Abstract
Myelodysplastic syndrome (MDS) is not a single disease, but a collection of hematopoietic disorders that require newer strategies. Currently, azacitidine, decitabine, and lenalidomide are approved by the US Food and Drug Administration for the treatment of MDS. A recent study demonstrated an improved overall survival (24.4 months vs 15 months) in high-risk MDS patients receiving azacitidine plus best supportive care vs conventional care which has resulted in an updated label for this product. Conventional care consisted of supportive care alone or either low-dose ara-C or standard chemotherapy plus best supportive care. While these data are encouraging, newer agents such as vorinostat, MGCD0103, MS-275, and tipifarnib are currently being studied as monotherapy or in combinations with approved treatments for MDS. The goal of combining pharmacotherapy, such as the combination of DNA methylation inhibitors and histone deacetylase inhibitors, in the management of MDS is to increase the response rates and decrease the toxicities associated with treatment. Clinical experience in the use of combination products has given practitioners the empirical knowledge necessary to better treat patients with MDS. Utilizing convergent or complementary molecular mechanisms with in vitro or in vivo evidence of synergy is a fresher and maybe a more efficacious approach to combination therapy.
Introduction
Myelodysplastic syndrome (MDS) encompasses a collection of malignant bone marrow failure states. MDS affects hematopoietic stem cells resulting in ineffective hematopoiesis. The results lead to a myriad of problems including infection, transfusion-dependent anemia, and progression to acute myeloid leukemia (AML). Currently, three agents are approved by the US Food and Drug Administration for the treatment of MDS: azacitidine1 (Vidaza®, Celgene Corp, Summit, New Jersey), decitabine2 (Dacogen®, Eisai Inc, Woodcliff Lake, NY), and lenalidomide3 (Revlimid®, Celgene Corp) (Table 1). Monotherapy has resulted in improved remission rates and azacitidine appears to improve overall survival; however, MDS remains fatal if not cured through allogeneic stem cell transplantation. Thus, the development of new strategies remains critical, and because MDS includes biologically heterogeneous diseases, one strategy will not likely benefit all patients.
Table 1.
FDA-Approved Drugs for MDS
| Drug (Trade Name, Manufacturer) | FDA-Approved Indication | Dosage |
|---|---|---|
| Azacitidine1 (Vidaza®, Celgene Corp, Summit, NJ) | Treatment of patients with the following MDS subtypes: refractory anemia or refractory anemia with ringed sideroblasts (if accompanied by neutropenia or thrombocytopenia or requiring transfusions), refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia. | 1st cycle: 75 mg/m2 subcutaneously or intravenously, daily for 7 days. Cycles should be repeated every 4 weeks. The dose may be increased to 100 mg/m2 if no beneficial effect is seen after 2 treatment cycles and if no toxicity other than nausea and vomiting has occurred. Recommended minimum of 4 cycles. However, complete or partial response may require more than 4 treatment cycles. |
| Decitabine2 (Dacogen®, MGI Pharma Inc, Bloomington, MN) | Patients with MDS including previously treated and untreated, de novo and secondary MDS of all French-American-British subtypes (refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia) and intermediate- 1, intermediate-2, and high-risk International Prognostic Scoring System groups. | 15 mg/m2 administered by continuous intravenous infusion over 3 hours repeated every 8 hours for 3 days. Patients may be premedicated with standard antiemetic therapy. The above cycle should be repeated every 6 weeks. It is recommended that patients be treated for a minimum of 4 cycles; however, a complete or partial response may take longer than 4 cycles. |
| Lenalidomide3 (Revlimid®, Celgene Corp, Summit, NJ) | Transfusion-dependent anemia due to low- or intermediate-1-risk MDS associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities. | Orally with a dose of 10 mg daily. Dosing is continued or modified based on clinical and laboratory findings. |
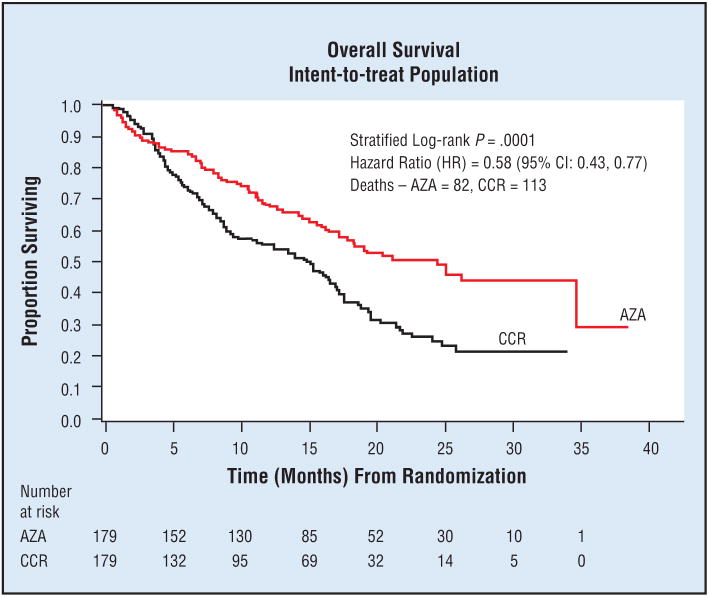
Fenaux et al4 recently compared overall survival using azacitidine (75 mg/m2 per day for 7 days every 28 days) vs preassigned conventional care regimens in 358 patients also receiving best supportive care (BSC) with high-risk MDS in a phase III, international, multi-center, randomized, prospective study. BSC consisted of transfusions, antibiotics, and hematopoietic growth factors. Conventional care regimens consisted of BSC alone, BSC plus low-dose ara-C 20 mg/m2 per day for 14 days every 28 days, or BSC plus standard chemotherapy with conventional induction and consolidation. Azacitidine demonstrated a significant improvement in disease progression and in median overall survival (24.4 months vs 15 months, respectively, P = .0001) (Table 2 and Fig 1).
