Abstract
Thiolate and selenolate complexes of CYP101 (P450cam) and the H25A proximal cavity mutant of heme-bound human heme oxygenase-1 (hHO-1) have been examined by UV-visible spectroscopy. Both thiolate and selenolate ligands bound to the heme distal side in CYP101 and gave rise to characteristic hyperporphyrin spectra. Thiolate ligands also bound to the proximal side of the heme in the cavity created by the H25A mutation in hHO-1, giving a Soret absorption similar to that of the H25C hHO-1 mutant. Selenolate ligands also bound to this cavity mutant under anaerobic conditions, but reduced the heme iron to the ferrous state as shown by formation of a ferrous-CO complex. Under aerobic conditions, the selenolate but not thiolate ligand was rapidly oxidized. These results indicate that selenocysteine-coordinated heme proteins will not be stable species in the absence of a redox potential stabilizing effect.
Cytochrome P450 enzymes are a superfamily of heme proteins that employ a cysteine thiolate as the proximal ligand to the heme.1 The P450-catalyzed reaction has been thought for three decades to be mediated by a Compound I-like ferryl species that is generated by reductive activation of molecular oxygen at the prosthetic heme iron center. Despite support by a variety of data for a Compund I-like ferryl species in the P450 catalytic cycle, it has remained elusive towards spectroscopic detection.2,3 Conflicting results have also been obtained from determinations of the lifetime of the ferryl species using radical clock substrates.4 Direct detection and characterization of the reactive oxidizing species in P450 enzymes would therefore be highly desirable. Recent computational studies have suggested that a seleno-CYP101 mutant in which the cysteine ligand is replaced by selenocysteine might provide an approach to investigation of the elusive P450 oxidizing species.5 The Se-Compound I intermediate was predicted to form faster than the wild-type S-Compound I, and to be consumed more slowly. The increased lifetime of the intermediate suggested by the calculations indicated that it might become spectroscopically detectable.
Targeted insertion of selenocysteine into a protein sequence through genetic code manipulations is a complex cotranslational process not easily adapted to P450 enzymes.6,7 An alternative and straightforward approach to investigate the effect of ligands in heme proteins has been to use a cavity mutant in which the proximal amino acid ligand is replaced by a small and noncoordinating amino acid such as an alanine or glycine, creating a pocket that can accommodate exogenous unnatural ligands. A successful example of this is provided by the sperm whale myoglobin (Mb) H93G mutant, which has been extensively used for this purpose.8,9 The crystal structures of β-mercaptoethanol and acetate-bound Mb H93G have recently confirmed that the exogenous ligands bind in the proximal ligation site.10 In this report, we report an initial evaluation of the coordination of selenolate ligands to the heme in both CYP101 and the heme oxygenase-1 H25A cavity mutant. The results shed considerable light on the parameters that circumscribe the design of a selenocysteine coordinated heme protein.
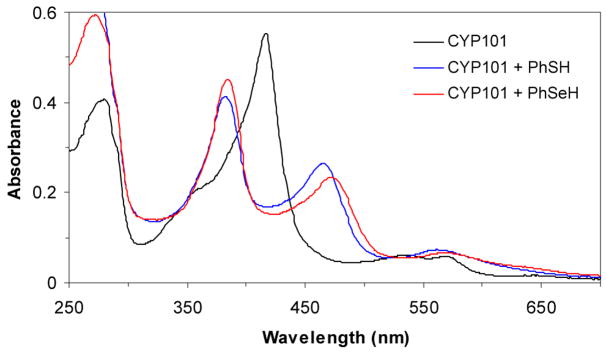
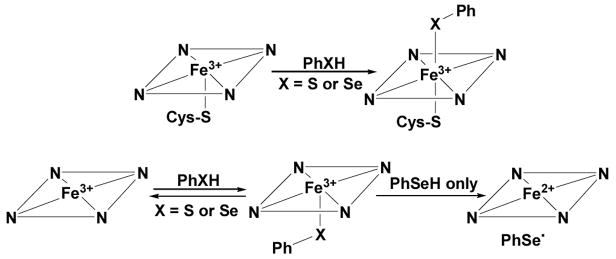
The UV-visible spectrum of CYP101 (camphor free) has a Soret maximum at ~417 nm and Q bands at 535 and 569 nm (Table 1 and Figure 1). Addition of thiophenol (~ 4 equivalents) to CYP101 led to a complex with two Soret bands at 383 and 465 nm and a broad band at 562 nm (Figure 1). The split Soret absorption observed here is known as a hyperporphyrin spectrum and is attributed to the coordination of ferric heme by two axial mercaptide ligands (Scheme 1).11 Similarly, the addition of benzeneselenol (~ 4 equivalents) to CYP101 under aerobic or anaerobic conditions resulted in a split Soret with maxima at 384 and 472 nm and a broad band at 570 nm (Figure 1). Compared to the bis-thiolate-ligated CYP101 complex, the seleno-thiolate complex has the same Soret band at 384 nm but the other Soret absorbance is red shifted by ~8 nm, as is the broad absorption band that appears at longer wavelengths. Both the bisthiolate-and thiolate/selenolate bound CYP101 complexes were found to be stable to air at room temperature, with no changes observed in their UV-vis spectra over 30 min. The split Soret or hyperporphyrin spectrum, which is characteristic of bis-thiolate or thiolate/phosphine coordinated ferric low spin systems,12 has been attributed to mixing and splitting of the normal porphyrin π, π* transition by a sulfur p†→ porphyrin π* transition of the correct energy and symmetry. Addition of benzeneselenol to CYP101 produced a split Soret spectrum similar to that obtained with thiophenol, demonstrating that the selenolate bound to the heme iron in the distal side of CYP101 has the same effect as a similarly bound thiolate ligand (Scheme 1). The red shift in the selenolate complex is consistent with the lower ionization energy of selenium and has been observed for a non-heme metalloprotein in which a selenolate replaces a thiolate.13
Table 1.
