutually exclusive subtypes based on cognitive features. Amnestic MCI required memory impairment defined as a score <1.5 SD below demographically corrected mean on an average composite measure comprising the following learning and memory measures: 1) total recall from the SRT, 2) delayed free recall from the SRT, and 3) recognition from the BVRT, with or without deficits in other cognitive domains. Non-amnestic MCI was demonstrated in the absence of the criterion for memory impairment but in the presence of the following: executive impairment, demonstrated on an average composite measure comprising Letter Fluency, Category Fluency, and the WAIS-R Similarities subtest; language impairment, demonstrated on an average composite measure comprising Boston Naming Test, Boston Diagnostic Aphasia Examination (BDAE) Repetition, and the BDAE Comprehension test; and visuospatial impairment, demonstrated on an average composite score comprising Rosen Drawing and BVRT matching.
Cognitive scores.
Composite scores were developed using an exploratory factor analysis approach, with confirmatory factor analysis that tested for invariance of the factor structure across English- and Spanish-speaking participants.8 An exploratory factor analysis using principal axis factoring and oblique rotation was performed on 15 neuropsychological test score variables in the English-speaking sample only. This factor analysis identified factors of memory, language, processing speed, and visual-spatial ability. Three SRT variables (total recall, delayed recall, and delayed recognition) loaded on the memory factor. The language factor was comprised of the naming total variable, the category and letter fluency tests, the Wechsler Adult Intelligence Scale–Revised (WAIS-R) similarities subtest, and the BDAE repetition and comprehension subtests. The processing speed factor was comprised of the 2 timed sequencing tasks, Color Trails 1 and Color Trails 2. The BVRT recognition and matching variables, the Rosen, and the Identities and Oddities subtest loaded on the visual-spatial ability factor. Z scores for each of the cognitive measures were created and then averaged to create a composite score for each factor.
Covariates.
Age was calculated at the time of MRI. Sex was self reported. Education was estimated in years. History of type 2 diabetes was ascertained by self-report or by the use of diabetes medications. Hypertension, heart disease, and smoking were defined by self report. Heart disease included a history of atrial fibrillation, other arrhythmias, congestive heart failure, myocardial infarction, and angina pectoris. Smoking was classified into never, current, and past smoking. History of self-reported stroke was also ascertained. APOE genotypes were determined using a modification of the method described by Hixson and Vernier.9 We classified persons as homozygous or heterozygous for the APOE ɛ4 allele or not having any ɛ4 allele.
MRI protocol.
Acquisition.
MRI was performed on a 1.5-T Philips Intera scanner at Columbia University Medical Center and transferred electronically to the University of California–Davis for morphometric analysis (Imaging of Dementia and Aging Laboratory). For measures of total brain volume, ventricular volume, and WMH volume, fluid-attenuated inverse recovery (FLAIR)–weighted images were acquired in the axial orientation.
WMH quantification.
User-operated-image analysis was performed on a Sun Microsystems Ultra 5 workstation using the Quantum 6.2 package. WMH volumes were derived on FLAIR-weighted images following a 2-step process.10,11 The dura mater was manually traced within the cranial vault, including the middle cranial fossa but not the posterior fossa and cerebellum. Intracranial volume was defined as the number of voxels contained within the tracings, multiplied by voxel dimensions and slice thickness. These tracings also defined the border between brain and non-brain elements for removal of the latter.
Nonuniformities in image intensity were removed12 and two Gaussian probability functions, representing brain matter and CSF, were fitted to the skull-stripped image.12 Once brain matter was isolated, a single Gaussian distribution was fitted to image data and a segmentation threshold for WMH was set a priori at 3.5 SDs in pixel intensity above the mean of the fitted distribution of brain matter. Erosion of 2 exterior image pixels was applied to the brain matter image before modeling to remove partial volume effects and ventricular ependyma on WMH determination. WMH volume was calculated as the sum of voxels greater than or equal to 3.5 SD above the mean intensity value of the image and multiplied by voxel dimensions and slice thickness.
Infarcts.