Table 2.
Azacitidine: Overall Survival Data
| Group | % Patients | Median Overall Survival (mos) | HR (95% CI) | Log-Rank P | |
|---|---|---|---|---|---|
| AZA | CCR | ||||
| Good | 46 | Not reached | 17.1 | 0.61 (0.39, 0.96) | .030 |
| Intermediate | 21 | 26.3 | 17.0 | 0.43 (0.21, 0.88) | .017 |
| Poor | 28 | 17.2 | 6.0 | 0.52 (0.32, 0.87) | .011 |
This research was originally published in Blood. Fenaux P, Mufti GJ, Santini V, et al. Azacitidine (AZA) treatment prolongs overall survival in higher-risk MDS patients compared with conventional care regimens (CCR): results of the AZA-001 phase III study. Blood. 2007;110:250a. Abstract 817. © American Society of Hematology. Reprinted with permission.
Fig 1.
Overall survival of patients with higher-risk MDS using azacitidine. This research was originally published in Blood. Fenaux P, Mufti GJ, Santini V, et al. Azacitidine (AZA) treatment prolongs overall survival in higher-risk MDS patients compared with conventional care regimens (CCR): results of the AZA-001 phase III study. Blood. 2007;110:250a. Abstract 817. © American Society of Hematology. Reprinted with permission.
Like azacitidine, decitabine (2′-deoxy-5-azacytidine) inhibits DNA methyltransferases, the enzymes responsible for maintenance of the cell’s specific pattern of cytosine methylation. Treatment of cancer cells with either of these DNA methyltransferase inhibitors in vitro leads to reversal of aberrant promoter methylation and concomitant re-expression of transcriptionally silenced genes. Decitabine has demonstrated similar clinical response rates to azacitidine in MDS patients; however, the single randomized trial comparing decitabine to supportive care did not show a statistically significant survival increase.5
Therapies combining azacitidine or decitabine with agents with a different mechanism of action may improve complete remission (CR), partial remission (PR), hematologic improvement (HI), or overall survival. This article discusses strategies designed to improve outcomes with DNA methyltransferase inhibitors and newer agents under development alone or in combination for the treatment of MDS.
New Agents, Rationale, Published Experience, and Ongoing Studies
Despite improved survival in high-risk MDS patients treated with azacitidine, the overall hematologic response rate to azacitidine monotherapy is 40% to 50%.6,7 Recent survival data, if validated, will lead to the use of azacitidine as a new gold standard against which newer upfront regimens must be tested.4 Azacitidine may also serve as the backbone for newer regimens in the treatment of high-risk MDS. Developing higher hematologic responses and improving quality of life remain important goals in addition to prolonging survival.
Several newer agents, including histone deacetylase (HDAC) inhibitors and farnesyltransferase inhibitors, have shown promise in the MDS treatment arsenal. Classes of targets under investigation for treatment of MDS include transcription signaling, angiogenesis, cytokine milieu, apoptosis, and immune modulation. HDAC and DNA methyltransferase inhibitors putatively affect gene transcription through reversal of methylation of cytosine residues in CpG-rich promoter regions.8 Farnesyltransferase inhibitors modify the signaling transduction pathways.9 The cytokine milieu may be modified by tumor necrosis factor (TNF) inhibitors such as etanercept and antithymocyte globulins, and lenalidomide may affect cytokines while inducing apoptosis directly10,11 (Table 3).
Table 3.
New Agents for MDS
| Class/Mechanism of Action | Agent (Trade) |
|---|---|
| DNA methyltransferase inhibitor | Azacitidine (Vidaza®) Decitabine (Dacogen™) |
| HDAC | Phenylbutyrate Valproic acid MS-275 MGCD0103 Vorinostat (Zolinza®) |
| Farnesyltransferase inhibitor | Tipifarnib Lonafarnib |
| TNF inhibitor | Etanercept (Enbrel®) |
| Suppression of the abnormal 5q31 clone | Lenalidomide (Revlimid®) |
| Retinoid | All-trans retinoic acid (ATRA) |
| Nucleoside analog | Sapacitabine |
| Glutathione analog | TLK199 |
HDAC Inhibitors
HDAC inhibitors impact chromatin conformation by altering the pattern of acetylation of lysine residues in nucleosomal histones. However, HDAC inhibitors aggravate many other aspects of cellular physiology, including induction of reactive oxygen species, inhibition of protein chaperone function, alteration of death receptor pathways, and alterations of the NFκB pathway.12 Early-phase studies of HDAC inhibitors in myeloid malignancies, including MDS but focusing on AML, suggest that HDAC inhibitors have modest activity when given as single agents.13–19
The FDA has approved vorinostat (suberoylanilide hydroxamic acid, SAHA, Zolinza®, Merck & Co Inc, Whitehouse Station, New Jersey) as the first commercially available HDAC inhibitor for use in oncology. It has been approved for use in patients with cutaneous T-cell lymphoma who have progressive, persistent, or recurrent disease on or following two systemic therapies.20 The maximum tolerated dose of vorinostat was evaluated recently in an open-label nonrandomized phase I study in 41 patients with advanced leukemia or MDS. The maximum tolerated dose of vorinostat was 200 mg 2 times daily or 250 mg 3 times daily for 14 days followed by 1 week off. Seven patients had a hematologic response or improvement. Common mild to moderate adverse effects included diarrhea, nausea, fatigue, and anorexia. However, patients experienced grade 3/4 fatigue (27%), thrombocytopenia (12%), and diarrhea (10%). Further studies evaluating vorinostat in MDS are needed.17
While vorinostat inhibits HDACs of class I and II, the benzamides SNDX-275 (formerly MS-275) and MGCD0103 specifically target class I HDACs. While it is not clear whether HDAC class specificity is particularly desirable, class I-specific drugs do not impact HSP90 acetylation (although they may impact HSP70 activity and thereby disrupt chaperone function). Theoretically, class I-specific drugs could affect epigenetic changes without impacting cytoplasmic proteins and could therefore be more epigenetic-specific and less toxic. Lancet et al21 evaluated twice-weekly MGCD0103 (40 to 83 mg/m2 per day) in 19 patients with advanced leukemia or MDS in a phase I study. Patients received between 1 and 6 cycles, with stable disease being reported in 4 patients and significant inhibition of whole-cell total HDAC activity occurring in a majority of the patients. SNDX-275 was administered weekly for 4 doses in a phase I study in AML and demonstrated modest improvement in WBC in isolated patients.19
