Abstract
Activation of innate inflammatory pathways, marked by increased production of pro-inflammatory cytokines, has been proposed as a potential mechanism linking poor sleep and inflammatory disease risk. In the present study, we examined associations of self-reported sleep quality and duration, and a calculated measure of sleep debt with the production of pro-inflammatory cytokines, interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α among a community sample of 156 healthy adults. Bivariate correlations revealed an inverse association between sleep quality and production of all three pro-inflammatory cytokines that was retained for IL-1β after controlling for demographic and health characteristics. Hierarchical linear regressions also revealed that higher sleep debt scores predicted greater production of IL-1β and IL-6 after adjusting for covariates. Secondary analyses showed an interaction between sleep debt and body mass index (BMI) in the prediction IL-1β, suggesting that the impact of sleep debt on cytokine production is greater among participants with lower BMI scores. Further exploration of this potential psychophysiological pathway linking sleep difficulty and inflammatory disease susceptibility is warranted.
Disrupted sleep is a significant predictor of future health problems, with prospective epidemiologic investigations linking shortened sleep duration and poor sleep quality to chronic disease morbidity and mortality among relatively healthy (Ayas et al., 2003; Gangwisch et al., 2007; Hublin et al., 2007; Jennings et al., 2007) and medically-ill populations (Elder et al., 2008; Mallon et al., 2002; Theadom et al., 2007). Sleep debt, which is broadly defined as the disparity between the amount of sleep naturally required to function optimally and the amount actually obtained, has also been associated with increased risk of physical disease (Fujino et al., 2006; Knutson et al., 2006). For example, epidemiologic evidence suggests that rotating shift workers, a population characterized as carrying a large sleep debt, are at significantly greater risk of death from ischemic heart disease (O.R.=2.23) than day workers, with sleep debt contributing risk independently of more conventional cardiovascular risk factors (Fujino et al., 2006).
In an effort to determine the underlying mechanisms linking sleep to increased disease risk, researchers have begun to examine relationships between sleep and inflammation, a pathophysiologic process that plays a primary and essential role in the incidence and course of a number of chronic diseases, including cardiovascular disease (Feldmann et al., 1996; Libby et al., 2002; Ross, 1999). Although elevated levels of circulating inflammatory mediators, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, predict the development of inflammatory diseases, the interpretation of the circulating levels of these proteins is complicated by the multiple cell types that produce them, including adipocytes and endothelial cells (Mohamed-Ali et al., 1997; Papanicolaou et al., 1998), raising the possibility that they may not reflect a primary inflammatory response. In order to more directly assess inflammatory competence, researchers have turned to examining the associations between sleep parameters and the functional ability of immune cells to produce pro-inflammatory cytokines when stimulated in vitro.
An inflammatory response begins when monocytes/macrophages are activated by pathogens or tissue damage to release the pro-inflammatory cytokines, IL-6, IL-1β, and TNF-α. Pro-inflammatory cytokines, in turn, stimulate the local recruitment and activation of leukocytes, and the systemic release of acute phase proteins, such as C-reactive protein (CRP) and fibrinogen (Black and Garbutt, 2002). The magnitude of the pro-inflammatory cytokine response to immune activation is critical; insufficient response may leave the organism vulnerable to infection, whereas excessive response can increase risk for inflammatory diseases (Nathan, 2002; Pavlov and Tracey, 2004). Thus, it has been proposed that early pro-inflammatory responses may provide a physiologic mechanism linking sleep disruption to risk of inflammatory disease (Opp et al., 2007).
