Abstract
This study used mixed-effects modeling of data from a national sample of 6,476 US adults born before 1924, who were tested 5 times between 1993 and 2002 on word recall, serial 7's, and other mental status items to determine demographic and socioeconomic predictors of trajectories of cognitive function in older Americans. Mean decline with aging in total cognition score (range, 0–35; standard deviation, 6.00) was 4.1 (0.68 standard deviations) per decade (95% confidence interval: 3.8, 4.4) and in recall score (range, 0–20; standard deviation, 3.84) was 2.3 (0.60 standard deviations) per decade (95% confidence interval: 2.1, 2.5). Older cohorts (compared with younger cohorts), women (compared with men), widows/widowers, and those never married (both compared with married individuals) declined faster, and non-Hispanic blacks (compared with non-Hispanic whites) and those in the bottom income quintile (compared with the top quintile) declined slower. Race and income differences in rates of decline were not sufficient to offset larger differences in baseline cognition scores. Educational level was not associated with rate of decline in cognition scores. The authors concluded that ethnic and socioeconomic disparities in cognitive function in older Americans arise primarily from differences in peak cognitive performance achieved earlier in the life course and less from declines in later life.
Keywords: aged, cognition, health status disparities, longitudinal studies, social class
Cognitive decline exacts an enormous toll on older adults, their families, and society. Although gradual decline is common in late life (1–3), the rate of cognitive decline varies substantially (4–7). In cross-sectional studies, demographic and socioeconomic variables account for 22%–26% of the variance in cognitive test scores (8), much more than chronic medical conditions (9–11); yet, there is debate about whether this reflects demographic and socioeconomic differences in the rates of cognitive decline or only differences in maximum achieved cognitive function (12–15).
Longitudinal studies that have found socioeconomic and demographic differences in cognitive decline have been either small (5, 16–18), conducted in special populations (1, 19), based on 1 or 2 follow-ups (where cognitive decline is confounded by differences in the practice effect) (5, 18–23), or have used cognition tests prone to ceiling effects that mask declines in high performers (17–19, 21, 23–26). Moreover, in nearly all studies, cross-sectional associations with demographic variables are larger than longitudinal associations (25, 26), and some studies have found no differences in cognitive decline by gender (12, 13, 15, 23, 25), birth year (22, 27), or socioeconomic status (SES) (2, 12, 15, 28).
Thus, it is not clear whether there truly are demographic and socioeconomic differences in rates of cognitive decline with aging. Accordingly, the objective of this study was to determine the average trajectory of cognitive functioning in older Americans, and its demographic and socioeconomic predictors, using a nationally representative cohort serially tested for cognitive performance over 9 years.
MATERIALS AND METHODS
Data were derived from The Study of Assets and Health Dynamics Among the Oldest Old (AHEAD), started in 1993 as a national survey of a probability sample of 8,222 US noninstitutionalized persons born before 1924, with oversampling of minority ethnic groups (29). The response rate was 80%. Demographic, socioeconomic, and cognitive performance assessments were made at baseline (1993) and were repeated in 1995, 1998, 2000, and 2002. Our analytic sample consisted of 6,476 participants who, at baseline, were at least 69 years and 10 months of age, had nonproxy cognition testing, and were missing at most one cognition subscale. More than 80% of the sample (n = 5,272) had cognition scores at 2 or more visits, and 2,353 (36.3%) had cognition scores at all 5 visits.
Measurements
Demographic information collected included self-reported sex, age, marital status (married, widowed, separated/divorced, never married), and race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, non-Mexican Hispanic American, other). Socioeconomic measures considered were highest year of school/college completed, household wealth (the sum of all components—e.g., primary residence, retirement accounts, savings—minus all debt), and annual household income (e.g., individual and spouse's earnings, pensions, Social Security). To allow for nonlinear associations with cognition (12, 13, 30), we categorized age (70–79, 80–89, ≥90 years), education (<8, 8–11, 12–14, >14 years of formal schooling), wealth, and annual income (each as ≤20th, 21st–50th, 51st–80th, >80th percentile). In follow-up visits, changes from baseline were recorded for marital status (dichotomized: lost partner vs. no change or gained partner), wealth, and income (each classified: ≥15% increase, ≥15% decrease, <15% change).