Spectroscopic comparison of CYP101, the hHO-1/H25A heme complex, and their corresponding thiolate and selenolate complexes
| Complex | Soret (nm) | CT (nm) |
|---|---|---|
| H25A heme complex | 399 | 498, 537, 620 |
| H25A + heme (ferrous) | 427 | 553, 585 |
| H25A heme complex + PhSH | 388 | 527, 651 |
| H25A heme complex + PhSeH | 388, 427 | 549, 583 |
| H25A heme complex + BnSeH | 427 | 553, 585 |
| CYP101 | 417 | 536, 569 |
| CYP101 + PhSH | 383, 465 | 562 |
| CYP101 + PhSeH | 384, 472 | 570 |
Figure 1.
UV-vis spectra of CYP101, thiolate-ligated CYP101 and selenolate-ligated CYP101: CYP101 (417, 535, 589 nm), PhSH-CYP101 (383 nm, 465 nm, 562 nm), PhSeH-CYP101 (384, 472, 570 nm). PhSH-CYP101 and PhSeH-CYP101 were prepared by adding, respectively, PhSH or PhSeH (~4 equivalents) in ethanol to a 1 mL solution of CYP101 (~5 μM) in 100 mM phosphate (pH 7.4) at 20 °C.
Scheme 1.
Binding of thiolate or selenolate to CYP101 (top) and the H25A proximal cavity mutant of heme-bound hHO-1 (bottom). The hHO-1 mutant does not bind large ligands on the distal side.
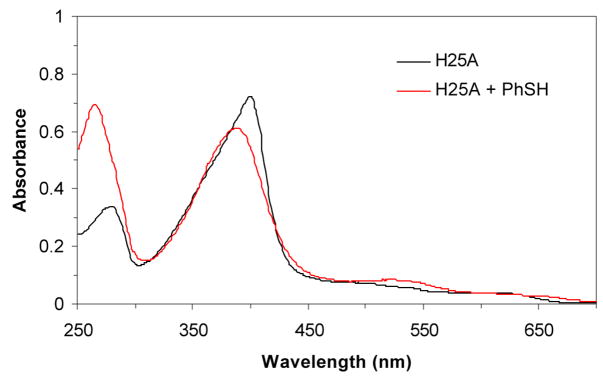
Heme oxygenase-1 catalyzes the NADPH- and cytochrome P450 reductase-dependent conversion of heme to biliverdin.14 The enzyme employs heme as both the prosthetic group and substrate. His25 has been identified as the proximal iron ligand in the heme:heme oxygenase complex by site-directed mutagenesis, RR spectroscopy, and X-ray crystallography. The H25A heme oxygenase-1 cavity mutant binds heme but lacks catalytic activity, which can be restored by the addition of exogenous imidazole.15 Based on the reasonable assumption that other exogenous ligands, like imidazole, will bind to the proximal side of the heme, the mutant provides a system that can be used to investigate proximal ligand binding to the heme iron. The H25A heme complex has a Soret maximum at ~398 nm (Figure 2). Addition of thiophenol (~ 4 equivalents) to the H25A heme complex gave a spectrum with a Soret maximum at 388 nm, about 10 nm blue shifted from that of the H25A-heme complex alone. The Soret at 388 nm of the thiolate-H25A heme complex is close to that at 385 nm of the H25C heme complex and is consistent with a thiolate-ligated heme.16 The complex was stable in air. No Soret split spectrum was observed for the H25A-heme complex in the presence of excess (up to l0 equivalents) of thiophenol, indicating that the complex only accepts one thiolate ligand, probably for steric reasons.