Accumulating experimental evidence suggests substantial variation in enumerative and functional aspects of the innate and adaptive immune system across phases of normal sleep(Born et al., 1997; Dimitrov et al., 2007; Dimitrov et al., 2004; Lange et al., 2006) and in response to sleep deprivation (Born et al., 1995; Dimitrov et al., 2007; Dimitrov et al., 2004; Dinges et al., 1994; Frey et al., 2007; Irwin et al., 1994; Irwin et al., 1996; Lange et al., 2006; Meier-Ewert et al., 2004; Palmblad et al., 1979; Shearer et al., 2001). With regard to inflammatory competence, sleep restriction covaries positively with the in vitro production of pro-inflammatory mediators by peripheral blood cells stimulated with endotoxins (Born et al., 1997; Irwin et al., 2006; Moldofsky et al., 1989; Uthgenannt et al., 1995). Here, experimental findings show that total and partial sleep deprivation results in increased production of IL-6, TNF-α, and IL-1β and an increase in IL-6 and TNF-α mRNA transcription, when compared with levels measured during uninterrupted sleep (Irwin et al., 2006; Moldofsky et al., 1989; Uthgenannt et al., 1995). To date, studies exploring the relationship of sleep with innate inflammatory responses have been limited to the laboratory setting and it is not yet known whether naturally occurring disruptions in sleep are similarly associated with inflammatory potential. Accordingly, the primary goal of the present study of healthy community volunteers was to examine relationships between self-reported sleep quality, duration, and debt and the capacity of immune cells to produce the pro-inflammatory cytokines, IL-1 β, IL-6, and TNF-α in response to in vitro stimulation with endotoxin.
Another issue that requires clarification in the literature linking sleep disturbance to inflammation is the role of adiposity. Body mass index (BMI) covaries positively with risk of sleep disorders (e.g. sleep apnea) and chronic inflammatory conditions (Bogers et al., 2007; Kopelman, 2000). Moreover, initial animal and human evidence shows that obesity covaries positively with the magnitude of cytokine production following in vitro stimulation (Mito et al., 2002; Yamakawa et al., 1995), raising the possibility that associations between sleep disruption and inflammatory competence are accounted for by body mass. A secondary goal of the current study was to explore this possibility. Based on the existing literature, we hypothesized that poorer self-reported sleep quality, shorter sleep duration and greater reported sleep debt would be associated with enhanced production of pro-inflammatory cytokines and that these relationships would be moderated by BMI.
Methods
Participants
Data for the present study were derived from the University of Pittsburgh Adult Health and Behavior (AHAB) project, a registry of behavioral and biological measurements on non-Hispanic Caucasian and African American individuals (30–54 years old) recruited via mass-mail solicitation from communities of southwestern Pennsylvania, USA (principally Allegheny County). Exclusion criterion for entry into the AHAB study included a reported history of atherosclerotic cardiovascular disease, chronic kidney or liver disease, cancer treatment in the preceding year, and major neurological disorders, schizophrenia or other psychotic illness. Other exclusions included pregnancy and the use of insulin, glucocorticoid, antiarrhythmic, psychotropic, or prescription weight-loss medications. For the current analyses, we identified 156 AHAB participants on whom we had measures of sleep and stimulated cytokine production, as well as, demographic and health behavior information. Additional exclusion criterion for this sub-study included any current symptoms of infection. Data collection occurred over multiple laboratory sessions, and informed consent was obtained in accordance with approved protocol guidelines of the University of Pittsburgh Institutional Review Board.
Sleep Measures
Self-reported sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989). The PSQI is widely used and reliable measure of global sleep quality (Cronbach alpha=.83). Higher PSQI global sleep-quality scores are indicative of poorer overall sleep. Sleep duration was assessed by trained interviewers as a component of the Stanford Five-City Physical Activity Interview (Sallis et al., 1985). Participants were asked to report the number of hours they slept on weekdays and weekend days in the past week. This information was used to calculate a weighted average sleep duration [((# hours slept during a weekday*5)+(# hours slept during a weekend day*2))/7]. Finally, sleep debt was calculated by subtracting the average number of hours slept on a weekday from hours slept on weekend days [(average # of hours slept during a weekend day; Friday–Saturday)-(average # of hours slept during a weekday; Sunday–Thursday)]. This measure of sleep debt is based on the assumption that individuals attempt to recoup lost sleep during the weekdays on weekend days.
Cytokine assessment
Blood samples for the measurement of stimulated production of inflammatory cytokines were collected on a different day approximately 2 weeks after sleep assessment. On this occasion, participants were asked to fast for 8 hours and avoid exercise for 12 hours and alcohol for 24 hours before attending a session scheduled between 7:30 and 10:30 AM. At this visit the project nurse completed a medical history interview, recorded any current symptoms of infection and medication use, obtained measurements of height and weight for the determination of BMI (kg/m2), and collected a blood sample used for the cytokine assessment. Determination of stimulated cytokine production was conducted by stimulating citrate-treated whole blood with lipopolysaccharide (LPS; serotype 026:B6, Sigma) at a final concentration of 2.5 ug/ml without antibiotics in polypropylene tubes under sterile conditions. Control samples, containing whole blood without LPS, were set up in parallel to measure spontaneous levels of cytokine production. The samples were incubated at 37°C for 24 hours. Following incubation, the tubes were centrifuged at 1000g for 10 minutes and supernatants were frozen at −80°C until the completion of the study.