Cognitive performance testing, based on the Telephone Interview for Cognitive Status (a validated assessment tool comparable to the Mini-Mental State Examination (31)), was administered at all visits. Participants were assigned to telephone interviews if aged 79 years or younger and to in-person interviews if older than age 79 years, but they were allowed to switch mode of administration. Testing included immediate and 5-minute delayed-recall test of 10 high-frequency nouns (each scored 0–10); serial 7’s subtraction test, as a test of working memory, attention, and calculation (scored 0–5); and other mental status items (scored 0–10) that assess orientation to time (date, month, year, day of the week; 4 points), attention (counting backward from 20; 2 points), language (object naming; 2 points), and knowledge of current affairs (president and vice president of the United States; 2 points). The total score (range, 0–35) has been validated (32); has a near-normal distribution; and, because of the verbal memory component, is sensitive to early changes and less susceptible to ceiling effects (33, 34).
Total cognition scores for participants missing 1 of the 4 subscales were computed as the sum of the 3 available subscales. An indicator variable for imputed score was included in all models. The proportions of scores imputed were 1.8% (n = 120) in 1993, 5.0% (n = 321) in 1995, 3.3% (n = 215) in 1998, 2.9% (n = 191) in 2000, and 1.9% (n = 121) in 2002. Recall scores (range, 0–20) were computed by summing immediate and delayed-recall scores.
In the second (1995) and third (1998) waves, to compare telephone with face-to-face administration, AHEAD randomly assigned participants aged 78–81 years to one or the other; average scores did not differ significantly by mode of administration (35). Test-retest reliability has also been documented for similar instruments. In one study, the intraclass correlation coefficients between the comprehensive test score from telephone interview and in-person interview of the same individuals by different clinicians varied between 0.92 and 0.98 (36).
Statistical analyses
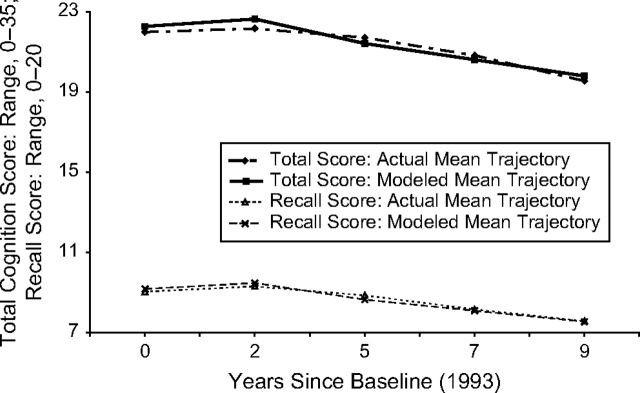
After visual examination of the trajectory of visit-specific mean cognition scores for the 2,353 participants with scores at all 5 visits, we decided to fit a 3-parameter growth curve, with intercept (baseline score), practice effect (step increase after first testing that reflects learning and increased self-assurance (25, 26)), and linear aging-related decline (constant slope, starting from baseline). The 3-parameter model fit the mean trajectory well for both total cognition score (R2 = 0.89) and recall score (R2 = 0.95) (Figure 1).
Figure 1.
Mean cognition score trajectories among Study of Assets and Health Dynamics Among the Oldest Old cohort participants with complete follow-up: 1993, 1995, 1998, 2000, and 2002.
We used a mixed-effects model to fit growth curves to the repeated measurements of cognition score (using HLM software, version 6.01 with full maximum likelihood (37)), wherein each trajectory parameter (intercept, practice effect, and slope) was allowed to vary from individual to individual (as random effects) and to vary by baseline demographic and socioeconomic characteristics (as fixed effects). Additionally, change from baseline in marital status, household income, and wealth were modeled as affecting cognition score contemporaneously—also as fixed effects. This approach allows changes in cognitive performance to be initiated by changes in socioeconomic or marital status and to persist as long as the change in predictor status is maintained.
