Abstract
Objective
To describe trends in incidence rates of AIDS-defining cancers (ADCs) and non-AIDS-defining cancers (NADCs) during the HIV epidemic and to evaluate predictors, including the impact of antiretroviral therapy, of cancer development.
Design
Retrospective analysis of a multicenter, prospective natural history study including 4,498 HIV-infected U.S. military beneficiaries with 33,486 person-years of follow-up.
Methods
Predictors evaluated included demographics, clinical data, time-updated CD4 cell counts, HIV viral loads, and antiretroviral history. Time periods were classified as early pre- (1984-1990), late pre- (1991-1995), early post- (1996-2000), and late post-(2001-2006) HAART eras. Cox proportional hazard models were used to evaluate the association of specific factors with cancer.
Results
Ten percent of HIV-infected persons developed cancer. ADC rates increased between the early and late pre-HAART eras (7.6 and 14.2 cases per 1000 person years) and have since declined from 5.4 to 2.7 in the early and late HAART eras, respectively (p<0.001). Rates of NADCs have risen over the four time periods (2.9, 2.8, 4.2, 6.7, p=0.0004). During the late HAART era, 71% of cancers were NADCs. Predictors for ADCs included low CD4 cell count, non-cancer AIDS diagnosis, and lack of HAART. NADCs were predicted by increasing age, Caucasian race (due to skin cancers), and lack of HAART.
Conclusions
Although the rate of ADCs continues to fall, the rate of NADCs is rising and now accounts for the majority of cancers in HIV-infected persons. The development of NADCs is associated with increasing age among HIV patients. HAART is protective for both ADCs and NADCs.
Keywords: Cancer, malignancy, HIV, military, epidemiology, HAART
Cancers such as Kaposi’s sarcoma (KS) were among the initial clinical diagnoses that led to the recognition of human immunodeficiency virus (HIV) infections in 1981 [1]. Some experts in the 1980s suggested that malignancies would cause a second epidemic, which was realized with the occurrence of KS and lymphoma [2]. Subsequently, three cancers were classified as AIDS-defining cancers (ADCs), including KS, non-Hodgkin’s lymphoma (NHL), and invasive cervical carcinoma (ICC) [3, 4].
With the advent of highly active antiretroviral therapy (HAART) in 1996, the rates of KS and NHL of the central nervous system have dramatically fallen, with less effect on ICC and systemic NHL rates [5-10]. Simultaneously, non-AIDS-defining cancers (NADCs) have accounted for an increasing proportion of cancer cases reported in HIV-infected individuals. Recent studies have reported that NADCs represented 13% of deaths during the HAART era, compared to less than 1% in the pre-HAART era [11], and that fatal NADCs are now more common than fatal ADCs [12]. However, other research has shown conflicting results regarding incidence rates of NADCs [13, 14]. Further evaluation of cancer trends in large and diverse HIV positive cohorts that include early-stage HIV patients is needed.
We evaluated prospectively collected data from the 23-year observational Tri-Service AIDS Clinical Consortium (TACC) HIV Natural History Study (NHS) to further investigate trends in the rates of ADCs and NADCs among HIV-infected persons. Further, given the availability of individual patient data, we assessed whether CD4 cell counts, HIV viral loads, or antiretroviral medications were predictors of cancer occurrence among HIV-infected persons.
Methods
We examined data collected from the TACC NHS, a multicenter, prospective, observational study, which enrolled 4,566 HIV positive persons from 1984-2006 at seven geographic locations in the U.S. Participants were military beneficiaries (active duty members, retirees, and dependents) evaluated on a biannual basis utilizing standardized data collection procedures, and all have free access to care, including medications. Our study was approved by central and local Institutional Review Boards, and patients provided informed consent.
All active duty U.S. military personnel undergo routine HIV screening every one to five years; prior to enlistment, all members are confirmed HIV-negative. For this study, among those with a last known HIV negative date (58%), the median time from last HIV negative date to first HIV positive date (ELISA confirmed by a Western Blot test) was 16 months. Baseline was defined as the time of HIV seroconversion, conservatively estimated as six months prior to the first documented HIV positive test. Cancer cases were only included if they occurred after this point. The diagnosis of cancer was based on physician diagnosis supported by laboratory, radiologic, and/or histopathologic results. Cancer events were identified in our database by searching for specific cancer codes and for the terms “cancer”, “malignancy”, “tumor” or “neoplasm”. Cancers were defined as ADCs (KS, NHL, or ICC) or NADCs (all others). Participants excluded from this analysis were those without a documented HIV positive test (n=10), with cancer prior to HIV seroconversion (n=51), and with a benign tumor or a clinical diagnosis that could not be confirmed as malignant (n=7), yielding 4,498 participants for our study (Figure 1).
Figure 1.
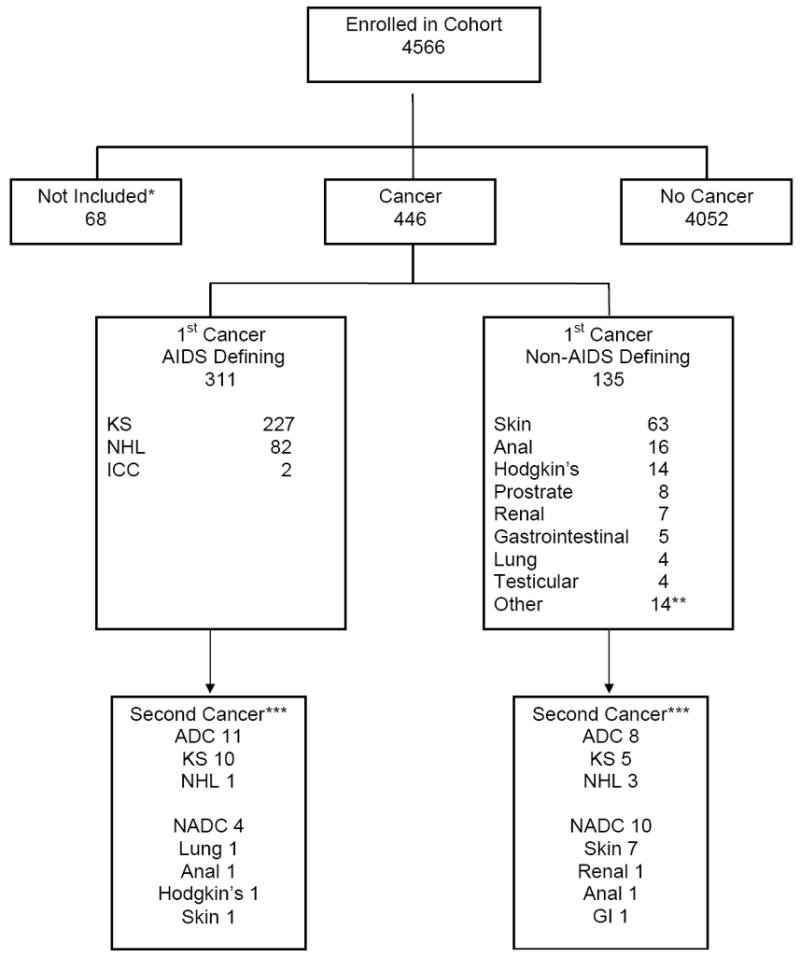
Flow diagram of cancer cases in the study cohort.