Valproic acid (VPA), the anticonvulsant, is an HDAC inhibitor that has more recently been evaluated for its use in the management of MDS. In a clinical trial evaluating VPA as monotherapy, response rates varied from 16%22 to 44%.23 Patients with low-risk MDS had a significantly higher response rate to VPA monotherapy than other risk groups had (low risk = 70%, intermediate-1 risk = 24%, intermediate-2 risk = 11%, and high risk = 18%, P = .002).24
Farnesyltransferase Inhibitors
Farnesyltransferase (Ftase) is a key enzyme regulating cancer cell growth and is involved in cell signaling, proliferation, and differentiation. It catalyzes the transfer of a farnesyl moiety to the cystine terminal residue of a substrate protein. Farnesyltransferase inhibitors selectively target intercellular Ftase; however, they inhibit a multitude of pathways, thereby affecting angiogenesis, cellular adhesion, mitosis, and eventually cellular survival. Their oral bioavailability has encouraged their development for many hematologic malignancies.9 The two farnesyltransferase inhibitors currently under development are tipifarnib and lonafarnib. A majority of tumor cell lines are sensitive to tipifarnib. Farnesyl-transferase inhibitors suppress RAS activity resulting in a decrease in vascular endothelial growth factor (VEGF) expression and secretion.25
Kurzrock et al25 evaluated tipifarnib 600 mg b.i.d. for 4 weeks, followed by a 2-week rest in 28 patients with MDS in a phase II study. Three patients responded to tipifarnib, with a CR in 2 patients and a PR in 1 patient. Doses were reduced to 300 mg b.i.d. within the first month due to rash or severe cytopenia in the responders. Myelosuppression, fatigue, and nausea were the most common side effects observed.
Fenaux et al26 evaluated tipifarnib 300 mg orally b.i.d. for 21 days per cycle in 82 patients with intermediate- to high-risk MDS in a single-arm open label multicenter phase II study. A CR occurred in 12 patients (14.6%) after a median of 4 weeks and a duration of 12.5 months. Fourteen (17.1%) achieved an HI for greater than 2 months. The median overall survival was 11.7 months. The most common treatment-related adverse effects where grade 3/4 neutropenia (18% of patients), thrombocytopenia (32%), and anemia (18%). While these response rates may not seem impressive, the CR rate is in fact comparable to that of azacitidine in a similar patient population.
Etanercept
Etanercept (Enbrel®, Immunex Corp, Thousand Oaks, California) is a soluble fusion protein that blocks tumor necrosis factor alpha (TNF-α). In MDS, increased levels of TNF-α and Fas-ligand occur and are associated with increased apoptosis. Deeg et al11 evaluated etanercept 25 mg given subcutaneously twice a week for 16 weeks in a pilot study in MDS patients. Of the 12 patients who were considered evaluable in the study, 3 had hemoglobin increases of 1 to 1.5 g/dL and 1 had a decrease in transfusion requirements. In addition, 2 patients had increased neutrophil counts and 2 had increased platelet counts. Adverse effects reported included neutropenia, sore throat, calf cellulitis, and pneumonia. The study concluded that the limited blood cell count differences observed suggest that TNF-α is directly responsible for only a portion of observable dysregulation of hemopoiesis in patients with MDS.
TLK199
TLK199 is a glutathione analog that binds to glutathione S-transferase (GST) P1-1, dissociating it from Jun kinase (JNK) and resulting in a decrease in kinase activity. This activation of JNK caused cellular growth and maturation resulting in significant myelostimulant activity in bone marrow cell cultures. Raza et al27 evaluated the hematologic response of 52 patients with refractory MDS to TLK199 (600 mg/m2 per day given intravenously for 5 days every 3 weeks) in a phase II study. Positive responses were considered to be decreases in the number of red blood cell and platelet transfusions, as well as improvements in bone marrow differentiation. Adverse effects included infusion reactions, back pain, nausea, chills, and bone pain. Thirty-two patients (82%) experienced an HI accompanied by decreased need for transfusions of both red blood cells and platelets. This study concluded that TLK199 is safe, well tolerated, and active in the treatment of MDS. In an ongoing phase IIa study, 9 of 25 patients with MDS experienced improvement in at least one blood lineage, and 6 patients had documented HI.28
Sapacitabine
Sapacitabine is an oral nucleoside analog that induces G2 cell cycle arrest. A phase I study in 29 patients with advanced leukemia or MDS established the recommended dose to be 325 mg twice daily for 7 days every 21 days. The dose-limiting toxicity noted during the study was gastrointestinal toxicity. In addition, 7 patients (5 AML and 2 MDS) had a clinical response in bone marrow blast counts, including 1 MDS patient with a CR.29
Combination Therapy: Rationale, Published Experience, and Ongoing Studies
The goal of combining drugs in the management of MDS is to increase response rates, prolong response duration, and decrease the toxicities associated with treatment. In selecting therapy, the clinician has two approaches. Traditionally, agents are combined based on the absence of overlapping or synergistic toxicities leading to empiric combinations. A smarter approach may be combining agents based on an understanding of convergent or complementary molecular mechanisms with in vitro or in vivo evidence of synergy. Many of the combinations under investigation are utilizing azacitidine based on the promising results seen in overall survival rates from Fenaux et al.4 Mechanistically, combining agents such as HDAC inhibitors with DNA methyltransferase inhibitors is based on models of epigenetic biology.