To capture the potential impact of poor functioning at baseline (21), we included a binary indicator for low performance (based on being in the bottom quartile of baseline total cognition score in one's educational peer group) as a predictor of both practice effect and slope. We used educational-level-specific cutpoints to identify low performers (total cognition score ≤13 if less than a high school education, ≤18 if only a high school education, and ≤19 if more than a high school education) because of the strong influence of education on cognition test performance and to allow for the fact that, in the less educated, low scores may not reflect lower cognitive abilities (38, 39).
To minimize bias due to differential attrition (12, 40), we included a binary indicator of continued participation (at least one follow-up) as well as the number of cognition assessments (integer; range, 1–5). Both terms were modeled to influence the intercept (baseline score) and the integer term to also influence practice effect and slope. These terms reflect, among other factors, proximity to death or serious illness (15).
Because education effects on cognitive decline could vary across groups (41), we tested modification of education effects (on trajectory parameters) by decade of age, ethnicity, gender, and marital status, adding interactions (education × demographic variable), one variable at a time, to the models. We also tested for differences in education effects on learning (practice) and decline (slope) by baseline low performance status, using education × “low performance” interactions. Because studies have found that being married is more protective for men than for women (42, 43), we also tested gender × marital status interactions.
For all analyses, we used normalized sample weights to estimate population averages for noninstitutionalized, older adults in the United States. All statistical tests were 2-sided.
RESULTS
Compared with persons excluded from the study, the study sample was younger, more educated, wealthier, and more likely to be female and non-Hispanic white (Table 1). Similar differences were seen when we compared those in the study sample for whom follow-up was complete complete with the rest. In addition, those persons with complete follow-up were also more likely to be married (Table 2). Mean number of cognition assessment visits was 3.3 (median, 4).
Table 1.
Descriptive Baseline (1993) Demographic and Socioeconomic Statistics for the Study Sample and the Rest of the AHEAD Cohort
| Study Sample (n = 6,476) | Rest of the AHEAD Cohort (n = 1,746) | P Value for Test of Differencea | |
| Age, mean years | 77.1 | 80.2 | <0.001 |
| Gender, % | <0.001 | ||
| Male | 38.7 | 49.6 | |
| Female | 61.3 | 50.4 | |
| Marital status, % | <0.05 | ||
| Married | 51.8 | 56.6 | |
| Widow/widower | 39.9 | 38.1 | |
| Separated/divorced | 5.0 | 2.3 | |
| Never married | 3.3 | 3.0 | |
| Ethnicity, % | <0.001 | ||
| Non-Hispanic white | 88.2 | 80.1 | |
| Non-Hispanic black | 7.6 | 10.4 | |
| Mexican Hispanic | 1.7 | 4.4 | |
| Other Hispanic | 1.4 | 2.1 | |
| Other | 1.1 | 3.0 | |
| Educational level, mean years | 11.3 | 9.3 | <0.001 |
| Wealth, median US dollars | 96,000 | 50,500 | <0.01 |
| Annual income, median US dollars | 17,500 | 13,000 | <0.01 |
Abbreviation: AHEAD, The Study of Assets and Health Dynamics Among the Oldest Old.
Tests of difference between groups: t test for age and education, chi-square test for marital status and ethnicity, and rank sum test for wealth and income. All tests were 2-sided.
Table 2.