The presence of infarcts was determined according to previously published protocols from the size, location, and imaging characteristics of the lesion.13 The image analysis system allowed superimposition of the subtraction image, the proton density image, and the T2-weighted image at 3 times magnified view to assist in interpretation of lesion characteristics. Signal void, best seen of T2-weighted image, was interpreted as indicative of a vessel. Only lesions 3 mm or larger qualified as brain infarcts. Infarcts were classified by brain hemisphere (left/right), brain region (28 anatomic regions), and size (small [3 to 9 mm] or large [≥10 mm]). Previously published Kappa values for agreement among raters have been good, ranging from 0.73 to 0.90.13
Statistical analysis.
First, we conducted univariate analyses. WMH volume was adjusted for total cranial size and expressed as a percentage. The distribution of the adjusted WMH was skewed and required logarithmic transformation. Secondly, we conducted bivariate analyses examining the relation of WMH and infarcts with MCI and its subtypes, amnestic and non-amnestic. We used t test for WMH and χ2 for infarcts. Third, we conducted multivariable analyses using logistic regression. In all analyses, MCI and its subtypes were compared to persons with normal cognition. There were separate models for WMH and infarcts. We conducted additional analyses relating WMH and infarcts to cognitive scores using multivariable linear regression. We report 3 models for all multivariable analyses: 1) adjusted for age and gender; 2) additionally adjusted for years of education, ethnic group, and APOE ɛ4 genotype; 3) adjusted for either infarcts or WMH. Model 2 is reported in the text unless otherwise indicated. The rationale for the second model was to adjust for other risk factors and confounders of cognitive impairment, and to explore the independent contribution of WMH or stroke in the third model. Other vascular conditions (diabetes, hypertension, heart disease, stroke history) were not included in the models because they share the pathway relating CVD and MCI. All analyses were conducted using SAS 9.1 for Windows.
RESULTS
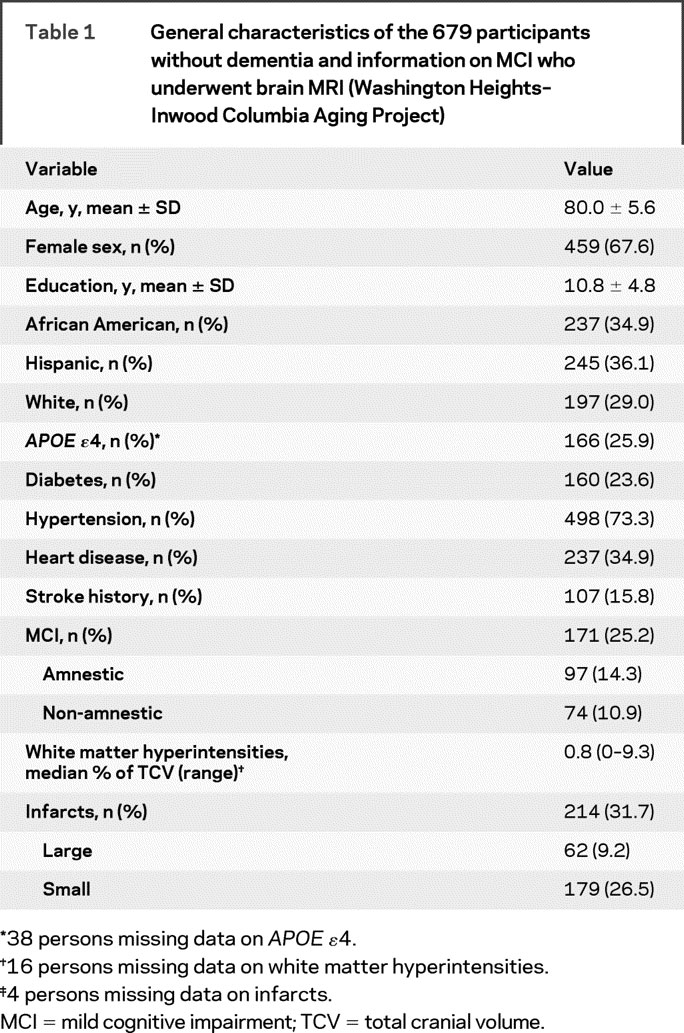
Table 1 shows the characteristics of the sample. The odds of an infarct increased 3.6 times for each increase in logarithmically transformed adjusted WMH (95% CI: 2.4–5.3). Only 1 person had more than 1 infarct.