*Cancer more than 6 months prior to HIV seroconversion (N=51); HIV seroconversion date unknown (N=10); benign tumor or diagnosis not confirmed as malignant (N=7).
** Including one case of hepatocellular carcinoma.
***Second cancers were malignancies which developed after the first cancer and that were due to a different type of cancer.
Data collected included: demographics, including self-reported race/ethnicity; CD4 cell counts and HIV viral load tests at baseline and sequentially over time; history of AIDS-defining conditions other than cancer; and antiretroviral therapy. HAART was defined as: two or more nucleoside reverse transcriptase inhibitors (NRTIs) in combination with at least one protease inhibitor (PI) or one non-nucleoside reverse transcriptase inhibitors (NNRTI); one NRTI in combination with at least one PI and at least one NNRTI; or an abacavir or tenofovir containing regimen of three or more NRTIs. For those with cancer, the event date was determined by the first cancer diagnosis date for the specific type of cancer considered. For those without cancer, the censoring date was the last study visit or the date of death. Follow-up for this report ended 31 December 2006.
Statistical analyses included descriptive statistics to compare those with and without cancer events. Medians are presented with interquartile ranges (IQR). Kruskal-Wallis tests were used to compare medians, and Chi-square tests were used to compare proportions. The number of events, person years at risk, and rates of events (per 1,000 person years of follow-up) were calculated for the overall study period and for specific time intervals: the early pre-HAART era (1984-1990), the late pre-HAART era (1991-1995), the early post-HAART era (1996-2000), or the late post-HAART era (2001-2006). Each participant contributed to the person years at risk for every time interval from HIV diagnosis until the event or censoring time. Poisson regression analyses were used to test the hypothesis that the cancer rates remained constant over those intervals. In order to compare the risk of cancer in our HIV positive cohort with the risk seen in the general population, age-adjusted incidence (per 1,000) over the study period (1984-2006) was calculated for any cancer event (excluding basal cell and squamous) and then separately for a NADC event (excluding basal cell and squamous), KS, NHL, Hodgkin’s disease, and anal carcinoma. For each event, the incidence was age-adjusted to the U.S. 2000 standard population [15] and calculated for the overall cohort and for males only. The age-adjusted incidence was compared to data provided in the National Cancer Institute Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review [16] for the period 1974-2004; for KS, SEER results from 1975-1979 were used, as most cases of KS occurred among HIV-patients after 1980. Basal cell and squamous were excluded since SEER does not collect data on those events.
Participants were classified by HIV diagnosis era: pre-HAART (prior to 1996) or post-HAART (at or after 1996). Univariate and multivariate Cox proportional hazard models, stratified by HIV diagnosis eras, were used to evaluate the association of specific factors with cancer. The multivariate models were adjusted for demographics at the time of HIV diagnosis (age, gender, ethnicity, and year of HIV diagnosis). Variables which may change during follow-up (CD4 cell count, HIV RNA levels, HAART use, and non-cancer AIDS event) were considered as time-updated covariates, using all available data between HIV diagnosis time and the event or censoring time. For those with cancer, time from cancer diagnosis to death was evaluated with unadjusted proportional hazards models and Kaplan-Meier survival estimates. Hazard ratios (HR) are reported with 95% confidence intervals (CI). All analyses were conducted using SAS (version 8.2, Cary, NC).
Results
Between 1984 and 2006, 4,498 participants were followed for a total of 33,486 person years. The study cohort had a median age at HIV diagnosis of 28 (IQR 24-33) years; 91% were male. Race was reported as: African-American for 45%, Caucasian/non-Hispanic for 44% and other for 11% (Table 1). HIV seropositive date was prior to 1996 for 2,443 (77%) of participants. The median length of follow-up was 6.6 (IQR 3.7-10.1) years. Median baseline CD4 cell count at HIV diagnosis was 510 (IQR 353-680) cells/mm3. During the study period, 24% experienced an AIDS-defining event other than cancer (Table 1).
Table 1.
Cohort characteristics at time of HIV diagnosis and during follow-up.