Descriptive Baseline (1993) Statistics for AHEAD Study Participants Who Had Complete Follow-up (All 5 Cognitive Assessments) and for the Rest of the Study Sample
| AHEAD Study Sample With Complete Follow-up Data (n = 2,353) | Rest of the AHEAD Study Sample (n = 4,123) | P Value for Test of Differencea | |
| Socioeconomic/demographic variable | |||
| Age, mean years | 74.9 | 78.5 | <0.001 |
| Gender, % | 0.08 | ||
| Male | 36.6 | 40.0 | |
| Female | 63.4 | 60.0 | |
| Marital status, % | <0.001 | ||
| Married | 58.0 | 48.0 | |
| Widow/widower | 34.7 | 43.1 | |
| Separated/divorced | 4.8 | 5.1 | |
| Never married | 2.5 | 3.8 | |
| Ethnicity, % | <0.001 | ||
| Non-Hispanic white | 91.6 | 86.0 | |
| Non-Hispanic black | 4.9 | 9.3 | |
| Mexican Hispanic | 1.3 | 2.0 | |
| Other Hispanic | 1.3 | 1.5 | |
| Other | 0.8 | 1.2 | |
| Educational level, mean years | 12.1 | 10.8 | <0.001 |
| Wealth, median US dollars | 129,500 | 80,500 | <0.001 |
| Annual income, median US dollars | 21,500 | 15,250 | <0.001 |
| Mean cognition score (range) | |||
| Immediate recall (0–10) | 5.3 | 4.2 | <0.001 |
| Delayed recall (0–10) | 3.9 | 2.7 | <0.001 |
| Serial 7’s (0–5) | 3.7 | 2.9 | <0.001 |
| Other mental status items (0–10) | 9.5 | 8.7 | <0.001 |
| Total cognition score (0–35) | 22.3 | 18.3 | <0.001 |
Abbreviation: Abbreviation: AHEAD, The Study of Assets and Health Dynamics Among the Oldest Old.
Tests of difference between groups: t test for age, education, and cognition scores; chi-square test for marital status and ethnicity; and rank sum test for wealth and income. All tests were 2-sided.
Mean total cognition score at baseline (range, 0–35) was 19.84 (95% confidence interval (CI): 19.69, 19.99; standard deviation, 6.00), mean practice effect was 0.93 (95% CI: 0.78, 1.08)—thus, average improvement on repeat testing was almost 1 point—and mean slope was −0.41 per year (95% CI: −0.44, −0.38)—thus, mean decline was slightly more than 4 points (0.68 standard deviations) per decade. Random-effect variances (in the null model) were 24.26 in intercept, 1.32 in practice effect, and 0.13 in annual slope. Mean recall score (range, 0–20) at baseline was 7.78 (95% CI: 7.68, 7.88; standard deviation, 3.84), mean practice effect was 0.73 (95% CI: 0.61, 0.86), and mean slope was −0.23 per year (95% CI: −0.25, −0.21), representing a mean decline of 2.3 points (0.60 standard deviations) per decade; random-effect variances were 8.82 in intercept, 1.52 in practice effect, and 0.04 in annual slope.
Differences by length of participation and baseline cognitive functioning
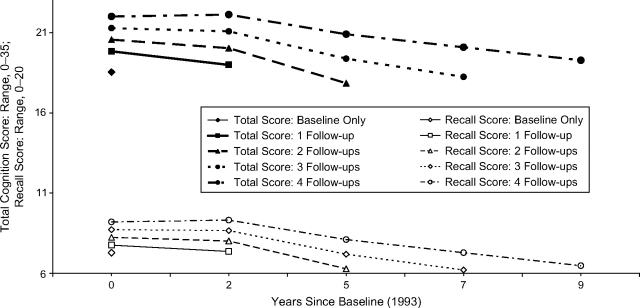
Compared with those persons with only a baseline cognition assessment, those with 2 cognition assessments had a 1.30-point higher total cognition score at baseline (95% CI: 0.81, 1.79). Participating in each additional assessment was associated with a 0.72-point higher baseline score (95% CI: 0.61, 0.84; P < 0.001) and a 1.61-point slower decline per decade (95% CI: 1.16, 2.06; P < 0.001). We found no association between number of assessments and practice effect (P = 0.4). Differences in recall score trajectory by length of participation were similar (Figure 2).
Figure 2.
Predicted cognition score trajectories as a function of length of follow-up (1–5 visits; 0–9 years) among Study of Assets and Health Dynamics Among the Oldest Old cohort participants.