Table 1 General characteristics of the 679 participants without dementia and information on MCI who underwent brain MRI (Washington Heights–Inwood Columbia Aging Project)
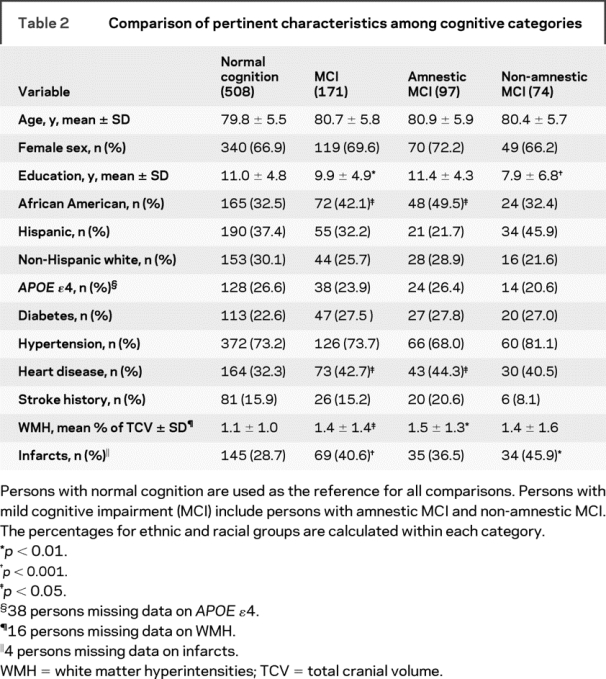
Persons with amnestic MCI had a higher proportion of African Americans, a higher proportion of heart disease, and higher WMH (table 2). Persons with non-amnestic MCI were less educated and had more infarcts.
Table 2 Comparison of pertinent characteristics among cognitive categories
Relation of WMH to MCI.
The adjusted odds of MCI increased with higher WMH (table 3). This relation was mildly attenuated and became nonsignificant after adjustment for infarcts. A square term for WMH was not statistically significant (p = 0.11), indicating that the relation of WMH and MCI was linear. WMH were more strongly associated with amnestic MCI than with non-amnestic MCI. After adjusting for infarcts, the association persisted for amnestic MCI and disappeared for non-amnestic MCI.
Table 3 Odds ratios (OR) from logistic regression relating logarithmically transformed adjusted white matter hyperintensity (WMH) volume percentage and brain infarcts with mild cognitive impairment (MCI)
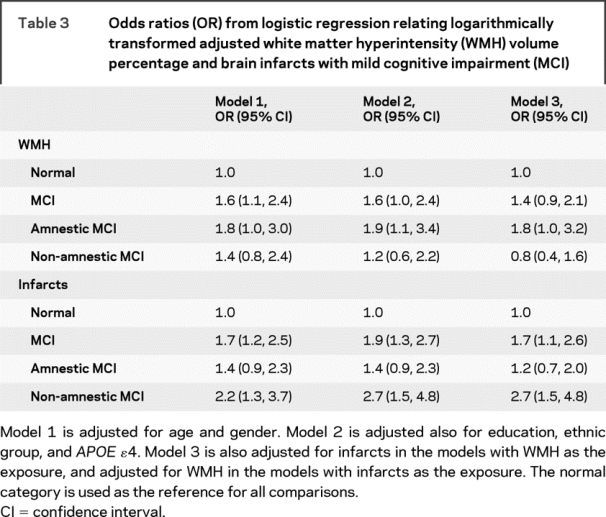
Relation of infarcts to MCI.
The adjusted odds of MCI increased with the presence of infarcts (table 3). The OR changed little after adjusting for WMH. This relation was stronger for non-amnestic MCI compared to amnestic MCI. Only the association with non-amnestic MCI remained significant after adjusting for WMH.