DNA Methyltransferase Inhibitors Plus HDAC Inhibitors
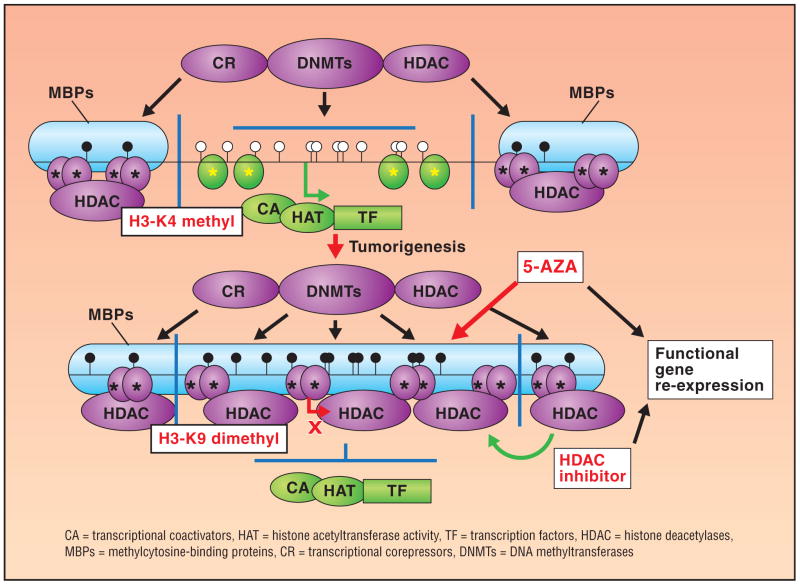
Numerous HDAC inhibitors are being studied in combination with DNA methyltransferase inhibitors for treatment of MDS. The sequential use of DNA methyltransferase inhibitors and HDAC inhibitors leads to synergistic reactivation of gene expressions (Fig 2).30 Researchers postulate that a combination of an HDAC and a hypomethylating agent may be associated with producing a reversal of epigenetic markers that are thought to cause gene repression or silencing resulting in reactivation of suppressed anticancer genes.31 The HDAC inhibitors include the short-chain fatty acids phenylbutyrate and VPA, hydroxamic acids including vorinostat, belinostat, and LBH589, the cyclic depsipeptide romidepsin, and the benzamides SNDX-275 and MGCD0103. Other HDAC inhibitors are undergoing earlier-stage development.
Fig 2.
Effects of DNA methylation and chromatin structure on gene transcription in normal and tumor cells. From Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349(21):2042–2054. Copyright © 2008 Massachusetts Medical Society. All rights reserved. Reprinted with permission.
Decitabine/VPA
Garcia-Manero et al31 evaluated the combination of decitabine and VPA in 54 patients with advanced leukemia in a phase I/II study. Of the 54 patients treated, 10 had a diagnosis of MDS. The therapeutic drug regimen consisted of decitabine 15 mg/m2 IV daily for 10 days concomitantly with increasing doses of VPA over 10 days. Of the 10 MDS patients, 5 had a response (4 CR, 1 PR). In addition, reactivation of p15 was noted and was in proportion to the amount of gene demethylation occurring. This study proves that the epigenetic viability of the combination of decitabine and VPA is safe and effective in treating MDS.
Azacitidine/SNDX-275
A phase I study to evaluate the combination of azacitidine and SNDX-275 (previously MS-275) included 31 patients: 13 patients with a diagnosis of MDS, 4 with chronic myelomonocytic leukemia (CMML), and 14 with AML. They received azacitidine 30,40, or 50 mg/m2 per dose subcutaneously as a self-administered injection daily for 10 days and MS-275 2,4,6, or 8 mg/m2 per dose on days 3 and 10 on a 28-day cycle. Twelve of 27 patients (7 MDS, 1 CMML, and 4 AML) responded with 2 CRs, 4 PRs, and 6 HIs. There was a 2.5-fold increase in H3 acetylation and a 4-fold increase in H4 acetylation. Additionally, there was a median 5.3-fold increase in H2AXγ expression with the combination. Adverse events included laryngeal edema, asthenia, delayed neutrophil recovery (> 21 days), and fatigue. This study concluded that azacitidine plus MS-275 is clinically tolerated and has shown positive cytogenetic remissions.32
Decitabine/Vorinostat
Ravandi et al33 conducted a phase I sequential dosing study of decitabine and vorinostat in 31 patients with relapsed and refractory leukemia (5 MDS patients progressed to AML). One patient did not receive drug due to the rapid progression of the disease. Five cohort groups consisting of 6 patients each received escalating doses of decitabine (10, 10, 15, 20 and 25 mg/m2 intravenously daily × 5 days). Cohort 1 received vorinostat 100 mg p.o. t.i.d. × 14 days and cohorts 2–5 received 200 mg p.o. t.i.d. × 14 days. Of the 30 patients, 1 had a CR lasting 5.5 weeks, 4 had significant reductions in bone marrow blasts, 4 had stable disease, 14 had no response or disease progression, and 7 were still being evaluated. Adverse effects included pulmonary emboli, dose-dependent diarrhea, neutropenic fever, fatigue, renal failure, rash, nausea, thrombosis, and angioedema. These early results suggest that decitabine plus vorinostat is safe and has shown some efficacy in the treatment of relapse/refractory leukemia. In addition, the combination of decitabine or azacitidine with vorinostat was effective in 3 elderly patients with secondary AML after an initial diagnosis of MDS. After at least 6 months of combination therapy, all 3 patients had no disease progression.34
Azacitidine/MGCD0103
MGCD0103 is a selective HDAC inhibitor that has shown promise as a single agent in the treatment of patients diagnosed with relapsed/refractory MDS and AML. A phase I/II study evaluated MGCD0103 (at increasing dosages between 35 and 135 mg 3 times per week) and azacitidine (75 mg/m2 SC once daily × 7 days of a 28-day cycle) in 37 patients. Due to the dose-limiting toxicities of nausea, vomiting, diarrhea, and dehydration, the dosage of MGCD0103 was set at 90 mg. Some clinical response was seen in 11 patients (30%), with 4 CRs, 5 CR-I, and 2 PRs. With a CR-I, the bone marrow blasts decrease to a range consistent with complete response but peripheral blood counts do not recover completely. The phase II portion of the study included 27 patients, of which 10 (37%) achieved a response. Additional combination studies are planned.35
Other Combinations
In addition to HDAC inhibitors, other agents are being combined with DNA methyltransferase inhibitors including lenalidomide and etanercept. Unlike DNA methyltransferase inhibitor/HDAC combinations, these combinations do not derive from biological models but represent empiric combinations of drugs that are active individually.