People with imputed scores had total scores that were 4.25 points lower on average than predicted by their demographic and socioeconomic characteristics (95% CI: 3.89, 4.61; P < 0.001). This finding suggests that they would have scored an average of 4.25 points on the missing test.
When we adjusted for length of participation, imputed scores, and demographic and socioeconomic variables, low performers at baseline had a practice effect for total cognition score similar to that for those who performed better at baseline (P = 0.3). They also had similar slopes for both total and recall scores (P = 0.96 and P = 0.3, respectively), but they had a smaller practice effect for recall score (0.67 points smaller, P < 0.001).
Demographic and socioeconomic predictors
Adjusted for length of participation, imputed scores, baseline low performance, and each other, all baseline demographic and socioeconomic variables and their changes since baseline were independently associated with cognition score trajectories (Tables 3 and 4). There was a significant cohort effect, with older individuals scoring lower at baseline and declining faster than younger individuals (more negative slopes). Age at baseline did not influence the magnitude of the practice effect (Tables 3 and 4). Women scored higher than men at baseline, and the practice effect was similar in the 2 groups, but total cognition score declined faster over time (more negative slopes) among women than among men, so that the gender difference diminished with aging (Tables 3 and 4). Compared with non-Hispanic whites, non-Hispanic blacks had lower baseline scores, had similar practice effects, and experienced slower declines (more positive slopes), so that the black-white difference diminished with aging. Mexican Americans also had lower total cognition scores at baseline but had practice effects and slopes similar to those of the majority ethnic group (Tables 3 and 4). Marital status at baseline had no independent association with baseline scores, but widowed and never-married participants had larger practice effects and faster declines than married participants did. Loss of marital partner during the study did not have an independent association with cognition scores (Tables 3 and 4).
Table 3.
Demographic and Socioeconomic Associationsa With Trajectories of Total Cognition Score for Older Americans
| Contemporaneous Association With Cognition Scoreb |
Association With Practice Effect |
Association With Slope (per Decade) |
||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Baseline (1993) socioeconomic/demographic variable | ||||||
| Age, years | ||||||
| 80–89 vs. 70–79 | −2.04*** | −2.31, −1.76 | 0.32 | −0.04, 0.69 | −1.45*** | −2.18, −0.71 |
| >89 vs. 70–79 | −3.42*** | −4.18, −2.66 | 0.85 | −0.14, 1.85 | −3.79** | −6.23, −1.35 |
| Gender (female vs. male) | 0.40** | 0.14, 0.66 | 0.23 | −0.11, 0.57 | −0.64* | −1.25, −0.03 |
| Marital status (vs. married) | ||||||
| Widow/widower | 0.05 | −0.26, 0.36 | 0.49* | 0.10, 0.89 | −0.79* | −1.50, −0.08 |
| Separated/divorced | 0.19 | −0.38, 0.76 | 0.28 | −0.44, 1.00 | 0.71 | −0.58, 2.00 |
| Never married | 0.33 | −0.34, 1.01 | 0.99* | 0.06, 1.91 | −1.00 | −2.82, 0.81 |
| Ethnicity (vs. non-Hispanic white) | ||||||
| Non-Hispanic black | −3.09*** | −3.48, −2.70 | 0.06 | −0.47, 0.60 | 1.63** | 0.67, 2.60 |
| Mexican Hispanic | −1.24** | −2.07, −0.40 | 0.73 | −0.42, 1.87 | −1.16 | −3.02, 0.69 |
| Non-Mexican Hispanic | −0.75 | −1.56, 0.05 | −0.06 | −1.28, 1.15 | −0.37 | −2.31, 1.58 |