Large infarcts were related to amnestic MCI but not non-amnestic MCI (table e-2), although this association was attenuated after adjustment for WMH (OR = 1.6; 95% CI: 0.8, 3.4). Small infarcts were strongly related to non-amnestic MCI, even after adjusting for WMH (OR = 3.3; 95% CI: 1.8, 6.0).
Infarct side was not related to MCI. Analyses of infarct location were limited by small numbers.
Relation of WMH to cognitive domains.
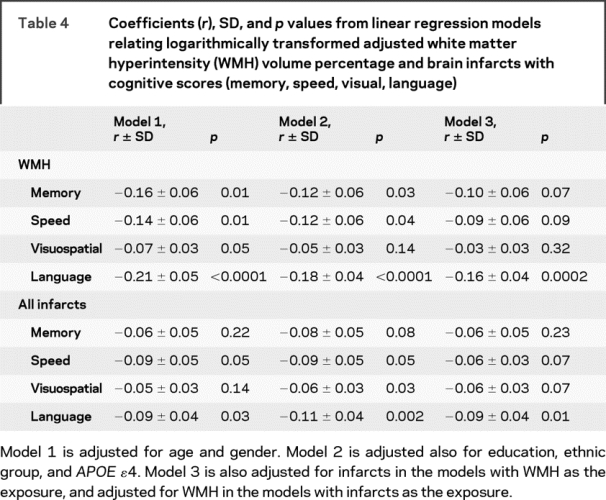
WMH were related to memory, speed, and language (table 4). These associations were attenuated after adjusting for infarcts and only the association with language remained statistically significant.
Table 4 Coefficients (r), SD, and p values from linear regression models relating logarithmically transformed adjusted white matter hyperintensity (WMH) volume percentage and brain infarcts with cognitive scores (memory, speed, visual, language)
Relation of WMH to memory encoding and retrieval deficits.
Our findings linking WMH to amnestic MCI could be explained by memory retrieval deficits, traditionally considered to be the mechanism for amnestic problems in vascular cognitive impairment.14 Amnestic disorders related to consolidation, more typical of Alzheimer disease (AD) and amnestic MCI, are usually identified by deficits in delayed recall with impaired recognition, while retrieval deficits are characterized by impaired delayed recall with preserved recognition. Delayed recall and total recognition from the SRT were moderately correlated (Pearson coefficient [c] = 0.6; p < 0.0001), and they were highly correlated with the memory score (c = 0.9 [p < 0.0001] and 0.8 [p < 0.0001], respectively). Recognition from the BVRT was less strongly correlated with the memory score, SRT delayed recall, and SRT delayed recognition (c = 0.3; p < 0.0001 for all 3 comparisons). WMH were related similarly to delayed recall (coefficient [r] ± SD = −0.7 ± 0.2 words; p = 0.004) and recognition (r ± SD = −0.7 ± 0.2 words; p = 0.004) of the SRT, and recognition of the BVRT (r ± SD = −0.5 ± 0.2 words; p = 0.01). Thus, our data suggest that WMH in our population is at least partially related to an amnestic impairment profile suggestive of a consolidation deficit.
Relation of infarcts to cognitive domains.
Infarcts were related to speed, visuospatial abilities, and language (table 4). The only association that remained statistically significant after adjustment for WMH was with language.
DISCUSSION
WMH and infarcts on brain MRI were related to MCI. WMH was more strongly related to amnestic MCI than non-amnestic MCI, while infarcts were more strongly related to non-amnestic than amnestic MCI. Supporting these observations, WMH were related to a memory score, while infarcts were not, and the association between infarcts and non-amnestic cognitive scores was stronger than for WMH. WMH was related to word recognition deficits, suggesting that the association between WMH and memory was not completely explained by retrieval deficits.
CVD is the main culprit in vascular cognitive impairment,15 but CVD may be important in AD. CVD alone can result in cognitive impairment,16 but recent postmortem data suggest that CVD may interact with AD to increase the likelihood of dementia.17,18 Infarctions and WMH are 2 CVD manifestations that can be identified during life.19 Both stroke history20 and CVD on MRI21 are related to higher AD risk. MCI is increasingly used in research and clinical practice. MCI has been characterized into subtypes.2 Amnestic MCI is thought to be more specific to AD, while non-amnestic MCI seems to be related to other causes including CVD.