Figure 2.
UV-vis spectra of the hHO-1/H25A heme complex alone (400 nm) and in the presence of PhSH (388 nm). No CO-bound form was observed after the solution was flushed with CO. The thiolatehHO-1/H25A heme complex was prepared by adding PhSH (~4 equivalents) in ethanol to a 1 mL solution of the hHO-1/H25A heme complex (~5 μM) in 100 mM phosphate (pH 7.4) at 20 °C.
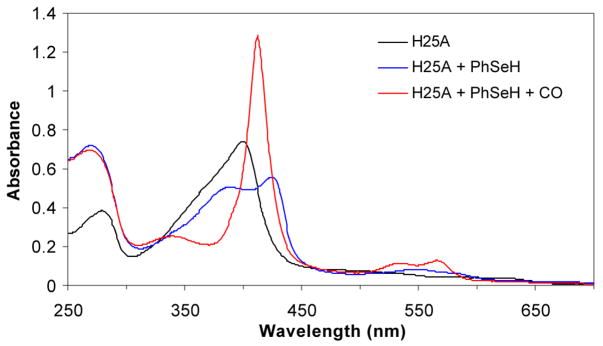
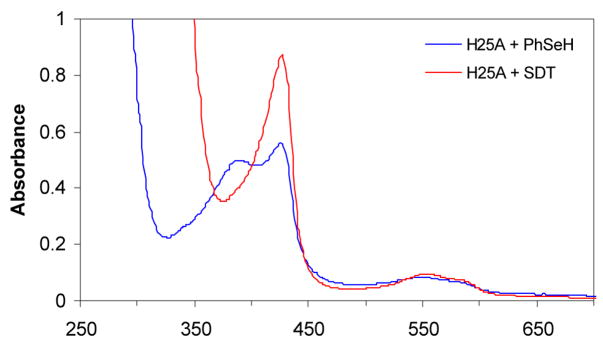
Addition of benzeneselenol to the H25A heme complex under anaerobic conditions gave a UV-vis spectrum with two Soret bands at 388 nm and 427 nm and two broad bands at 553 and 585 nm (Figure 3). At first glance, the two Soret bands suggest a hyperporphyrin spectrum due to binding of a selenolate ligand to the heme. However, comparison of the UV-vis spectra indicates that the heme is partially reduced by the selenolate as the Soret band at 427 nm is the same as that of the ferrous H25A heme complex (Figure 4). Furthermore, flushing of the solution with CO produced a spectrum identical to that of the ferrous H25A heme-CO complex. Under the same conditions, no ferrous-CO spectrum was observed for the thiolate-ligated H25A heme complex. The same result was obtained when the H25A heme complex was flushed with CO followed by addition of benzeneselenol. Exposure of the selenolate H25A heme complex to air resulted in recovery of the ferric H25A Soret at 400 nm with a slight intensity decrease, indicating both heme and selenolate oxidation. The two Soret bands of the benzeneselenolate-H25A heme complex also indicate that the heme in H25A hHO-1 was partially reduced by benzeneselenol (Scheme 1). When the more reductive benzylselenol was added to the H25A heme complex, heme was found to be completely reduced, giving only the ferrous Soret at 427 nm (Table 1). These results suggest that the selenolate is oxidized by the heme to the corresponding selenyl radical that dissociates from the enzyme and recombines with another selenyl radical to form a diselenide.
Figure 3.
UV-vis spectra of the hHO-1/H25A heme complex alone (400 nm) and in the presence of PhSeH (388 and 427 nm) and CO (412 nm). The benzeneselenolate hHO-1/H25A heme complex was prepared by adding PhSeH (~4 equivalents) in ethanol to a 1 mL solution of the hHO-1/H25A heme complex (~5 μM) in 100 mM phosphate (pH 7.4) at 20 °C under anaerobic conditions.
Figure 4.
Comparison of the UV-vis spectrum of the hHO-1/H25A-PhSeH heme complex with that of the ferrous hHO-1/H25A heme complex. The ferrous hHO-1/H25A heme complex was prepared by adding sodium dithionite (SDT, 1 mM) into a 1 mL solution of the hHO-1/H25A heme complex (~5 μM) in 100 mM phosphate (pH 7.4) at 20 °C under anaerobic conditions.