Samples were assayed in one batch using a multiplex analysis system as previously described (Marsland et al., 2007). Briefly, multiplex bead kits, based on the principle of solid phase sandwich immunoassays, were employed and samples were read within 24 hours using Bio-Plex reader (Luminex 100™). Stimulated levels of IL-6, IL-1β, and TNF-α were determined using the Bio-Plex Manager Software (Bio-rad Corporation, Hercules, CA), interpolating from the standard curve (Logistic-5PL curve fit). Inter- and intra- assay coefficients of variability were less than 10%. Stimulated cytokine production was quantified by subtracting unstimulated from stimulated levels. A complete blood count was performed to determine the absolute numbers of monocytes in peripheral circulation.
Covariates
All multivariate analyses included a standard group of covariates, including age, gender, race, use of reproductive hormones, and monocyte count. Secondary analyses also examined a number of lifestyle factors that might explain associations between sleep and inflammatory potential. In addition to BMI, these included smoking status (current smoker versus ex/non-smoker) and physical activity (estimated kilocalories expended per week by the Paffenbarger Physical Activity Questionnaire; Paffenbarger et al., 1993).
Statistical Analyses
All analyses were performed using SPSS for windows (version 16.0). Stimulated pro-inflammatory cytokines, BMI, physical activity, and PSQI scores were log transformed prior to analyses to better approximate normal distributions. Pearson product-moment and point-biserial correlations were performed to examine associations among cytokine levels, demographic characteristics, lifestyle factors, and sleep measures. Next, hierarchical linear regression analyses were performed examining whether sleep measures predicted stimulated pro-inflammatory cytokine levels after adjustment for standard covariates and related lifestyle factors. For these analyses, the standard covariates, physical activity, and smoking status were entered in the first step of the model followed by the sleep measure of interest (sleep quality, duration, or debt). Finally, a series of analyses were conducted to determine whether BMI moderated associations between sleep characteristics and stimulated cytokine levels. Here standard controls, physical activity, and smoking status were entered in step 1, BMI and the sleep measure of interest in step 2, and the interaction of BMI with the sleep measure in step 3 of the regression equation.
RESULTS
Sleep measures and pro-inflammatory cytokine production
Mean values of IL-6, IL-1β, and TNF-α with and without stimulation with LPS are presented in Table 1. As expected, all 3 inflammatory mediators were highly correlated with one another (r’s=.63–.71). Initial analyses revealed significant positive associations between PSQI scores and stimulated production of all three pro-inflammatory cytokines (r’s=.17–.29, p’s<.05; see Table 2). No significant associations were observed between sleep duration or sleep debt and stimulated levels of any of the cytokines. Additional associations between stimulated levels of cytokines and demographic and lifestyle factors are presented in Table 2.
Table 1.
Mean values of stimulated and unstimulated pro-inflammatory cytokines
| Measure | Stimulated levels (pg/ml) | Unstimulated levels (pg/ml) | ||
|---|---|---|---|---|
| M | SD | M | SD | |
| IL-6 | 71,506 | 21,771 | 1,455 | 4,163 |
| IL-1β | 31,205 | 12,085 | 462 | 826 |
| TNF-α | 54,340 | 23,127 | 638 | 1,607 |
Table 2.