| Characteristic | Overall (N=4498) | No Cancer (N=4052) | ADC1 (N=311) | NADC1 (N=135) |
|---|---|---|---|---|
| Age at HIV diagnosis, years | ||||
| Median (IQR) | 28 (24-33) | 28 (23-33) | 28 (25-33) | 34 (27-42) |
| Distribution, % | ||||
| < 20 | 2.9 | 3.1 | 1.3 | 0.7 |
| 20 - 34 | 76.0 | 76.6 | 78.5 | 52.6 |
| 35 - 55 | 19.9 | 19.3 | 18.3 | 41.5 |
| > 55 | 1.3 | 1.1 | 1.9 | 5.2 |
| Male gender, % | 91.0 | 90.3 | 97.4 | 95.6 |
| Race, % | ||||
| Caucasian | 43.5 | 42.1 | 51.8 | 68.2 |
| African American | 45.3 | 46.5 | 37.9 | 24.4 |
| Other | 11.2 | 11.4 | 0.3 | 7.4 |
| Follow-up time | ||||
| Median (IQR) years | 6.6 (3.7-10.1) | 6.7 (3.8-10.3) | 5.6 (3.6-7.9) | 6.0 (2.8-11.7) |
| HIV diagnosis date, % | ||||
| Before 1996 | 76.6 | 74.7 | 96.8 | 86.7 |
| 1996 or after | 23.4 | 25.3 | 3.2 | 13.3 |
| CD4 count at HIV diagnosis | ||||
| Median (IQR) cells/mm3 | 510 (353-680) | 520 (360-686) | 432 (250-602) | 480 (337-668) |
| Distribution, % | ||||
| < 200 | 5.7 | 5.1 | 14.2 | 5.2 |
| 200-500 | 31.6 | 31.4 | 32.5 | 34.8 |
| > 500 | 40.2 | 41.4 | 26.4 | 34.1 |
| Unknown | 22.6 | 22.1 | 27.0 | 25.9 |
| HIV viral load at HIV diagnosis | ||||
| Median (IQR) log10 copies/ml | 4.4 (3.8-4.8) | 4.4 (3.7-4.9) | 4.7 (4.3-50) | 4.5 (3.9-5.0) |
| Distribution, % | ||||
| <1,000 | 2.9 | 3.1 | 0.3 | 1.5 |
| 1,000 – 10,000 | 6.7 | 7.2 | 1.0 | 4.4 |
| >10,000 | 19.3 | 20.2 | 10.0 | 13.3 |
| Unknown | 71.2 | 69.5 | 88.8 | 80.7 |
| Non-cancer AIDS event, % | 3.1 | 2.7 | 8.7 | 3.0 |
| At baseline | 23.8 | 22.5 | 45.0 | 15.6 |
| Prior to censoring | ||||
| CD4 count at censoring2 | ||||
| Median (IQR) cells/mm3 | 404 (152-621) | 420 (185-638) | 72 (17-288) | 430 (258-578) |
| Distribution, % | ||||
| < 200 | 28.6 | 26.1 | 65.6 | 16.3 |
| 200-500 | 32.9 | 33.5 | 21.5 | 41.5 |
| > 500 | 37.3 | 39.9 | 5.8 | 32.6 |
| Unknown | 1.3 | 0.5 | 7.1 | 9.6 |
| HIV RNA viral load at censoring2,3 | ||||
| Median (IQR) log10 copies/ml | 3.5 (2.2-4.6) | 3.4 (2.1-4.5) | 4.8 (4.4-5.1) | 3.4 (2.0-4.4) |
| Distribution, % | ||||
| < 3.0 | 28.3 | 30.3 | 1.9 | 26.7 |
| 3.0 – 4.0 | 13.9 | 14.8 | 2.9 | 14.1 |
| > 4.0 | 27.9 | 27.6 | 35.1 | 19.3 |
| Unknown | 30.0 | 27.3 | 60.1 | 40.0 |
| Undetectable (<400 copies/ml) HIV RNA viral load at censoring2,3, % | 31.4 | 32.6 | 0 | 34.5 |
Defined by first cancer type: AIDS defining cancer (ADC) or non-AIDS defining cancer (NADC)
Censoring is the date of first cancer occurrence or last follow-up visit
Number with an available HIV viral load was 3,157 overall; for those with cancer, n=2946, ADC, n=125, and NADC, n=84
At least one cancer event was recorded for 446 individuals (10%). The first cancer was AIDS-defining for 311 (70%) and non-AIDS-defining for 135 (30%). The median time from HIV diagnosis to an ADC was 5.6 (IQR 3.6-7.9) years and for a NADC was 6.0 (IQR 2.8-11.7) years. Figure 1 reports the specific types of cancers diagnosed. Of those whose first cancer was AIDS-defining, KS was the most frequent (73%; n=227); the most common NADC was skin cancer (47%; n=63). Skin cancers were mostly basal carcinomas (n=48), followed by melanoma (n=10) and squamous (n=5). Thirty-three persons (7.4% of those with cancer) developed two different cancers during the study period as shown in Figure 1. Eleven people had two different ADCs: 10 had KS followed by NHL, and one person had NHL followed by KS. Four people with an initial ADC subsequently developed a NADC (lung, anal, Hodgkin’s, and skin cancer). Eight people with an initial NADC later developed an ADC (5 KS and 3 NHL cases), which was most commonly an initial skin cancer followed by KS. Finally, 10 people had a two NADCs develop; most commonly this was the development of two different types of skin cancer.
Of those who developed cancer, the diagnosis occurred in the pre-HAART era for 302 (68%) and in the post-HAART era for 144 (32%), resulting in pre- and post-HAART cancer rates (per 1,000 person years) of 16.1 and 9.8, respectively. The rate of ADCs increased significantly between the early and late pre-HAART eras (7.6 and 14.2, respectively), then declined significantly during the early and late post-HAART intervals (5.4 and 2.7, respectively) (Table 2). The rates for NADCs were stable in the pre-HAART era at approximately 3 cases per 1,000 person years. However, since the availability of HAART, NADC rates increased to 4.2 and 6.7 in the early and late post-HAART era, respectively (p=0.004). The rates of non-skin NADC were less than 2 per 1,000 person years in the pre-HAART and early post-HAART eras, but increased to 4.1 per 1,000 person years during the late post-HAART era (p=0.0003). Furthermore, the proportion of cancers that were NADCs significantly increased from 20% in the pre-HAART era to 36% in the early post-HAART era and 71% in the late post-HAART era (p<0.0001).
Table 2.