WMH is related to impairment in frontal-executive abilities22 and our finding of a stronger relation between WMH and amnestic MCI was unexpected. A number of studies have previously reported increased WMH in association with MCI.23,24 Few studies have explored WMH in relation with MCI subtypes. Most are small and clinic-based. Some studies found an association of WMH with amnestic MCI and AD.24,25 Others found that WMH is associated with non-amnestic MCI.26,27 One study found no relation between WMH and MCI subtypes.28 Most studies examining the association of infarcts and MCI are hospital or clinic-based and address MCI following clinical stroke.15,29–34 Cognitive impairment after a stroke is frequent,34 and is characterized by deficits in frontal-executive abilities,32 but persons with dementia after stroke also demonstrate memory problems.32 The Cardiovascular Health Study reported an association between infarcts on MRI and amnestic MCI35 in a large community-based sample.
Our findings suggest that higher WMH is more specific to amnestic than non-amnestic MCI, while infarcts are more specific to non-amnestic MCI. A traditional view is that WMH causes frontal-subcortical pathways disruption resulting in frontal-executive impairment.22 WMH in this context are interpreted as an expression of cerebral small vessel disease,36 possibly of ischemic nature,37 and are proposed as a surrogate marker of small vessel ischemia.36 Thus, our findings for WMH seem paradoxical because amnestic MCI is considered an AD precursor,2,38 while non-amnestic MCI is commonly associated with vascular disease. However, white matter disease is an important correlate of AD.39 WMH are frequent in AD39 and in cerebral amyloid angiopathy (CAA). CAA is related to higher WMH, WMH progression, and cognitive impairment.40 It is possible that some of the WMH we observed in amnestic MCI are not due to cerebral ischemia but could be part of a process accompanying AD, such as CAA or other neurodegenerative changes. Disruption of frontal subcortical pathways by WMH can result in memory deficits due to retrieval impairment, but we found that WMH were associated with recognition deficits. Finally, it is possible that WMH are entirely ischemic, not related to a degenerative pathology, and they are markers for ischemic insults that precipitate the manifestations of AD, memory impairment, and amnestic MCI. We cannot make inferences about the underlying pathology of WMH in our sample, but our results for WMH could be explained by its heterogeneity. That is, some WMH may be ischemic in nature, and some may be secondary to CAA or other degenerative processes. Thus, we believe that the nature of WMH requires further exploration, and that assuming that they are a surrogate marker of cerebral ischemia and systemic vascular burden may not be accurate.
There are alternative explanations for our findings. The apparent stronger association between WMH or infarcts with either amnestic or non-amnestic MCI could be due to chance due to sample variability and multiple comparisons, or to a lack of power to find statistical significance. Our results need to be reproduced in a larger sample.
Our study has several limitations. One is that we selected a group of elderly individuals without dementia to undergo MRI, which may have excluded persons with a higher CVD burden and cognitive impairment. Another limitation is the cross-sectional study design, which limits the inferences that can be made. Strengths of the study include the quantitative measures of WMH and infarcts, the detailed characterization of MCI, and the large community-based sample.