Given that a selenol is a highly reducing species with a redox potential more than 2-fold higher than that of a thiol,17, 18 it is not surprising that a thiolate forms a stable heme complex with the heme-bound H25A mutant but a selenolate reduces the heme. The dramatic difference between selenolate-bound CYP101 and the H25A heme complex indicates that the consequences of selenolate ligation to a heme group depend on the redox potential of the heme. Compared to substrate-free CYP101 with a redox potential of −300 mV, heme-bound hHO-1 has a nearly 5-fold lower redox potential of −65 mV.16,19 The electron donating effect of the cysteine ligand in CYP101 increases the redox potential of the heme and thus helps to stabilize the selenolate sixth ligand. The absence of a similar electron donating ligand in the H25A hHO-1 mutant, however, makes it possible for the selenolate to reduce the heme. Our findings indicate that stabilizing structural factors such as an electron donating sixth-iron ligand are required for the construction of a sustainable selenocysteine-ligated hemeprotein. If the preparation of selenocysteine CYP101 reported in a brief meeting abstract is confirmed,20 structural factors other than the presence of a distal electron donating substituent may contribute to this stabilizing effect.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants GM25515 and DK30297. We thank Dr. Hugues Ouellet for advice and discussions.
Footnotes
Supporting Information Available: Experimental methods. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ortiz de Montellano PR. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3. Plenum Klewer; New York: 2005. [Google Scholar]
- 2.Davydov R, Makris TM, Kofman V, Werst DE, Sligar SG, Hoffman BM. J Am Chem Soc. 2001;123:1403–1415. doi: 10.1021/ja003583l. [DOI] [PubMed] [Google Scholar]
- 3.Denisov IG, Makris TM, Sligar SG. J Biol Chem. 2001;276:11648–11652. doi: 10.1074/jbc.M010219200. [DOI] [PubMed] [Google Scholar]
- 4.Newcomb M, Toy PH. Acc Chem Res. 2000;33:449–455. doi: 10.1021/ar960058b. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S, Kumar D, Shaik S. J Am Chem Soc. 2006;128:2649–2653. doi: 10.1021/ja056586c. [DOI] [PubMed] [Google Scholar]
- 6.Atkins JF, Gesteland RF. Nature. 2000;407:463–465. doi: 10.1038/35035189. [DOI] [PubMed] [Google Scholar]
- 7.Johansson L, Gafvelin G, Arner ES. Biochim Biophys Acta. 2005;1726:1–13. doi: 10.1016/j.bbagen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Barrick D. Biochemistry. 1994;33:6546–6554. doi: 10.1021/bi00187a023. [DOI] [PubMed] [Google Scholar]
- 9.Perera R, Dawson JHJ. Porphyrins Phthalocyanines. 2004;8:246–254. [Google Scholar]
- 10.Qin J, Perera R, Lovelace LL, Dawson JH, Lebioda L. Biochemistry. 2006;45:3170–3177. doi: 10.1021/bi052171s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruf HH, Wende P. J Am Chem Soc. 1977;99:5499–5500. doi: 10.1021/ja00458a054. [DOI] [PubMed] [Google Scholar]
- 12.Dawson JH, Sono M. Chemical Review. 1987;87:1255–1276. [Google Scholar]
- 13.Ralle M, Berry SM, Nilges MJ, Gieselman MD, van der Donk WA, Lu Y, Blackburn NJ. J Am Chem Soc. 2004;126:7244–7256. doi: 10.1021/ja031821h. [DOI] [PubMed] [Google Scholar]
- 14.Unno M, Matsui T, Ikeda-Saito M. Nat Prod Rep. 2007;24:553–570. doi: 10.1039/b604180a. [DOI] [PubMed] [Google Scholar]
- 15.Wilks A, Sun J, Loehr TM, Ortiz de Montellano PR. J Am Chem Soc. 1995;117:2925–2926. [Google Scholar]
- 16.Liu Y, Moenne-Loccoz P, Hildebrand DP, Wilks A, Loehr TM, Mauk AG, Ortiz de Montellano PR. Biochemistry. 1999;38:3733–3743. doi: 10.1021/bi982707s. [DOI] [PubMed] [Google Scholar]
- 17.Jacob C, Giles GI, Giles NM, Sies H. Angew Chem, Int Ed Engl. 2003;42:4742–4758. doi: 10.1002/anie.200300573. [DOI] [PubMed] [Google Scholar]
- 18.Beld J, Woycechowsky KJ, Hilvert D. Biochemistry. 2007;46:5382–5390. doi: 10.1021/bi700124p. [DOI] [PubMed] [Google Scholar]
- 19.Sligar SG, Gunsalus IC. Proc Natl Acad Sci U S A. 1976;73:1078–1082. doi: 10.1073/pnas.73.4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gromov I, Garcia-Rubio I, Aldag C, Schweiger A, Hilvert D. 6th European Federation of EPR Groups Meeting Abstract, OT3.2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.