Descriptive, health characteristics, and cytokine production levels for sample (n= 156)
| Variable | Mean (SD) or % | Correlation Coefficient | ||
|---|---|---|---|---|
| Stimulated IL-6 | Stimulated IL-1β | Stimulated TNF-α | ||
| Age | 45.1 (6.34) | .03 | .07 | .06 |
| Gender | 58.3% Female | −.35** | −.04 | −.16* |
| Race | 94% Caucasian, 6% Other | .16* | −.04 | .07 |
| BMI (kg/m2) | 26.2 (4.6) | .10 | .11 | .07 |
| % taking reproductive hormones | 5.1% | −.07 | −.07 | −.10 |
| % current smokers | 17.9% | .26** | .08 | .16* |
| Physical activity (kilocalories/week) | 2658 (1883) | −.15 | −.23** | −.17* |
| PSQI global sleep score | 4.7 (2.1) | .18* | .29** | .17* |
| Sleep Duration (hrs/night) | 6.9 (.93) | −.09 | .01 | −.03 |
| Sleep Debt | .28 (1.12) | .14 | .15 | .04 |
p<.05,
p<.01
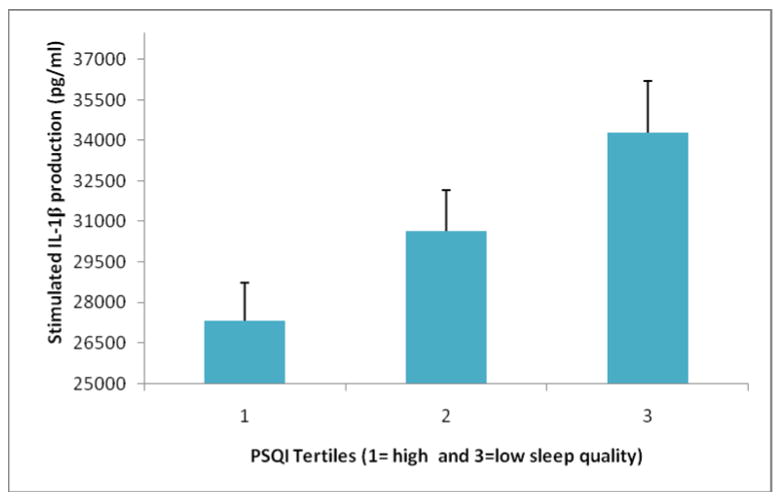
Hierarchical linear regressions analyses were employed to examine the relationship of sleep quality with stimulated cytokine production after adjusting for standard covariates and lifestyle characteristics (exercise and smoking status). After entering the covariates, PSQI scores continued to predict stimulated production of IL-1β, but not IL-6 or TNF-α (see Table 3). Figure 1 summarizes the relationship between sleep quality and IL-1β production. To simplify the presentation, PSQI scores were categorized into tertiles for this figure (Group 1 (n=50): mean PSQI score= 2.34 (SD=.75); Group 2 (n=56): mean=4.48 (SD=.50); Group 3 (n=50): mean=7.22 (SD=1.2). Hierarchical linear regression analyses also revealed that, after controlling for standard covariates and health behaviors, higher sleep debt scores predicted higher IL-1β and IL-6 production (see Table 4).
Table 3.
Results of hierarchical linear regressions examining sleep quality as a predictor of stimulated IL-6, IL-1β, and TNF-α production with demographic and health behaviors entered in step 1 and sleep quality (PSQI scores) in step 2.
| Stimulated IL-6 |
Stimulated IL-1β |
Stimulated TNF-α |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | Beta | P-value | Adj R2 | Beta | P-value | Adj R2 | Beta | P-value | Adj R2 |
| Step 1 | .257 | .119 | .191 | ||||||
| Gender | −.210 | .008 | .078 | .363 | −.007 | .935 | |||
| Age | −.064 | .367 | .007 | .926 | −.018 | .805 | |||
| Race | .078 | .302 | −.036 | .664 | .037 | .635 | |||
| Monocyte count | .411 | .001 | .355 | .001 | .457 | .001 | |||
| Reproductive Hormone Use | .072 | .333 | −.008 | .925 | −.005 | .951 | |||
| Physical Activity | −.039 | .591 | −.153 | .057 | −.063 | .407 | |||
| Smoking Status | .036 | .658 | −.029 | .743 | −.012 | .890 | |||
|
| |||||||||
| Step 2 | .254 | .159 | .187 | ||||||
| Sleep Quality (PSQI) | .046 | .523 | .220 | .005 | .048 | .524 | |||
Figure 1.
Poorer PSQI sleep quality predicts higher stimulated IL-1β production after adjusting for age, gender, race, physical activity, smoking status, and absolute monocyte count.
Table 4.