DISCLOSURE
Dr. Luchsinger receives research support from the NIH [AG07232, AG028506, AG026413, MD00206 CMS: 95-C-90998], the Institute for the Study of Aging [270901], the American Diabetes Association [7-08-CR-41], and the Alzheimer’s Association [IIRG-05-15053]. Dr. Brickman serves as consultant for ePharmaSolutions and ProPhase [education and training regarding neuropsychological and psychological/psychiatric assessment] and receives research support from the NIH [AG029949 (PI), AG024708 (PI)], Columbia University (PI), and the Alzheimer’s Association (PI). Dr. Reitz reports no disclosures. Dr. Cho reports no disclosures. Dr. Schupf serves as consultant for Elan Pharmaceuticals [literature review] and receives research support from the NIH [AG014673-05; U01 AG023749] and the Alzheimer’s Association [IIRG-08-90655]. Dr. Manly serves as Associate Editor for the Journal of the International Neuropsychological Association and receives research support from the NIA [R01 AG16206 (PI); 2P50 AG08702 (Co-Investigator); 5P01 AG07232 (Clinical Core Leader); 2P50 AG08702 (Co-Investigator); and R01 AG028786 (PI)] and the Alzheimer’s Association [IIRG 05-14236 (PI)]. Dr. Tang reports no disclosures. Dr. Small serves on the scientific advisory board of BCI, receives research support from the NIH [5R01AG025161-04: PI], and receives Board of Directors compensation and license fee payments from BCI. Dr. Mayeux receives research support from the NIH [PO1AG07232 (PI)]. Dr. DeCarli serves as Editor-in-Chief of Alzheimer Disease and Associated Disorders; receives speaker’s honoraria from UCI, UCSD Alzheimer’s Disease Center Meetings; holds corporate appointments with Eisai Pharmaceuticals and Merck [MRI analysis]; and receives research support from Eisai Pharmaceuticals, Merck Pharmaceuticals, and the Hillblom Foundation, Network for Cognitive Neuroscience of Diabetes and Aging. Dr. Brown reports no disclosures.
Supplementary Material
Address correspondence and reprint requests to Dr. José A. Luchsinger, 630 West 168th St., PH9E-105, New York, NY 10032 jal94@columbia.edu
Supplemental data at www.neurology.org
Supported by National Institutes of Health grants P01 AG07232 and AG029949.
Disclosure: Author disclosures are provided at the end of the article.
Received June 23, 2008. Accepted in final form May 1, 2009.
REFERENCES
- 1.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 2005;65:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luis CA, Barker WW, Loewenstein DA, et al. Conversion to dementia among two groups with cognitive impairment: a preliminary report. Dement Geriatr Cogn Disord 2004;18:307–313. [DOI] [PubMed] [Google Scholar]
- 3.Vliet EC, Manly J, Tang MX, Marder K, Bell K, Stern Y. The neuropsychological profiles of mild Alzheimer’s disease and questionable dementia as compared to age-related cognitive decline. J Int Neuropsychol Soc 2003;9:720–732. [DOI] [PubMed] [Google Scholar]
- 4.Luchsinger JA, Reitz C, Patel B, Tang M-X, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol 2007;64:570–575. [DOI] [PubMed] [Google Scholar]
- 5.Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol 2007;64:1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- 7.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 8.Siedlecki KL, Honig LS, Stern Y. Exploring the structure of a neuropsychological battery across healthy elders and those with questionable dementia and Alzheimer’s disease. Neuropsychology 2008;22:400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayeux R, Ottman R, Maestre G, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology 1995;45:555–557. [DOI] [PubMed] [Google Scholar]
- 10.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr 1992;16:274–284. [DOI] [PubMed] [Google Scholar]
- 11.DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 1995;45:2077–2084. [DOI] [PubMed] [Google Scholar]
- 12.DeCarli C, Murphy DG, Teichberg D, Campbell G, Sobering GS. Local histogram correction of MRI spatially dependent image pixel intensity nonuniformity. J Magn Reson Imaging 1996;6:519–528. [DOI] [PubMed] [Google Scholar]
- 13.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 14.Small SA, Mayeux R. A clinical approach to memory decline. J Pract Psychiatry Behav Health 1999;5:87–94. [Google Scholar]
- 15.Sachdev PS, Brodaty H, Valenzuela MJ, et al. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology 2004;62:912–919. [DOI] [PubMed] [Google Scholar]