Cancer event rates per 1,000 person-years (with 95% confidence intervals) for intervals formed by HAART availability dates.
| Cancer Diagnosis Date | |||||
|---|---|---|---|---|---|
| Event (No.) | Early Pre-HAART (1984-1990) |
Late Pre-HAART (1991-1995) |
Early Post-HAART (1996-2000) |
Late Post-HAART (2001-2006) |
P-value1 |
| Any cancer (n=446) | 10.6 (8.4, 12.9) | 17.2 (14.9, 19.5) | 8.5 (6.6, 10.4) | 9.7 (7.3, 12.0) | 0.01 |
| AIDS-defining cancer (n=311) | 7.6 (5.7, 9.6) | 14.2 (12.1, 16.3) | 5.4 (3.9, 6.9) | 2.7 (1.5, 3.9) | <0.0001 |
| Non-AIDS-defining cancer (n=135) | 2.9 (1.8, 4.1) | 2.8 (1.9, 3.8) | 4.2 (2.7, 5.8) | 6.7 (4.5, 9.0) | 0.0004 |
| Non-skin Non-AIDS defining cancer (n=72) | 1.6 (0.7, 2.5) | 1.1 (0.5, 1.7) | 1.8 (1.0, 2.7) | 4.1 (2.6, 5.7) | 0.0003 |
| Kaposi’s Sarcoma (n=227) | 6.3 (4.6, 8.0) | 10.2 (8.5, 12.0) | 3.5 (2.3, 4.7) | 1.8 (0.8, 2.8) | <0.0001 |
| Non-Hodgkin’s Lymphoma (n=82) | 1.3 (0.6, 2.1) | 3.8 (2.7, 4.9) | 1.7 (0.9, 2.5) | 0.8 (0.2, 1.5) | 0.07 |
| Skin Cancer (n=63) | 1.3 (0.6, 2.2) | 1.8 (1.1, 2.5) | 1.2 (0.5, 1.9) | 2.5 (1.4, 3.7) | 0.22 |
| Anal Cancer (n=16) | 0.1 (0.0, 0.4) | 0.2 (0, 0.4) | 0.4 (0, 0.8) | 1.3 (0.4, 2.1) | 0.001 |
| Hodgkin’s Disease (n=14) | 0.6 (0.1, 1.2) | 0.2 (0, 0.4) | 0.3 (0, 0.7) | 0.6 (0, 1.1) | 0.97 |
| Prostate Cancer (n=8) | 0.1 (0, 0.4) | 0.1 (0, 0.2) | 0.2 (0, 0.5) | 0.6 (0, 1.1) | 0.07 |
| Renal Carcinoma (n=7) | 0.2 (0, 0.6) | 0.1 (0, 0.2) | 0.1 (0, 0.3) | 0.4 (0, 0.9) | 0.45 |
From the null hypothesis that the event rates were constant over the intervals listed
The rates over time for the most common cancer types are shown in Table 2 and Figure 2. Rates for both KS and NHL increased significantly before HAART, but have steadily declined since 1996. The rate of anal cancer was stable in the pre-HAART era (0.1-0.2 cases per 1,000 person-years), but significantly increased to a rate of 1.3 in the late post-HAART era (p=0.001). Skin, renal, and prostate cancer also had the highest rates during the late post-HAART era.
Figure 2.
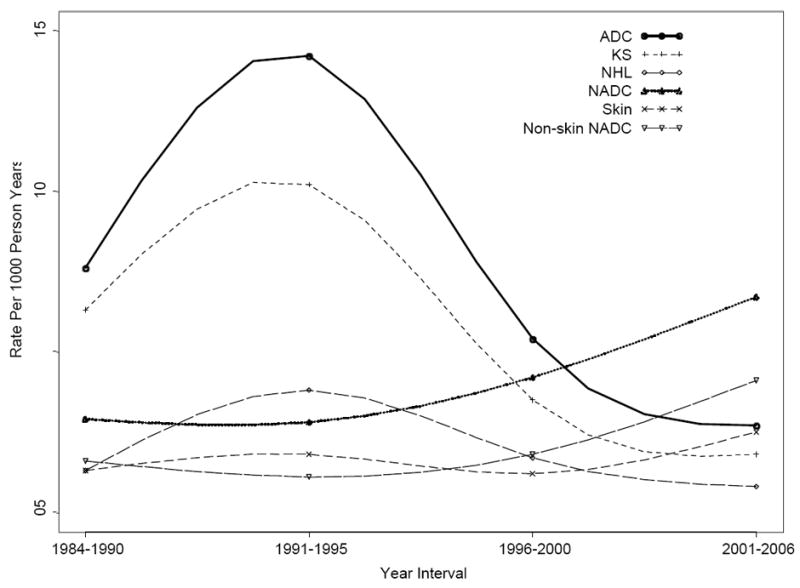
Cancer incidence rates per 1000 person-years by HAART availability time periods.
The age-adjusted incidence among males in our HIV cohort was 13.0 per 1,000 person years for any cancer event (excluding basal cell and squamous), compared to 5.5 for males in the general population. Limiting the events to NADCs (excluding basal cell and squamous), the age-adjusted incidence among males in our cohort was 6.5 per 1,000 person years. The age-adjusted incidence among HIV positive males (compared to the general population) was 4.0 (vs. 0.004) for KS, 2.7 (vs. 0.2) for NHL, 1.6 (vs. 0.03) for Hodgkin’s disease, and 0.2 (vs. 0.01) for anal carcinoma. For each type of event, the age-adjusted incidence for our overall cohort was similar to the age-adjusted incidence for the males (data not shown).
The univariate proportional hazards regression models for any cancer, ADCs, NADC, and non-skin NADCs are shown in Table 3. For all models, increased age was significantly associated with an increased risk of a cancer event, while HAART use was associated with a significantly decreased risk. African American race (compared to Caucasians) and increased CD4 cell count throughout follow-up were significantly associated with a decreased risk of an ADC or NADC event, but were not associated with a non-skin NADCs. ADCs were also associated with male gender, occurrence of a non-cancer AIDS diagnosis, and higher HIV viral loads. The association of ADCs and male gender was due to KS (data not shown).
Table 3.