Azacitidine/Lenalidomide
Researchers theorized that the use of an antiangiogenic agent, such as lenalidomide, in combination with a hypomethylating agent, such as azacitidine, would result in positive clinical outcomes greater than those achieved with the use of each agent alone. A phase I study evaluated lenalidomide in combination with azacitidine in 7 patients with a diagnosis of advanced MDS. Of the 7 patients enrolled, 4 patients were evaluated for response and 2 achieved a CR, 1 had an erythroid response, and 1 had disease progression. Six patients were evaluable for toxicities that included fatigue, injection site reaction, rash, pruritus, constipation or diarrhea, dizziness, mucositis, and febrile neutropenia. However, initial results suggest a positive safety and efficacy profile.36
Azacitidine/Etanercept
Given the variety of mechanisms causing MDS and the observed phenomenon of a shift favoring TNF-α2 receptors, the combination of azacitidine and etanercept in the treatment of MDS was evaluated in a phase II single-arm open-label study. Twenty-three patients with advanced MDS were treated with azacitidine (75 mg/m2 per day for 7 days) and etanercept (25 mg SC twice a week for 2 weeks every 28 days). Utilizing International Working Group (IWG) response criteria, 14 patients (78%) responded, with 5 (28%) experiencing a CR, 8 (44%) a PR, and 1 (6%) a positive HI response. Three patients receiving therapy for 24 months have had sustained positive responses. Adverse events included hematologic toxicity (78%).37
Other combinations with HDAC inhibitors have also been studied. VPA, an HDAC inhibitor, has been combined with all-trans retinoic acid (ATRA), a putative inducer of terminal cell differentiation. In two different studies, the addition of ATRA to VPA did not modify any positive outcomes seen from the use of VPA alone.14,,38 In one study, 2 patients with secondary AML from MDS experienced a PR.14 Another combination consisting of VPA, ATRA, and azacitidine has been studied. Soriano et al39 evaluated the triple combination of azacitidine (75 mg/m2 per day for 7 days), VPA (escalating doses 50, 62.5 and 75 mg/kg per day × 7 days), plus ATRA (45 mg/m2 for 5 days starting on day 3 of treatment) in 53 patients with AML and MDS in a phase I/II trial. Overall, 42% of patients responded to therapy, with a higher percentage of therapy-naive elderly patients responding (52%). Evaluation of data revealed a significant decrease (P <.005) in global DNA methylation and histone acetylation. Despite promising phase I data, randomized trials will be required to establish incremental benefit of the VPA and ATRA compared to azacitidine alone.
Anemia in MDS
Anemia is widespread in patients with MDS, with more than 50% of patients having an active diagnosis of anemia at presentation.40 As many as 90% will develop anemia as their disease progresses and 80% will require transfusions to control their disease process.40 Lenalidomide, a congener of thalidomide, has demonstrated erythroid stimulatory activity in MDS patients. Empirically, it has proven to be active against MDS patients with deletions of chromosome 5q band 31.1; for such patients, lenalidomide may be considered a “targeted therapy” despite the lack of identification of a specific molecular target to date. List et al10 evaluated the hematologic and cytogenic response to lenalidomide 10 mg orally daily in 102 patients or 10 mg daily for 21 days every 4 weeks in 46 patients. Among these 148 MDS patients with 5q31 deletion,112 (76%) reduced their need for transfusions, while 99 patients (67%) no longer required transfusions. In addition to erythroid responses, 62 (73%) of 85 patients evaluated showed cytogenetic improvement, while 38 (61%) of those 62 patients demonstrated a CR of the cytogenic abnormality. Overall, lenalidomide was beneficial to MDS patients, decreasing reliance on transfusions.
Erythropoietin-stimulating agents (ESAs) have also been utilized in the management of anemia in MDS patients. Spiriti et al40 evaluated the quality of life and HI of epoetin alfa 40,000 U twice weekly in 133 low-risk MDS patients. At week 8, 68% of patients had responded; the mean change in Hg was 1.43. However, these differences were not statistically significant. Quality of life improved over the 8 weeks, with the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire scores showing a statistically significant improvement over baseline. ESAs have been most effective in patients whose endogenous serum erythropoietin levels were less than 500 and who require little transfusional support. A patient should not be treated with an ESA without prior determination of the baseline endogenous serum erythropoietin level. Recent data have raised safety concerns with the use of ESA in oncology.41,42 There are no data from MDS studies suggesting that ESA administration increases thromboembolic events in MDS patients or promotes acceleration of disease. However, the MDS studies have not had extensive follow-up to enable definitive analysis of similar safety issues. Availability of ESAs for the treatment of Medicare-insured MDS patients varies among states. Most authors currently recommend a 2- to 4-month trial of ESAs for MDS patients whose serum erythropoietin level is < 500 and whose primary problem is anemia, similar to NCCN guidelines.
Another approach is the utilization of heme supplementation with hemin (Panhematin®, Ovation Pharmaceuticals, Deerfield, Illinois) (Table 4). Hemin is an iron-containing metalloporphyrin. Therapy with heme supplementation utilizing heme arginate has been previously studied with some limited success in a small number of patients,43,44 and is undergoing phase II evaluation in MDS (MDS 2005–01).