Results of hierarchical linear regressions examining sleep debt as a predictor of stimulated IL-6, IL-1β, and TNF-α production with demographic and health behaviors entered in step 1 and sleep debt in step 2.
| Stimulated IL-6 |
Stimulated IL-1β |
Stimulated TNF-α |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | Beta | P-value | Adj R2 | Beta | P-value | Adj R2 | Beta | P-value | Adj R2 |
| Step 1 | .257 | .119 | .191 | ||||||
| Gender | −.210 | .008 | .078 | .363 | −.007 | .935 | |||
| Age | −.064 | .367 | .007 | .926 | −.018 | .805 | |||
| Race | .078 | .302 | −.036 | .664 | .037 | .635 | |||
| Monocyte count | .411 | .001 | .355 | .001 | .457 | .001 | |||
| Reproductive Hormone Use | .072 | .333 | −.008 | .925 | −.005 | .951 | |||
| Physical Activity | −.039 | .591 | −.153 | .057 | −.063 | .407 | |||
| Smoking Status | .036 | .658 | −.029 | .743 | −.012 | .890 | |||
|
| |||||||||
| Step 2 | .280 | .140 | .189 | ||||||
| Sleep Debt | .168 | .019 | .165 | .034 | .058 | .437 | |||
The role of BMI
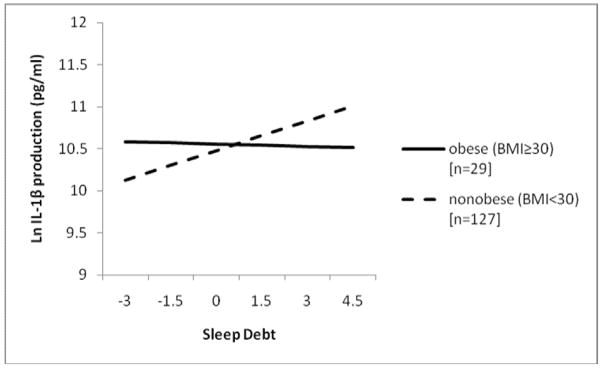
Finally, secondary analyses were performed to examine whether BMI moderated relationships between sleep measures (quality, duration, and debt) and cytokine production. Here, initial bivariate analyses revealed that higher BMI was associated with shorter sleep duration (r=−.18, p<.05) but was unrelated to the other sleep measures or any of the inflammatory mediators. Results of hierarchical linear regressions revealed that, after adjusting for standard covariates and health behaviors, sleep debt interacted with BMI to predict IL-1β [F(10,144)=3.94, p<.001; ΔR2=.03; b=−3.23, p=.024], and approached significance on analysis of IL-6 production [F(10,144)=7.12, p<.001; ΔR2=.01; b=−2.23, p=.090]. Figure 2 illustrates that the impact of sleep debt on IL-1β production was greater among those with lower BMI scores. For presentation purposes, the sample was stratified by obesity status (i.e. those with BMI scores of ≥ 30). There was no significant interaction between sleep debt and BMI in the prediction of TNF-α production nor were there any significant interactions between BMI and either PSQI scores or sleep duration in the prediction of pro-inflammatory cytokine production.
Figure 2.
Sleep debt interacts with obesity status to predict IL-1β production adjusting for age, gender, race, monocyte count, reproductive hormone use, physical activity, and smoking status.
DISCUSSION
This study provides initial evidence for an association between perceived sleep quality and sleep debt and LPS-stimulated production of the pro-inflammatory cytokines among relatively healthy midlife adults. Specifically, we found that individuals who report poorer sleep quality show greater stimulated production of the all three inflammatory mediators. The inverse covariance of sleep quality and production of IL-1β was independent of demographic and lifestyle risk factors, including age, sex, race, physical exercise, smoking and monocyte count. Similarly, after controlling for covariates, higher sleep debt scores predicted greater production of IL-1β and IL-6. These findings are consistent with existing experimental evidence showing that total and partial sleep deprivation primes immune cells to produce higher levels of pro-inflammatory cytokines when stimulated in vitro. For instance, Uthgenannt and colleagues (1995) showed that modest sleep restriction (one night of 3.5 hours of sleep) was associated with a significant increase in monocyte-derived production of IL-1β and TNF-α. Similarly, partial sleep deprivation has been associated with early morning upregulation of IL-6 and TNF-α mRNA expression (Irwin et al., 2006) and increased activation of nuclear factor (NF)-κB, a transcription factor known to play a key role in controlling pro-inflammatory genes (Irwin et al., 2008). Thus, the current findings generalize prior laboratory findings to show that variability in sleep is associated with inflammatory competence (Opp et al., 2007), suggesting a possible mechanism linking disrupted sleep to susceptibility to inflammatory disease.