Univariate analyses for time to first cancer event.
| Any Cancer1 | AIDS Defining Cancer1 | Non-AIDS Defining Cancer1 | Non-Skin Non-AIDS DefiningCancer1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | |
| Age at HIV diagnosis (per 10 years) | 1.54 (1.39, 1.72) | <0.0001 | 1.22 (1.06, 1.40) | 0.007 | 2.18 (1.87, 2.56) | <0.0001 | 1.98 (1.59, 2.46) | <0.0001 | |
| Male gender | 2.73 (1.60, 4.65) | 0.0002 | 3.38 (1.67, 6.82) | 0.0007 | 1.82 (0.80, 4.13) | 0.15 | 0.97 (0.42, 2.24) | 0.94 | |
| Race | |||||||||
| Caucasian | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| African American | 0.55 (0.45, 0.68) | <0.0001 | 0.70 (0.55, 0.89) | 0.003 | 0.32 (0.22, 0.48) | <0.0001 | 0.85 (0.53, 1.37) | 0.52 | |
| Other | 0.66 (0.48, 0.92) | 0.01 | 0.82 (0.56, 1.20) | 0.31 | 0.42 (0.22, 0.80) | 0.009 | 0.88 (0.41, 1.88) | 0.73 | |
| CD4 count at HIV diagnosis (per 50 cells) | 0.91 (0.89, 0.93) | <0.0001 | 0.89 (0.86, 0.91) | <0.0001 | 0.96 (0.92, 1.00) | 0.04 | 0.94 (0.89, 1.00) | 0.04 | |
| HIV RNA viral load at HIV diagnosis (per ½ log) | 2.88 (1.43, 5.77) | 0.003 | 5.14 (1.90, 13.87) | 0.001 | 1.57 (0.62, 4.02) | 0.34 | 0.72 (0.22, 2.38) | 0.59 | |
| Time-updated CD4 cell count (per 50 cells) | 0.81 (0.79, 0.83) | <0.0001 | 0.72 (0.69, 0.75) | <0.0001 | 0.96 (0.93, 1.00) | 0.04 | 0.96 (0.92, 1.01) | 0.12 | |
| Time-updated HIV RNA viral load (per ½ log) | 2.98 (2.32, 3.84) | <0.0001 | 9.69 (6.53, 14.39) | <0.0001 | 0.91 (0.61, 1.35) | 0.62 | 0.84 (0.50, 1.40) | 0.50 | |
| Non-cancer AIDS event2 | 1.62 (1.33, 1.97) | <0.0001 | 2.26 (1.80, 2.84) | <0.0001 | 0.59 (0.37, 0.95) | 0.03 | 2.28 (0.55, 9.44) | 0.25 | |
| Time-updated HAART use | 0.34 (0.26, 0.47) | <0.0001 | 0.06 (0.04, 0.09) | <0.0001 | 0.26 (0.17, 0.38) | <0.0001 | 0.75 (0.66, 0.85) | <0.0001 | |
Analyses are stratified by HIV diagnosis era (pre- or post-1996) and are adjusted for year of HIV diagnosis
In the multivariate model, the predictors of any cancer and an ADC included male gender and a non-cancer AIDS event, while factors associated with a reduced risk of a cancer event included African American race, increased CD4 cell counts, and HAART (Table 4). HIV viral load was not included in the multivariate models because measurements were unavailable for 30% of the cohort.
Table 4.
Multivariate analyses for time to first cancer event.
| All Participants | ||||||||
|---|---|---|---|---|---|---|---|---|
| Any Cancer1 | AIDS Defining Cancer1 | Non-AIDS Defining Cancer1 | Non-Skin Non-AIDS DefiningCancer1 | |||||
| Characteristic | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value |
| Male gender | 2.35 (1.37, 4.02) | 0.002 | 3.15 (1.55, 6.38) | 0.002 | 1.47 (0.64, 3.36) | 0.36 | 0.66 (0.28, 1.54) | 0.34 |
| Race | ||||||||
| Caucasian | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| African American | 0.63 (0.51, 0.79) | <0.001 | 0.69 (0.53, 0.88) | 0.004 | 0.31 (0.20, 0.47) | <0.001 | 0.99 (0.58, 1.68) | 0.97 |
| Other | 0.71 (0.49, 1.02) | 0.063 | 0.84 (0.56, 1.27) | 0.42 | 0.42 (0.21, 0.84) | 0.01 | 0.72 (0.28, 1.88) | 0.51 |
| Age at HIV diagnosis (per 10 years) | 1.28 (1.13, 1.44) | <0.001 | 1.08 (0.92, 1.26) | 0.34 | 1.99 (1.68, 2.36) | <0.001 | 1.84 (1.44, 2.4) | <0.001 |
| CD4 cell count (time updated; per 50 cells) | 0.89 (0.86, 0.91) | <0.001 | 0.77 (0.74, 0.81) | <0.001 | 0.98 (0.95, 1.02) | 0.27 | 0.99 (0.94, 1.04) | 0.67 |
| HAART use (time updated; yes/no) | 0.53 (0.37, 0.76) | <0.001 | 0.11 (0.07, 0.16) | <0.001 | 0.28 (0.19, 0.43) | <0.001 | 0.32 (0.18, 0.57) | <0.001 |
| Non-cancer AIDS event (time updated) | 2.04 (1.61, 2.58) | <0.001 | 1.71 (1.30, 2.25) | <0.001 | 1.14 (0.68, 1.90) | 0.62 | 1.20 (0.58, 2.45) | 0.62 |
Analyses are stratified by HIV diagnosis era (pre- or post-1996) and are adjusted for year of HIV diagnosis
NADCs were associated with increasing age, Caucasian race, and lack of HAART in the multivariate model; there was no association with gender, CD4 cell counts or prior non-cancer AIDS events (Table 4). The relationship between Caucasian race and an elevated risk of NADCs was due to the high number of skin cancers; of the 63 cases of skin cancers, 93% occurred in the Caucasian race group. Models were repeated for non-skin NADCs (Tables 3 and 4). From the multivariate model for non-skin NADCs, age was still associated with cancer development and HAART remained protective. There were no associations with race, AIDS events, or CD4 cell counts.