Table 4.
Selected Therapies Under Investigation for Myelodysplastic Syndrome*
| Drug | Risk Factor | Study Phase | No. of Studies |
|---|---|---|---|
| Azacitidine combination | All MDS | I – III | 16 |
| Lonafarnib monotherapy | All MDS | II | 9 |
| Arsenic trioxide monotherapy or in combination | All MDS | I, II | 9 |
| TLK199 monotherapy | All MDS | I, II, IIa | 8 |
| Etanercept combination | All MDS | I, II | 4 |
| Alemtuzumab monotherapy and combination | All MDS | Pilot | 3 |
| SGN-33 plus lenalidomide | All MDS | I | 3 |
| Curcumin and gingeral | All MDS | Pilot | 1 |
| Cytarabine plus 17-allylamino-17-dernethoxygeldanamycin | All MDS | I | 1 |
| Sorafenib monotherapy | All MDS | II | 1 |
| Interleukin-2 monotherapy | All MDS | I | 1 |
| Prinomastat monotherapy | All MDS | II | 1 |
| Calcitrol plus dexamethasone | All MDS | II | 1 |
| PXD101 monotherapy | All MDS | II | 1 |
| VPA monotherapy or plus decitabine | MDS and AML | Pilot, II | 3 |
| VEGF monotherapy | High-grade MDS | I, II | 10 |
| Ara-C LDAC plus tipifarnib | High-risk MDS | I/II | 1 |
| ATRA plus MGCD0103 plus azacitidine | High-risk MDS | I, II | 2 |
| VNP40101M monotherapy | High-risk MDS | II | 1 |
| Fludarabine combination | Advanced MDS | II | 4 |
| CPX-351 monotherapy | Advanced MDS | I | 1 |
| Clofarabine monotherapy or combination | Acute MDS | II | 7 |
| AMG 531 monotherapy | Low- to intermediate-risk MDS | I, II | 5 |
| Vorinostat monotherapy: - plus decitabine - plus AraC plus etoposide |
Low- to intermediate I-risk MDS Refractory relapse MDS |
I, II I |
2 1 |
| Co-enzyme Q10 monotherapy | Low- to intermediate I-risk MDS | Pilot | 1 |
| CC-11006 monotherapy | Low- and intermediate I-risk MDS | I | 2 |
| Panhematin® monotherapy | Low- to intermediate I-risk MDS | II | 1 |
| Lenalidomide monotherapy or combination | MDS with primary or secondary non-5q deletion | I, II | 7 |
| Thalidomide monotherapy or combination | Idiopathic myelofibrosis or MDS overlap | II | 5 |
| GX15-70-MS obatoclax mesylate monotherapy | Untreated MDS | II | 3 |
| Cytosine arabinoside plus mitoxantrone plus flavopiridol | Poor-risk AML/MDS | I | 1 |
| MS-275 plus sargramostim | Relapse and refractory MDS | II | 1 |
| Sunitinib malate monotherapy | Intermediate II- to high-risk MDS | II | 1 |
MDS foundation, ClinicalTrials.gov
Thrombocytopenia in MDS
Thrombocytopenia is defined as a platelet count of less than 100 × 109/L. In a retrospective evaluation of 2,410 MDS patients, 1,605 (67%) had a diagnosis of thrombocytopenia at referral. In untreated patients with a primary diagnosis of MDS, 37% have thrombocytopenia. Unfortunately, many of the agents being utilized to manage MDS have the potential of increasing the incidence of thrombocytopenia in MDS patients. Hemorrhagic complications in MDS are directly attributed to thrombocytopenia, with hemorrhagic deaths occurring at a rate of 14% to 24%.45
AMG 531 is a thrombopoietin receptor ligand that safely and effectively increases platelet counts. It increases megakaryoctopoiesis using the same mechanism as thrombopoietin, although there is no sequential homology to endogenous thrombopoieten.46 Kantarjian et al47 evaluated 44 low-risk MDS patients treated with AMG 531 (300 to 1,500 μg), as 3 weekly SC injections) in a phase I/II open-label sequential cohort study. At baseline, platelet counts were less than 50 × 109. After 23 weeks, 18 patients (41%) showed positive platelet response for at least 8 weeks, according to IWG guidelines. Overall, the mean duration of response was 22.8 ± 13.3 weeks. The study concluded that AMG 531 was safe and well tolerated. The clinical impact of administration of AMG 531 on bleeding has not been presented.
Several ongoing trials are evaluating agents in the treatment of MDS (Table 4). Individual agents are being explored not only as monotherapy, but also in a variety of different combinations. While some of these studies are utilizing agents discussed in this review, a number of new agents are also being investigated.
Conclusions
The goal of pharmacotherapy in the management of MDS is to increase the overall survival of the patient. Fenaux et al4 demonstrated that an increase in overall survival in response to azacitidine is achievable compared with conventional care regimens and that while CR, PR, and HI data are encouraging, they may no longer be adequate to provide a complete picture of positive treatment in MDS patients. In evaluating the data, DNA methyltransferase inhibitors such as azacitidine will form the backbone of combination therapy that may continue to improve overall survival and will become the standard to which new therapies are compared. The clinical experience in using combination products has given practitioners the empirical knowledge necessary to improve treatment of patients with MDS. Utilizing convergent or complementary molecular mechanisms with in vitro or in vivo evidence of synergy is a fresher and maybe a more efficacious approach to combination therapy.
Clinical studies evaluating combination regimens need to be analyzed, evaluated, and published so practitioners can incorporate these regimens into their clinical practices. Utilization of this knowledge base can have a profound positive effect on the quality of life and the overall survival rates of MDS patients. Clinical trials of combination regimens have had improved positive outcomes regarding CR, PR, HI and, in some instances, overall survival rates for MDS patients. However, so that research can continue, patients with MDS should be referred to clinical trials. This will not only refine what has been learned, but also enable practitioners to explore new and novel approaches to treating patients with MDS with the aim of increasing overall survival rates.