| Other ethnic group | −2.48*** | −3.72, −1.25 | 1.98* | 0.40, 3.56 | 0.61 | −2.49, 3.72 |
| Educational level, years | ||||||
| <8 vs. 12–14 | −4.76*** | −5.20, −4.32 | 1.35*** | 0.75, 1.95 | −0.28 | −1.44, 0.87 |
| 8–11 vs. 12–14 | −2.00*** | −2.31, −1.69 | 0.31 | −0.08, 0.71 | 0.36 | −0.34, 1.06 |
| >14 vs. 12–14 | 0.99*** | 0.63, 1.36 | −0.22 | −0.65, 0.21 | 0.10 | −0.66, 0.85 |
| Wealth at baseline, percentile | ||||||
| <20th vs. >80th | −0.96*** | −1.43, −0.49 | 0.38 | −0.30, 1.07 | −0.70 | −1.96, 0.55 |
| 21st–50th vs. >80th | −0.31 | −0.70, 0.07 | −0.03 | −0.56, 0.50 | −0.63 | −1.54, 0.28 |
| 51st–80th vs. >80th | −0.03 | −0.37, 0.32 | −0.24 | −0.68, 0.19 | −0.21 | −0.94, 0.51 |
| Annual income at baseline, percentile | ||||||
| <20th vs. >80th | −1.40*** | −1.96, −0.84 | −0.44 | −1.18, 0.30 | 1.30 | −0.01, 2.61 |
| 21st–50th vs. >80th | −0.60** | −1.02, −0.17 | 0.16 | −0.40, 0.72 | −0.29 | −1.23, 0.64 |
| 51st–80th vs. >80th | −0.13 | −0.50, 0.23 | 0.03 | −0.44, 0.51 | −0.18 | −0.97, 0.61 |
| Change in socioeconomic/demographic variable | ||||||
| Change in annual incomec | ||||||
| Income increasing | 0.06 | −0.11, 0.22 | ||||
| Income decreasing | −0.13 | −0.30, 0.04 | ||||
| Change in wealthc | ||||||
| Wealth increasing | 0.03 | −0.15, 0.22 | ||||
| Wealth decreasing | −0.23* | −0.42, -0.04 | ||||
| Change in marital statusd | ||||||
| Lost a partner | 0.23 | −0.06, 0.52 | ||||
| Pseudo-R2e | 0.53 | 0.23 | 0.19 | |||
Abbreviations: AHEAD, The Study of Assets and Health Dynamics Among the Oldest Old; CI, confidence interval.
P < 0.05;
P < 0.01;
P < 0.001. All tests were 2-sided.
All associations were adjusted for other predictors in this table, survivorship, imputation of partially completed cognition tests, and baseline cognitive impairment; refer to the Materials and Methods section of the text for more information.
For baseline predictors, the association is with baseline cognition score; for change variables, the association is with cognition score at and after the time of the predictor change.
Change of at least 15% since baseline visit; reference group: no change or change less than 15%.
Change since baseline in marital status; reference group: no change or gained a partner.
Proportion of variance in random intercept, random practice effect, and random slope explained by predictors.
Table 4.
Demographic and Socioeconomic Associationsa With Trajectories of Recall Score for Older Americans
| Contemporaneous Association With Recall Scoreb |
Association With Practice Effect |
Association With Slope (per Decade) |
||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Baseline socioeconomic/ demographic variable | ||||||
| Age at baseline, years | ||||||
| 80–89 vs. 70–79 | −1.47*** | −1.67, −1.27 | 0.17 | −0.12, 0.46 | −0.49 | −0.99, 0.01 |
| >89 vs. 70–79 | −2.44*** | −2.92, −1.96 | 0.74 | −0.06, 1.54 | −2.01** | −3.48, −0.54 |
| Gender (female vs. male) | 0.90*** | 070, 1.09 | 0.09 | −0.20, 0.37 | 0.12 | −0.33, 0.58 |
| Marital status (vs. married) | ||||||
| Widow/widower | −0.03 | −0.26, 0.20 | 0.39* | 0.07, 0.71 | −0.64* | −1.15, −0.13 |
| Separated/divorced | 0.08 | −0.34, 0.49 | −0.01 | −0.59, 0.57 | 0.62 | −0.33, 1.56 |
| Never married | −0.06 | −0.57, 0.44 | 0.97* | 0.22, 1.73 | −1.42* | −2.58, −0.27 |
| Ethnicity (vs. non-Hispanic white) | ||||||
| Non-Hispanic black | −1.16*** | −1.43, −0.90 | 0.11 | −0.31, 0.53 | 1.03** | 0.37, 1.70 |
| Mexican Hispanic | −0.48 | −1.01, 0.05 | −0.07 | −0.86, 0.73 | 0.07 | −1.16, 1.29 |
| Non-Mexican Hispanic | −0.15 | −0.71, 0.40 | 0.03 | −0.82, 0.88 | −0.80 | −2.03, 0.42 |