Comparing those with NADCs to those with ADCs at the time of cancer diagnosis, those with a NADC were more likely to be older (median 42 vs. 35 years, p<0.0001) and Caucasian (68% vs. 52%, p=0.006); have an HIV diagnosis date after 1996 (13% vs. 3%, p<0.0001); have higher median CD4 counts (430 vs. 72 cells/mm3, p<0.0001) and lower median HIV viral loads (3.4 vs. 4.8 log, p<0.0001); have received HAART for a greater percentage of their follow-up time (15% vs. 3%, p<0.0001); and were less likely to have a prior AIDS defining event (16% vs. 45%, p<0.0001). The median CD4 cell counts at cancer occurrence for those with an ADC during the early pre-HAART, late pre-HAART, early post-HAART, and late post-HAART eras were 40, 80, 44, and 242 cells/mm3, respectively (p=0.14). For the same intervals, those with NADCs had median CD4 cell counts at cancer diagnosis of 410, 370, 361, and 474 cells/mm3, respectively (p=0.05).
During the study period, 1,523 (34%) participants of the overall cohort died. Death occurred among 85% of patients with an ADC, 40% of those with an NADC, 46% with a non-skin NADC, and 30% without cancer (p<0.0001). For patients with an ADC compared to a NADC, the hazard ratio for time from cancer diagnosis to death was 3.7 (95% CI 2.7 – 4.9, p<0.0001). The estimated mortality at one, three, and five years after cancer diagnosis was 51%, 76%, and 84%, respectively for those with an ADC; 15%, 29%, and 41% for those with a NADC; and 26%, 38%, and 49% for those with a non-skin NADC.
Discussion
This study, with extended follow-up in the late HAART era, provides current data on the trends for AIDS-defining and non-AIDS-defining cancers. We found that rates for ADCs have continued to decline well after the advent of HAART. Conversely, the rates of NADCs have continued to rise over time and now account for the majority of the cancers in our study cohort; the development of NADCs appears most related to increasing age. HAART use was protective for both ADCs and NADCs.
Studies after the advent of HAART noted dramatic declines in the rates of ADCs [5-10]; however, data in the late post-HAART era are more limited [17-19]. A recent study suggested that ADC rates have stabilized during the HAART era [9], while our study demonstrates that the rate of ADCs is progressively decreasing. These contrasting results may be due to the study periods considered (our study has follow-up through 2006, while other studies include follow-up only through 2002) and the general health of the cohorts considered (our cohort was composed primarily of people with earlier stages of HIV infection, while other cohorts included only those with AIDS). A more recent study (1996-2005) performed in an urban HIV clinic had findings similar to ours showing that ADC rates continue to decline [20]. The decreasing rates of ADCs may be related to the improving efficacy and tolerability of antiretroviral therapies.
The most frequent cancer in our study cohort was KS followed by NHL, both of which occurred most commonly during the pre-HAART era. Predictors for these cancers included low time-updated CD4 cell counts and the lack of HAART use. These data emphasize the importance of early HIV diagnosis and initiation of therapy before low CD4 cell counts occur.
The most common NADCs in our cohort were non-KS skin cancers and anal cancer. For both of these cancers, the rates were fairly stable from 1984 through 2000, with increased rates during the period 2001 through 2006. The increasing rate of anal carcinoma was statistically significant (13-fold over the study period). Of note, formal cancer screening practices did not change in our cohort during recent years to explain the increased rate; however, data on trends in the number of MSM in our study cohort is not available. Other cancers, such as renal and prostate carcinoma, reached their highest rates during the late post-HAART period, but the trends were not significant, likely due to the small sample size of the individual cancer types; other studies have also reported an increase in these cancers during the late HAART era [9, 13, 21]. We did not find an increase in the incidence of Hodgkin’s disease as seen in some studies. [9, 22, 23]
The proportion of cancer due to NADCs increased over the study period similar to other reports in the literature [13]. Reasons for the recent increased importance of NADCs among HIV-infected persons are likely several-fold. Increasing life expectancy [24, 25] and the reduction in competitive causes of death [26] are undoubtedly contributory. Viral co-infections such as the human papillomavirus, which may have a relatively long latency before their oncogenic effect, are prevalent in HIV patients and may play a role in cancer development [27-29]. In addition, HIV-infected patients may have higher rates of behaviors, such as tobacco use, which contributes to cancer development [30, 31]. Finally, HIV itself could play a role either by a direct oncogenic effect (e.g., HIV tat gene) [32] or as a consequence of immunosuppression with diminished tumor surveillance [33].
Factors associated with the development of an NADC in our study included increasing age, Caucasian race, and lack of HAART, although the association with race was restricted to skin cancers. We did not find an association with NADCs and prior non-cancer AIDS events or time-updated CD4 cell counts. In fact, in our study, patients with NADCs had robust CD4 cell counts at diagnosis (the median was 430 cells/mm3), and 79% of the NADC events in the late post-HAART era occurred at a CD4 cell count >350 cells/mm3. Other studies have also noted that NADCs, such as lung cancer, may not related to advanced immunosuppression as measured by CD4 cell counts or HIV viral loads [9, 11, 30, 34-36]. This suggests that strategies beyond achievement of high CD4 cell counts may be necessary for reduction in NADCs. Such strategies may include behavioral modifications, such as smoking cessation, safe-sex practices to reduce viral co-infections, and early recognition and management of viral hepatitis.
This is one of the first studies to show an association between HAART use and reduced rates of both ADCs and NADCs among individual HIV patients. A recent study suggested that HAART use may be associated with a lower risk of NADCs, but did not demonstrate statistical significance [23]. Other studies have traditionally lacked individual patient data regarding HAART use, and merely examined cancer trends during the HAART era [37]. Our data are important because they demonstrate the potential benefits of antiretroviral medications to the individual HIV patient and help dispel concerns that HAART may be mutagenic. Whether practitioners should initiate HAART earlier than advocated by previous guidelines [38] is unclear. Although HAART was protective, many of our cancer events occurred among persons with relatively high CD4 cell counts; hence the timing of HAART initiation based solely on CD4 cell levels may not be completely applicable for cancer prevention. The costs of initiating HAART earlier during the course of HIV infection may be offset by the potential cost-savings of preventing future complications such as malignancies. Research examining the effect of earlier initiation of antiretroviral therapy on the incidence of AIDS-defining and non-AIDS-defining conditions, such as cancers, is under development.