Abbreviations used in this paper
- MDS
myelodysplastic syndrome
- AML
acute myeloid leukemia
- CR
complete remission
- PR
partial remission
- HI
hematologic improvement
- VPA
valproic acid
- HDAC
histone deacetylase
Footnotes
Disclosures
Dr Gore is a consultant for Celgene Corp, MGI Pharma Inc (now Eisai Inc), Pharmion Corp (now Celgene), Novartis Corp, and Johnson & Johnson Services, Inc. He receives research support from Celgene and Johnson & Johnson, and has stock ownership in Celgene.
Dr Hermes-DeSantis reports no significant relationship with the companies/organizations whose products or services may be referenced in this article.
References
- 1.Vidaza® (azacitidine) [package insert]. Summit, NJ: Celegene Corp; 2008.
- 2.Dacogen® (decitabine) [package insert]. Woodcliff Lake, NY. Eisai Inc; 2006.
- 3.Revlimid® (lenalidomide) [package insert]. Summit, NJ: Celgene Corp; 2007.
- 4.Fenaux P, Mufti GJ, Santini V, et al. Azacitidine (AZA) treatment prolongs overall survival (OS) in higher-risk MDS patients compared with conventional care regimens (CCR): results of the AZA-001 phase III study. Blood. 2007;110:250a. Abstract 817. [Google Scholar]
- 5.Kantarjian HM, O’Brien S, Shan J, et al. Update of the decitabine experience in higher risk myelodysplastic syndrome and analysis of prognostic factors associated with outcome. Cancer. 2007;109(2):265–273. doi: 10.1002/cncr.22376. [DOI] [PubMed] [Google Scholar]
- 6.Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24(24):3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 7.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the Cancer and Leukemia Group B. J Clin Oncol. 2002;20(10):2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 8.Gore SD. Combination therapy with DNA methyltransferase inhibitors in hematologic malignancies. Nat Clin Pract Oncol. 2005;2(suppl 1):S30–S35. doi: 10.1038/ncponc0346. [DOI] [PubMed] [Google Scholar]
- 9.Lancet JE, Gojo I, Gotlib J, et al. A phase 2 study of the farnesyl-transferase inhibitor tipifarnib in poor-risk and elderly patients with previously untreated acute myelogenous leukemia. Blood. 2007;109(4):1387–1394. doi: 10.1182/blood-2006-04-014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 11.Deeg HJ, Gotlib J, Beckham C, et al. Soluble TNF receptor fusion protein (etanercept) for the treatment of myelodysplastic syndrome: a pilot study. Leukemia. 2002;16(2):162–164. doi: 10.1038/sj.leu.2402356. [DOI] [PubMed] [Google Scholar]
- 12.Rosato RR, Grant S. Histone deacetylase inhibitors: insights into mechanisms of lethality. Expert Opin Ther Targets. 2005;9(4):809–824. doi: 10.1517/14728222.9.4.809. [DOI] [PubMed] [Google Scholar]
- 13.Gore SD, Weng LJ, Zhai S, et al. Impact of the putative differentiating agent sodium phenylbutyrate on myelodysplastic syndromes and acute myeloid leukemia. Clin Cancer Res. 2001;7(8):2330–2339. [PubMed] [Google Scholar]
- 14.Bug G, Ritter M, Wassmann B, et al. Clinical trial of valproic acid and all-trans retinoic acid in patients with poor-risk acute myeloid leukemia. Cancer. 2005;104(12):2717–2725. doi: 10.1002/cncr.21589. [DOI] [PubMed] [Google Scholar]
- 15.Gore SD, Weng LJ, Figg WD, et al. Impact of prolonged infusions of the putative differentiating agent sodium phenylbutyrate on myelodysplastic syndromes and acute myeloid leukemia. Clin Cancer Res. 2002;8(4):963–970. [PubMed] [Google Scholar]
- 16.Raffoux E, Chaibi P, Dombret H, et al. Valproic acid and all-trans retinoic acid for the treatment of elderly patients with acute myeloid leukemia. Haematologica. 2005;90(7):986–988. [PubMed] [Google Scholar]
- 17.Garcia-Manero G, Yang H, Bueso-Ramos C, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008;111(3):1060–1066. doi: 10.1182/blood-2007-06-098061. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Manero G, Assouline S, Cortes J, et al. Phase I study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood. 2008;112:981–989. doi: 10.1182/blood-2007-10-115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gojo I, Jiemjit A, Trepel JB, et al. Phase 1 and pharmacological study of MS-275, a histone deacetylase inhibitor, in adults with refractory and relapsed acute leukemias. Blood. 2007;109(7):2781–2790. doi: 10.1182/blood-2006-05-021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zolinza® (vorinostat) [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2006.