The current study showed poor sleep quality and greater sleep debt were more strongly related to production of IL-1β and IL-6 than TNF-α. It is likely that different effects across cytokines reflect the kinetics of pro-inflammatory responses to endotoxin. Specifically, TNF-α is released quickly in response to LPS with peak levels occurring around 2 hours after stimulation and dropping off quickly. In contrast, IL-1β is released more slowly, peaking at around 6 hours, and IL-6 is last to peak, reaching maximal levels around 24 hours after LPS is introduced (Oliver et al., 1993). The current study employed a 24 hour post-stimulation incubation that was more likely to capture the longer-lasting IL-1β and IL-6 responses than the initial TNF-α response. In contrast, shorter incubation periods may favor other cytokine kinetic patterns. For instance, Irwin and colleagues (2006) employed a 4 hour incubation and showed that sleep disruption had a stronger impact on the production of TNF-α and IL-6 than IL-1β. Given the high intercorrelations between stimulated levels of all 3 cytokines, it is likely that disparate findings reflect the same response captured at different time points.
Secondary analyses revealed that the relationship of sleep debt with IL-1β was moderated by BMI. Although existing literature suggests that BMI is positively associated with both sleep complaints and risk of chronic inflammatory disease, we found that it was among individuals with lower BMI scores that sleep debt predicted cytokine production. It is possible that this reflects differential activation of the hypothalamic-pituitary adrenal (HPA) axis, with BMI being positively associated with levels of cortisol (Marin et al., 1992; Vgontzas et al., 2005), which is an endogenous anti-inflammatory hormone. Thus, individuals with lower BMI may have lower levels of cortisol and be more susceptible to inflammatory responses. Prospective studies are needed to further elucidate the role of BMI in the link between sleep and innate immunity.
A growing literature demonstrates that sleep duration predicts inflammatory disease, with individuals at either end of the sleep duration continuum being at increased risk (e.g., Ayas et al., 2003). In the current findings, we observed no association between self reported sleep duration and inflammatory competence. Although it is possible that cytokine production is not the mechanism linking sleep duration to inflammatory risk, our failure to find significant effects may also reflect the limited range of sleep durations reported by the current relatively healthy mid-life sample (mean = 7.0, SD =.94). Future research would benefit from larger samples and more objective measures of sleep duration, such as actigraphy or polysomnography.
There are a number of limitations of the current study. First, its cross-sectional design precludes causal inferences regarding relationships among sleep quality, sleep debt, and cytokine production. Although there is considerable evidence for a bi-directional relationship between peripheral pro-inflammatory cytokines and sleep disturbance (Bryant et al., 2004; Dantzer and Kelley, 2007), it is unlikely that inflammatory responses stimulated in vitro result in decreased sleep quality or increased sleep debt; however, it is plausible that they are independently related to a third factor, such as genetic predisposition. A further limitation of the current study is the single assessment of stimulated cytokine levels and sleep parameters. Although evidence suggests that individuals vary markedly in both the magnitude of stimulated cytokine production by peripheral leukocytes and sleep quality and that this variability is stable across time (Buysse et al., 1989; de Craen et al., 2005), multiple simultaneous assessments over time would provide a more reliable measure of individual differences. Future studies would also benefit from utilizing more ecologically valid sleep measures, such as electronic sleep diaries, rather than relying on retrospective reporting. Finally, the clinical significance of observed differences in stimulated production of pro-inflammatory cytokines remains to be determined. While in vitro measurement of pro-inflammatory cytokine production is assumed to provide a proxy for the functional ability of the body’s immune cells to mount an acute inflammatory response in the face of injury or an invading pathogen, research is needed to determine whether individual differences in the magnitude of the inflammatory response are related to clinical outcomes.