The protective effect of antiretroviral medications on cancer development was noted in the setting of increasing rates of NADCs during the HAART era. These data suggests that although HAART is protective of NADCs, HIV-infected persons are now living longer and not succumbing to deaths due to other causes resulting in a cumulative greater opportunity of a NADC event. The effect of HAART on decreasing cancers is due in part to improvement in the CD4 cell count, as seen for ADCs. The impact of HAART on NADC rates did not appear to be closely related to the CD4 cell count, suggesting that other mechanisms are occurring. Although this study did not evaluate the exact mechanism(s) of the protective effect of HAART, these may include decreased immune activation and cytokine levels, boosting immune responses unmeasured by the CD4 cell count, and HAART-related suppression of oncogenic viruses.
The strengths of our study include the long-term follow-up of HIV patients as part our 23-year Natural History Study. Given recommendations for early HIV detection through routine screening [39], this study of patients with early-stage HIV infection provides important data on cancer rates and trends. In addition, our study population was racially diverse, without barriers to healthcare access, and from varied geographical locations in the U.S. Moreover, our data included confirmed cancer diagnoses and individual patient data, including precisely defined use of antiretroviral therapy, AIDS defining events, and serial CD4 cell counts.
Several potential limitations of this study should be noted. Our cohort consisted primarily of non-drug users with a low prevalence of hepatitis C; hence, data regarding the effect of drug use or hepatitis co-infection on NADC rates could not be evaluated. Furthermore, we did not collect data on behaviors such as tobacco use, alcohol use, or on family history of cancer. In addition, our population was primarily male; hence, female-specific tumors could not be adequately studied. Finally, given the limited number of NADCs and that most were skin cancers, examining individual NADCs, such as anal cancer or Hodgkin’s disease, was not possible.
In conclusion, while the overall rate of cancer has declined since the HAART era, malignancies remain an important cause of morbidity among HIV-infected persons. The rates of ADCs have continued to fall since the advent of HAART, but the rates of NADCs are rising and now account for the majority of cancer cases. The increasing rates of NADCs appear most related to the aging of the HIV population. Antiretroviral therapy appears protective for the development of both ADCs and NADCs.
Acknowledgments
Support for this work was provided by the Infectious Disease Clinical Research Program (IDCRP), Uniformed Services University of the Health Sciences (USUHS), Bethesda, MD, of which the TriService AIDS Clinical Consortium (TACC) is a component. The IDCRP is a DoD tri-service program executed through USUHS and the Henry M. Jackson Foundation for the Advancement of Military Medicine in collaboration with HHS/NIH/NIAID/DCR through Interagency Agreement HU0001-05-2-0011.
Footnotes
Author Contributions: Drs. Crum-Cianflone and Huppler Hullsiek had full access to the all of the data and takes responsibility for the integrity accuracy of the data and its analyses.
Study concept and design: Crum-Cianflone
Acquisition of the data: Crum-Cianflone, Ganesan, Weintrob, Marconi, Barthel, Fraser, Wegner
Analysis and interpretation of data: Crum-Cianflone, Huppler Hullsiek, Marconi, Barthel, Fraser, Agan
Drafting of the manuscript: Crum-Cianflone, Huppler Hullsiek, Fraser
Critical revision of the manuscript for important intellectual content: Crum-Cianflone, Huppler Hullsiek, Ganesan, Weintrob, Marconi, Barthel, Fraser, Agan, Wegner
Statistical analysis: Crum-Cianflone, Huppler Hullsiek
Obtained funding: Crum-Cianflone, Agan.
Administrative, technical, or material support: Crum-Cianflone, Ganesan, Weintrob, Marconi, Barthel, Fraser, Agan, Wegner
Study supervision: Crum-Cianflone
The opinions or ascertains contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Departments of the Army, Navy, or Air Force, or the Department of Defense. The authors have no commercial or other association that might pose a conflict of interest in this work.
This work is original and has not been published elsewhere. Some data contained in this manuscript were presented at the 4th IAS Conference: “Trends in AIDS-Defining and Non-AIDS-Defining Cancers Among HIV-Infected Patients: A 20-year Study”. Sydney, Australia, July, 22-25, 2007.
References
- 1.Centers for Disease Control (CDC) Kaposi’s sarcoma and Pneumocystis pneumonia among homosexual men – New York City and California. MMWR Morb Mortal Wkly Rep. 1981;30:305–308. [PubMed] [Google Scholar]
- 2.Monfardini S, Vaccher E, Pizzocaro G, et al. Unusual malignant tumours in 49 patients with HIV infection. AIDS. 1989;3:449–452. doi: 10.1097/00002030-198907000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control (CDC) Update on acquired immune deficiency syndrome (AIDS) – United States. MMWR Morb Mortal Wkly Rep. 1982;31:507–14. [PubMed] [Google Scholar]
- 4.Centers for Disease Control (CDC) 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1992;41:961–962. [PubMed] [Google Scholar]
- 5.Grulich AE, Li Y, McDonald A, et al. Decreasing rates of Kaposi’s sarcoma and non-Hodgkin’s lymphoma in the era of potent combination antiretroviral therapy. AIDS. 2001;15:629–633. doi: 10.1097/00002030-200103300-00013. [DOI] [PubMed] [Google Scholar]
- 6.International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92:1823–1830. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson LP, Yamashita TE, Detels R, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi’s sarcoma and non-Hodgkin’s lymphomas among HIV-1 infected individuals. Multicenter AIDS Cohort Study. J Acquire Immune Defic Syndr Hum Retroviral. 1999;21:S34–S41. [PubMed] [Google Scholar]
- 8.Diamond C, Taylor TH, Aboumrad T, Anton-Culver H. Changes in acquired immunodeficiency syndrome-related non-Hodgkin’s lymphoma in the era of highly active antiretroviral therapy. Cancer. 2006;106:128–135. doi: 10.1002/cncr.21562. [DOI] [PubMed] [Google Scholar]
- 9.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 10.Louie JK, Hsu LC, Osmond DH, Katz MH, Schwarcz SK. Trends in causes of death among persons with acquired immunodeficiency syndrome in the era of highly active antiretroviral therapy, San Francisco, 1994-1998. J Infect Dis. 2002;186:1023–1027. doi: 10.1086/343862. [DOI] [PubMed] [Google Scholar]
- 11.Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101:317–324. doi: 10.1002/cncr.20354. [DOI] [PubMed] [Google Scholar]
- 12.D’Arminio Monforte A, Abrams D, Pradier C, et al. HIV-induced immunodeficiency and risk of fatal AIDS-defining and non-AIDS-defining malignancies: results from the D:A:D Study. Presented at the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. February 25-28; 2007. Abstract 84. [Google Scholar]
- 13.Bedimo R, Chen RY, Accortt NA, et al. Trends in AIDS-defining and non-AIDS-defining malignancies among HIV-infected patients: 1989-2002. Clin Infect Dis. 2004;39:1380–1384. doi: 10.1086/424883. [DOI] [PubMed] [Google Scholar]
- 14.Herida M, Mary-Krause M, Kaphan R, et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol. 2003;21:3447–3453. doi: 10.1200/JCO.2003.01.096. [DOI] [PubMed] [Google Scholar]
- 15.Census Scope: Your Portal to Census 2000 Data. [July 18, 2008]; http://www.censusscope.org.