- 21.Lancet JE, Nichols G, Assouline S, et al. A phase I study of MGCD0103 given as a twice weekly oral dose in patients with advanced leukemias or myelodysplastic syndromes (MDS) J Clin Oncol. 2007;25:18S. Abstract 2516. [Google Scholar]
- 22.Kuendgen A, Schmid M, Schlenk R, et al. The histone deacetylase (HDAC) inhibitor valproic acid as monotherapy or in combination with all-trans retinoic acid in patients with acute myeloid leukemia. Cancer. 2006;106(1):112–119. doi: 10.1002/cncr.21552. [DOI] [PubMed] [Google Scholar]
- 23.Kuendgen A, Strupp C, Aivado M, et al. Treatment of myelodysplastic syndromes with valproic acid alone or in combination with all-trans retinoic acid. Blood. 2004;104(5):1266–1269. doi: 10.1182/blood-2003-12-4333. [DOI] [PubMed] [Google Scholar]
- 24.Kuendgen A, Knipp S, Fox F, et al. Results of a phase 2 study of valproic acid alone or in combination with all-trans retinoic acid in 75 patients with myelodysplastic syndrome and relapsed or refractory acute myeloid leukemia. Ann Hematol. 2005;84(suppl 1):61–66. doi: 10.1007/s00277-005-0026-8. [DOI] [PubMed] [Google Scholar]
- 25.Kurzrock R, Albitar M, Cortes JE, et al. A phase II study of R115777, a farnesyl transferase inhibitor, in myelodysplastic syndrome. J Clin Oncol. 2004;22(7):1287–1292. doi: 10.1200/JCO.2004.08.082. [DOI] [PubMed] [Google Scholar]
- 26.Fenaux P, Raza A, Mufti GJ, et al. A multicenter phase 2 study of the farnesyltransferase inhibitor tipifarnib in intermediate- to high-risk myelodysplastic syndrome. Blood. 2007;109(10):4158–4163. doi: 10.1182/blood-2006-07-035725. [DOI] [PubMed] [Google Scholar]
- 27.Raza A, Callander N, Ochoa L, et al. Hematological improvement (HI) by TLK199 (Telintra™), a novel glutathione analog, in myelodysplastic syndrome: results of a phase 2 study results. Blood. 2005;106(11) Abstract 2520. [Google Scholar]
- 28.Callander N, Ochoa-Bayona JL, Piro L, et al. Hematologic improvement following treatment with TLK199 (Telintra™), a novel glutathione analogue inhibitor of GST P1–1, in myelodysplastic syndrome (MDS): interim results of a dose-ranging phase 2a study. Blood. 2004;104(11):400a. Abstract 1428. [Google Scholar]
- 29.Plunkett W, Garcia-Manero G, Faderl S, et al. Phase I study of sapacitabine, an oral nucleoside analogue, in patients with advanced leukemias or myelodysplastic syndromes. J Clin Oncol. 2007;25(18S):7063. [Google Scholar]
- 30.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349(21):2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, et al. Phase I/II study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108(10):3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gore SD, Jiemjit A, Silverman LB, et al. Combined methyltransferase/histone deacetylase inhibition with 5-azacitidine and MS-275 in patients with MDS, CMMoL, and AML: clinical response, histone acetylation and DNA damage . Blood. 2006;108:156a. Abstract 517. [Google Scholar]
- 33.Ravandi F, Faderl S, Thomas D, et al. Phase I study of suberoylanilide hydroxamic acid (SAHA) and decitabine in patients with relapse, refractory or poor prognosis leukemia. Blood. 2007;110(11) Abstract 897. [Google Scholar]
- 34.Thatikonda C, Rossetti JM, Shadduck RK, et al. Combination methyltransferase and histone deacetylase inhibition in elderly patients with secondary acute myelogenous leukemia. Blood. 2007;110(11) Abstract 4387. [Google Scholar]
- 35.Garcia-Manero G, Yang AS, Klimek V, et al. Phase I/II study of MGCD0103, an oral isotype-selective histone deacetylase (HDAC) inhibitor, in combination with 5-azacitidine in higher-risk myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML) Blood. 2007110:137a. Abstract 444. [Google Scholar]
- 36.Sekeres MA, List A, Cuthbertson D, et al. Preliminary results from a phase I study of Revlimid® (lenalidomide) in combination with Vidaza® (azacitidine) in patients with advanced myelodysplastic syndromes (MDS) Blood. 2007;110:437a. Abstract 1458. [Google Scholar]
- 37.Holsinger AL, Ramakrishnan A, Storer B, et al. Therapy of myelodysplastic syndrome (MDS) with azacitidine given in combination with etanercept: a phase II study. Blood. 2007;110:435a. Abstract 1452. [Google Scholar]
- 38.Pilatrino C, Cilloni D, Messa E, et al. Increase in platelet count in older, poor-risk patients with acute myeloid leukemia or myelodysplastic syndrome treated with valproic acid and all-trans retinoic acid. Cancer. 2005;104(1):101–109. doi: 10.1002/cncr.21132. [DOI] [PubMed] [Google Scholar]
- 39.Soriano AO, Yang H, Faderl S, et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110(7):2302–2308. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]
- 40.Spiriti MA, Latagliata R, Niscola P, et al. Impact of a new dosing regimen of epoetin alfa on quality of life and anemia in patients with low-risk myelodysplastic syndrome. Ann Hematol. 2005;84(3):167–176. doi: 10.1007/s00277-004-0961-9. [DOI] [PubMed] [Google Scholar]
- 41.National Comprehensive Cancer Network, Inc. [Accessed May 29, 2008];The NCCN Myelodysplastic Syndromes Clinical Practice Guidelines in Oncology (Version 2.2008) 2007 http://www.nccn.org/professionals/physician_gls/PDF/mds.pdf.
- 42.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299(8):914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 43.Timonen TT, Kauma H. Therapeutic effect of heme arginate in myelodysplastic syndromes. Eur J Haematol. 1992;49(5):234–238. doi: 10.1111/j.1600-0609.1992.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 44.Volin L, Ruutu T, Knuutila S, et al. Heme arginate treatment for myelodysplastic syndromes. Leukemia Res. 1988;12(5):423–431. doi: 10.1016/0145-2126(88)90062-8. [DOI] [PubMed] [Google Scholar]
- 45.Kantarjian H, Giles F, List A, et al. The incidence and impact of thrombocytopenia in myelodysplastic syndromes. Cancer. 2007;109(9):1705–1714. doi: 10.1002/cncr.22602. [DOI] [PubMed] [Google Scholar]
- 46.Wang B, Nichol JL, Sullivan JT. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clin Pharmacol Ther. 2004;76(6):628–638. doi: 10.1016/j.clpt.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Kantarjian H, Fenaux P, Sekeres MA, et al. Phase 1/2 study of AMG 531 in thrombocytopenic patients (pts) with low-risk myelodysplastic syndrome (MDS): update including extended treatment. Blood. 2007;110:81a. Abstract 250. [Google Scholar]