The presence of clinical sleep disorders, such as obstructive sleep apnea (OSA), was not assessed in the current sample. Epidemiological evidence suggests that approximately 6% of the U.S. population suffer from sleep apnea (Gliklich et al., 2000). Moreover, OSA has been associated with heightened levels of inflammation and increased cardiovascular risk (Alam et al., 2007), raising the possibility that OSA may account for the associations between sleep and cytokine production. That said, given the high correlation between clinical sleep disorders and obesity, we would have expected a moderating effect of BMI where the associations between poor sleep and greater cytokine production would be strengthened in individuals with higher BMI scores. However, this was not the case. Nevertheless, future studies should carefully assess for the presence of sleep disorders that may contribute to the interindividual variability in cytokine production associated with sleep quality and sleep debt.
Despite these limitations, our findings provide evidence that poorer self-reported sleep quality and greater sleep debt are associated with enhanced inflammatory responses to endotoxins. Furthermore, among individuals with lower BMI scores, sleep debt was also positively related to inflammatory response. Thus, interindividual variability in pro-inflammatory cytokine production may provide a pathway linking poor sleep to increased risk of inflammatory disease. Further testing is indicated using large, normative samples and longitudinal designs to examine whether sleep factors influence susceptibility to inflammatory disease, in part, through their effects on inflammatory competence.
Acknowledgments
This study was supported by a Central Research Development Grant provided by the Vice Provost for Research in the Office of the Provost at the University of Pittsburgh (ALM) and by grants P01HL40962 from the National Heart Lung and Blood Institute (SBM) and NR008237 from the National Institute of Nursing Research (ALM) and a National Institute of Health fellowship HL007560 (AAP). The expert technical assistance of Ramasri Sathanoori, MS and Adele Marrangoni at the University of Pittsburgh Cancer Institute Luminex Core Facility is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam I, Lewis K, Stephens JW, Baxter JN. Obesity, metabolic syndrome and sleep apnoea: all pro-inflammatory states. Obesity Reviews. 2007;8:119–127. doi: 10.1111/j.1467-789X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. Archives of Internal Medicine. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. Journal of Psychosomatic Research. 2002;52:1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Bogers RP, Bemelmans WJ, Hoogenveen RT, Boshuizen HC, Woodward M, Knekt P, van Dam RM, Hu FB, Visscher TL, Menotti A, Thorpe RJ, Jr, Jamrozik K, Calling S, Strand BH, Shipley MJ. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Archives of Internal Medicine. 2007;167:1720–1728. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. Journal of Immunology. 1997;158:4454–4464. [PubMed] [Google Scholar]
- Born J, Uthgenannt D, Dodt C, Nunninghoff D, Ringvolt E, Wagner T, Fehm HL. Cytokine production and lymphocyte subpopulations in aged humans. An assessment during nocturnal sleep. Mechanisms of Ageing and Development. 1995;84:113–126. doi: 10.1016/0047-6374(95)01638-4. [DOI] [PubMed] [Google Scholar]
- Bryant PA, Trinder J, Curtis N. Sick and tired: Does sleep have a vital role in the immune system? Nature Reviews. Immunology. 2004;4:457–467. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior, & Immunology. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Craen AJ, Posthuma D, Remarque EJ, van den Biggelaar AH, Westendorp RG, Boomsma DI. Heritability estimates of innate immunity: an extended twin study. Genes and Immunity. 2005;6:167–170. doi: 10.1038/sj.gene.6364162. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Nohroudi K, Born J. Number and function of circulating human antigen presenting cells regulated by sleep. Sleep. 2007;30:401–411. doi: 10.1093/sleep/30.4.401. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Tieken S, Fehm HL, Born J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain, Behavior, & Immunity. 2004;18:341–348. doi: 10.1016/j.bbi.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, Whitehouse WG, Orne EC, Kapoor SC, Icaza E, Orne MT. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. Journal of Clinical Investigation. 1994;93:1930–1939. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder SJ, Pisoni RL, Akizawa T, Fissell R, Andreucci VE, Fukuhara S, Kurokawa K, Rayner HC, Furniss AL, Port FK, Saran R. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrology, Dialialysis, Transplantation. 2008;23:998–1004. doi: 10.1093/ndt/gfm630. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annual Review of Immunology. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain, Behavior, & Immunity. 2007;21:1050–1057. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Fujino Y, Iso H, Tamakoshi A, Inaba Y, Koizumi A, Kubo T, Yoshimura T. A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers. American Journal of Epidemiology. 2006;164:128–135. doi: 10.1093/aje/kwj185. [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliklich RE, Taghizadeh F, Winkelman JW. Health status in patients with disturbed sleep and obstructive sleep apnea. Otolaryngology- Head and Neck Surgery. 2000;122:542–546. doi: 10.1067/mhn.2000.102579. [DOI] [PubMed] [Google Scholar]
- Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245–1253. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M, Mascovich A, Gillin JC, Willoughby R, Pike J, Smith TL. Partial sleep deprivation reduces natural killer cell activity in humans. Psychosomatic Medicine. 1994;56:493–498. doi: 10.1097/00006842-199411000-00004. [DOI] [PubMed] [Google Scholar]
- Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10:643–653. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Archives of Internal Medicine. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Sleep loss activates cellular inflammatory signaling. Biological Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30:219–223. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Archives of Internal Medicine. 2006;166:1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Fehm HL, Westermann J, Born J. Shift of monocyte function toward cellular immunity during sleep. Archives of Internal Medicine. 2006;166:1695–1700. doi: 10.1001/archinte.166.16.1695. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. Journal of Internal Medicine. 2002;251:207–216. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- Marin P, Darin N, Amemiya T, Andersson B, Jern S, Bjorntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41:882–886. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Sathanoori R, Muldoon MF, Manuck SB. Stimulated production of interleukin-8 covaries with psychosocial risk factors for inflammatory disease among middle-aged community volunteers. Brain, Behavior, & Immunolgy. 2007;21:218–228. doi: 10.1016/j.bbi.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. Journal of the American College of Cardiology. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- Mito N, Hiyoshi T, Hosoda T, Kitada C, Sato K. Effect of obesity and insulin on immunity in non-insulin-dependent diabetes mellitus. European Journal of Clinical Nutrition. 2002;56:347–351. doi: 10.1038/sj.ejcn.1601324. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. Journal of Clinical Endocrinology and Metabolism. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Moldofsky H, Lue FA, Davidson JR, Gorczynski R. Effects of sleep deprivation on human immune functions. FASEB J. 1989;3:1972–1977. doi: 10.1096/fasebj.3.8.2785942. [DOI] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Oliver JC, Bland LA, Oettinger CW, Arduino MJ, McAllister SK, Aguero SM, Favero MS. Cytokine kinetics in an in vitro whole blood model following an endotoxin challenge. Lymphokine and Cytokine Research. 1993;12:115–120. [PubMed] [Google Scholar]
- Opp MR, Born J, Irwin MR. Sleep and the immune system. In: Ader R, editor. Psychoneuroimmunology. Academic Press; New York, NY: 2007. pp. 570–618. [Google Scholar]
- Paffenbarger RS, Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Medicine and Science in Sports and Exercise. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- Palmblad J, Petrini B, Wasserman J, Akerstedt T. Lymphocyte and granulocyte reactions during sleep deprivation. Psychosomatic Medicine. 1979;41:273–278. doi: 10.1097/00006842-197906000-00001. [DOI] [PubMed] [Google Scholar]
- Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Annals of Internal Medicine. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cellular and Molecular Life Sciences. 2004;61:2322–2331. doi: 10.1007/s00018-004-4102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. New England Journal of Medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS., Jr Physical activity assessment methodology in the Five-City Project. American Journal of Epidemiology. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. Journal of Allergy and Clinical Immunology. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Theadom A, Cropley M, Humphrey KL. Exploring the role of sleep and coping in quality of life in fibromyalgia. Journal of Psychosomatic Research. 2007;62:145–151. doi: 10.1016/j.jpsychores.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Uthgenannt D, Schoolmann D, Pietrowsky R, Fehm HL, Born J. Effects of sleep on the production of cytokines in humans. Psychosomatic Medicine. 1995;57:97–104. doi: 10.1097/00006842-199503000-00001. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12:131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- Yamakawa T, Tanaka S, Yamakawa Y, Kiuchi Y, Isoda F, Kawamoto S, Okuda K, Sekihara H. Augmented production of tumor necrosis factor-alpha in obese mice. Clinical Immunology and Immunopathology. 1995;75:51–56. doi: 10.1006/clin.1995.1052. [DOI] [PubMed] [Google Scholar]