| Other ethnic group | −1.17** | −1.97, −0.37 | 1.41* | 0.12, 2.70 | 0.06 | −2.47, 2.59 |
| Educational level, years | ||||||
| <8 vs. 12–14 | −1.98*** | −2.28, −1.69 | 0.28 | −0.18, 0.73 | 0.31 | −0.45, 1.07 |
| 8–11 vs. 12–14 | −1.10*** | −1.33, −0.88 | 0.08 | −0.25, 0.40 | 0.41 | −0.11, 0.93 |
| >14 vs. 12–14 | 0.60*** | 0.31, 0.88 | −0.01 | −0.39, 0.36 | −0.12 | −0.71, 0.47 |
| Wealth at baseline, percentile | ||||||
| <20th vs. >80th | −0.50** | −0.86, −0.14 | 0.21 | −0.37, 0.79 | −0.45 | −1.38, 0.48 |
| 21st–50th vs. >80th | −0.18 | −0.48, 0.13 | 0.02 | −0.44, 0.47 | −0.46 | −1.18, 0.26 |
| 51st–80th vs. >80th | −0.01 | −0.28, 0.26 | −0.22 | −0.59, 0.15 | −0.13 | −0.68, 0.42 |
| Annual income at baseline, percentile | ||||||
| <20th vs. >80th | −0.76** | −1.19, −0.33 | −0.29 | −0.92, 0.34 | 0.91 | −0.06, 1.87 |
| 21st–50th vs. >80th | −0.40 | −0.72, −0.07 | 0.21 | −0.27, 0.69 | −0.16 | −0.87, 0.56 |
| 51st–80th vs. >80th | −0.12 | −0.40, 0.15 | 0.01 | −0.40, 0.42 | −0.07 | −0.68, 0.55 |
| Change in socioeconomic/demographic variable | ||||||
| Change in annual incomec | ||||||
| Income increasing | 0.06 | −0.07, 0.19 | ||||
| Income decreasing | −0.08 | −0.22, 0.06 | ||||
| Change in wealthc | ||||||
| Wealth increasing | −0.00 | −0.14, 0.15 | ||||
| Wealth decreasing | −0.14 | −0.29, 0.01 | ||||
| Change in marital statusd | ||||||
| Lost a partner | 0.17 | −0.04, 0.39 | ||||
| Pseudo-R2e | 0.41 | 0.00 | 0.12 | |||
Abbreviations: AHEAD, The Study of Assets and Health Dynamics Among the Oldest Old; CI, confidence interval.
P < 0.05;
P < 0.01;
P < 0.001. All tests were 2-sided.
All associations were adjusted for other predictors in this table, survivorship, and baseline cognitive impairment, as described in the text.
For baseline predictors, the association is with baseline cognition score; for change variables, the association is with cognition score at and after the time of the predictor change.
Change of at least 15% since baseline visit; reference group: no change or change less than 15%.
Change since baseline in marital status; reference group: no change or gained a partner.
Proportion of variance in random intercept, random practice effect, and random slope explained by all predictors.
SES was positively associated with baseline scores; in fact, education, income, and wealth were each associated with baseline scores, independent of each other and of other demographic variables (Tables 3 and 4). There was clear evidence for a dose response in every socioeconomic indicator, with high-SES individuals performing better on average than middle-SES individuals, and middle-SES individuals scoring better than low-SES individuals. Education gradients in baseline scores were larger than either income or wealth gradients. SES differences in baseline scores were comparable in magnitude to differences by decades of age (Tables 3 and 4). Despite these large SES differences in baseline scores, we found no independent SES associations with rates of cognitive decline (slopes), and there was only one statistically significant SES association with practice effect: Those with fewer than 8 years of education had a larger practice effect than those with more education. Additionally, those who experienced a decline of 15% or more in household wealth during the study scored 0.23 points (95% CI: 0.04, 0.42; P = 0.02) lower when they first reported the decline and on subsequent testing (Table 3). Increases in wealth and changes in annual household income during the study period were not associated with changes in cognition scores.