- 16.Ries LAB, Melbert D, Krapcho M, Mariotto A, Miller BA, Deuer EJ, Clegg L, Horner MN, Howlader N, Eisner MP, Reichman M, Edwards BK. SEER Cancer Statistics Review, 1975-2004. National Cancer Institute; Bethesda, MN: [July 18, 2008]. http://seer.cancer.gov/csr/1975_2004, based on November 2006 SEER data submission, posted to the SEER web site, 2007. [Google Scholar]
- 17.Stebbing J, Gazzard B, Mandalia S, Teague A, Waterston A, Marvin V, Nelson M, Bower M. Antiretroviral treatment regimens and immune parameters in the prevention of systemic AIDS-related non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22:2177–2183. doi: 10.1200/JCO.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 18.Bower M, Powles T, Nelson M, Mandalia S, Gazzard B, Stebbing J. Highly active antiretroviral therapy and human immunodeficiency virus-associated primary cerebral lymphoma. J Natl Cancer Inst. 2006;98:1088–1091. doi: 10.1093/jnci/djj302. [DOI] [PubMed] [Google Scholar]
- 19.Matthews GV, Bower M, Mandalia S, Powles T, Nelson MR, Gazzard BG. Changes in acquired immunodeficiency syndrome-related lymphoma since the introduction of highly active antiretroviral therapy. Blood. 2000;96:2730–2734. [PubMed] [Google Scholar]
- 20.Long JL, Engels EA, Moore RD, Gebo KA. Incidence and outcomes of malignancy in the HAART era in an urban cohort of HIV-infected individuals. AIDS. 2008;22:489–496. doi: 10.1097/QAD.0b013e3282f47082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rimland D, Guest J. Increasing incidence of prostate cancer in the Atlanta VA Cohort Study. Presented at the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. February 25-28, 2007. Abstract 874. [Google Scholar]
- 22.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786–3791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hessol NA, Pipkin S, Schwarcz S, Cress RD, Bacchetti P, Scheer S. The impact of highly active antiretroviral therapy on non-AIDS-defining cancers among adults with AIDS. Am J Epidemiol. 2007;165:1143–1153. doi: 10.1093/aje/kwm017. [DOI] [PubMed] [Google Scholar]
- 24.Lima VD, Hogg RS, Harrigan PR, et al. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS. 2007;21:685–692. doi: 10.1097/QAD.0b013e32802ef30c. [DOI] [PubMed] [Google Scholar]
- 25.Hogg RS, Heath KV, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 26.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 27.Forslund O, Iftner T, Andersson K, Lindelof B, Hradil E, Nordin P, Stenquist B, Kirnbauer R, Dillner J, de Villiers EM Viraskin Study Group. Cutaneous human papillomaviruses found in sun-exposed skin: Beta-papillomavirus species 2 predominates in squamous cell carcinoma. J Infect Dis. 2007;196:876–883. doi: 10.1086/521031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harwood CA, McGregor JM, Proby CM, Breuer J. Human papillomavirus and the development of non-melanoma skin cancer. J Clin Pathol. 1999;52:249–253. doi: 10.1136/jcp.52.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harwood CA, Surentheran T, McGregor JM, Spink PJ, Leigh IM, Breuer J, Proby CM. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289–297. doi: 10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Kirk GD, Merlo C, Driscoll PO, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45:103–110. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saves M, Chene G, Ducimetiere P, et al. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared to the general population. Clin Infect Dis. 2003;37:292–298. doi: 10.1086/375844. [DOI] [PubMed] [Google Scholar]
- 32.De Falco G, Bellan C, Lazzi S, et al. Interaction between HIV-1 Tat and pRb2/p130: a possible mechanism in the pathogenesis of AIDS-related neoplasms. Oncogene. 2003;22:6214–6219. doi: 10.1038/sj.onc.1206637. [DOI] [PubMed] [Google Scholar]
- 33.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 34.Mbulaiteye SM, Biggar RJ, Goedert JJ, Engels EA. Immune deficiency and risk for malignancy among persons with AIDS. J Acquir Immune Defic Syndr. 2003;32:527–533. doi: 10.1097/00126334-200304150-00010. [DOI] [PubMed] [Google Scholar]
- 35.Piketty C, Darragh TM, Heard I, et al. High prevalence of anal squamous intraepithelial lesions in HIV-positive men despite the use of highly active antiretroviral therapy. Sex Transm Dis. 2004;31:96–99. doi: 10.1097/01.OLQ.0000109515.75864.2B. [DOI] [PubMed] [Google Scholar]
- 36.Frisch M, Biggar RJ, Engels EA, et al. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;248:1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 37.Clifford G, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 38.Hammer SM, Saag MS, Schechter M, et al. International AIDS Society-USA panel. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 39.Branson BM, Handsfield HH, Lampe MA, et al. Centers for Disease Control and Prevention (CDC) Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR14):1–17. [PubMed] [Google Scholar]