- 16.Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz LM, Koschera A. Progression of cognitive impairment in stroke patients. Neurology 2004;63:1618–1623. [DOI] [PubMed] [Google Scholar]
- 17.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–2204. [DOI] [PubMed] [Google Scholar]
- 18.Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol 2007;62:59–66. [DOI] [PubMed] [Google Scholar]
- 19.Jagust WJ, Zheng L, Harvey DJ, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol 2008;63:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honig LS, Tang MX, Albert S, et al. Stroke and the risk of Alzheimer disease. Arch Neurol 2003;60:1707–1712. [DOI] [PubMed] [Google Scholar]
- 21.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 22.Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol Aging 2002;23:421–431. [DOI] [PubMed] [Google Scholar]
- 23.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Carmelli D. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol 2001;58:643–647. [DOI] [PubMed] [Google Scholar]
- 24.Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology 2006;67:2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YF, Wang H, Chu Y, Huang YC, Su MY. Regional quantification of white matter hyperintensity in normal aging, mild cognitive impairment, and Alzheimer’s disease. Dement Geriatr Cogn Disord 2006;22:177–184. [DOI] [PubMed] [Google Scholar]
- 26.Rossi R, Geroldi C, Bresciani L, et al. Clinical and neuropsychological features associated with structural imaging patterns in patients with mild cognitive impairment. Dement Geriatr Cogn Disord 2007;23:175–183. [DOI] [PubMed] [Google Scholar]
- 27.Grau-Olivares M, Bartres-Faz D, Arboix A, et al. Mild cognitive impairment after lacunar infarction: voxel-based morphometry and neuropsychological assessment. Cerebrovasc Dis 2007;23:353–361. [DOI] [PubMed] [Google Scholar]
- 28.Bombois S, Debette S, Delbeuck X, et al. Prevalence of subcortical vascular lesions and association with executive function in mild cognitive impairment subtypes. Stroke 2007;38:2595–2597. [DOI] [PubMed] [Google Scholar]
- 29.Rasquin SM, Lodder J, Verhey FR. Predictors of reversible mild cognitive impairment after stroke: a 2-year follow-up study. J Neurol Sci 2005;229–230:21–25. [DOI] [PubMed] [Google Scholar]
- 30.Rasquin SM, van Oostenbrugge RJ, Verhey FR, Lodder J. Vascular mild cognitive impairment is highly prevalent after lacunar stroke but does not increase over time: a 2-year follow-up study. Dement Geriatr Cogn Disord 2007;24:396–401. [DOI] [PubMed] [Google Scholar]
- 31.Sachdev PS, Brodaty H, Valenzuela MJ, et al. Clinical determinants of dementia and mild cognitive impairment following ischaemic stroke: the Sydney Stroke Study. Dement Geriatr Cogn Disord 2006;21:275–283. [DOI] [PubMed] [Google Scholar]
- 32.Stephens S, Kenny RA, Rowan E, et al. Neuropsychological characteristics of mild vascular cognitive impairment and dementia after stroke. Int J Geriatr Psychiatry 2004;19:1053–1057. [DOI] [PubMed] [Google Scholar]
- 33.Stephens S, Kenny RA, Rowan E, et al. Association between mild vascular cognitive impairment and impaired activities of daily living in older stroke survivors without dementia. J Am Geriatr Soc 2005;53:103–107. [DOI] [PubMed] [Google Scholar]
- 34.Srikanth VK, Thrift AG, Saling MM, et al. Increased risk of cognitive impairment 3 months after mild to moderate first-ever stroke: a community-based prospective study of nonaphasic English-speaking survivors. Stroke 2003;34:1136–1143. [DOI] [PubMed] [Google Scholar]
- 35.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol 2003;60:1394–1399. [DOI] [PubMed] [Google Scholar]
- 36.Pantoni L, Poggesi A, Inzitari D. The relation between white-matter lesions and cognition. Curr Opin Neurol 2007;20:390–397. [DOI] [PubMed] [Google Scholar]
- 37.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 1997;28:652–659. [DOI] [PubMed] [Google Scholar]
- 38.Luis CA, Loewenstein DA, Acevedo A, Barker WW, Duara R. Mild cognitive impairment: directions for future research. Neurology 2003;61:438–444. [DOI] [PubMed] [Google Scholar]
- 39.Lee JM, Markus HS. Does the white matter matter in Alzheimer disease and cerebral amyloid angiopathy? Neurology 2006;66:6–7. [DOI] [PubMed] [Google Scholar]
- 40.Chen YW, Gurol ME, Rosand J, et al. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology 2006;67:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