Because socioeconomic variables are highly correlated with each other, we also examined the effect of education, income, and wealth individually, without adjusting for the other 2. SES associations were larger in these models, but the pattern remained the same (Tables 5 and 6), with one exception: Those individuals in the bottom quintile of income declined less (more positive slope) than those in the top quintile, so that the difference in cognition scores between income groups diminished over time. To study the sensitivity of our findings to imputation of total cognition scores, we reestimated the model after excluding individuals with imputed scores. The pattern of associations with demographic and socioeconomic variables did not change (data not shown).
Table 5.
Adjusted Socioeconomic Associations, Isolated From Each Other: Total Cognition Scorea
| Contemporaneous Association With Cognition Scoreb |
Association With Practice Effect |
Association With Slope (per Decade) |
||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Educational level, years | ||||||
| <8 vs. 12–14 | −5.32*** | −5.74, −4.89 | 1.23*** | 0.69, 1.77 | 0.06 | −1.01, 1.13 |
| 8–11 vs. 12–14 | −2.27*** | −2.56, −1.97 | 0.31 | −0.06, 0.68 | 0.36 | −0.31, 1.02 |
| >14 vs. 12–14 | 1.21*** | 0.87, 1.55 | −0.22 | −0.63, 0.18 | 0.20 | −0.51, 0.91 |
| Wealth | ||||||
| Baseline wealth, percentile | ||||||
| ≤20th vs. >80th | −2.98*** | −3.44, −2.52 | 0.65* | 0.03, 1.27 | −0.32 | −1.46, 0.81 |
| 21st–50th vs. >80th | −1.82*** | −2.17, −1.46 | 0.20 | −0.25, 0.65 | −0.50 | −1.28, 0.29 |
| 51st–80th vs. >80th | −0.65*** | −0.98, −0.32 | −0.12 | −0.51, 0.28 | −0.25 | −0.92, 0.42 |
| Change in wealthc | ||||||
| Wealth increasing | 0.08 | −0.10, 0.26 | ||||
| Wealth decreasing | −0.24* | −0.44, −0.05 | ||||
| Annual income | ||||||
| Baseline income, percentile | ||||||
| ≤20th vs. >80th | −3.90*** | −4.42, −3.38 | −0.02 | −0.63, 0.60 | 1.12* | 0.05, 2.20 |
| 21st–50th vs. >80th | −2.16*** | −2.55, −1.78 | 0.38 | −0.08, 0.84 | −0.48 | −1.24, 0.29 |
| 51st–80th vs. >80th | −0.85*** | −1.20, −0.51 | 0.11 | −0.31, 0.52 | −0.34 | −1.03, 0.35 |
| Change in incomec | ||||||
| Income increasing | 0.16* | 0.01, 0.32 | ||||
| Income decreasing | −0.14 | −0.30, 0.03 | ||||
Abbreviation: CI, confidence interval.
P < 0.05;
P < 0.01;
P < 0.001. All tests were 2-sided.
All associations were adjusted for age, gender, ethnicity, marital status, change in marital status, survivorship, imputation of partially completed cognition tests, and baseline cognitive impairment, but not adjusted for other socioeconomic status (SES) variables (baseline or change).
For baseline SES, the association is with baseline cognition score; for change variables, the association is with cognition score at and after the time of the predictor change.
Change of at least 15% vs. smaller or no change, adjusted for baseline value.
Table 6.
Adjusted Socioeconomic Associations, Isolated From Each Other: Recall Scorea
| Contemporaneous Association With Recall Scoreb |
Association With Practice Effect |
Association With Slope (per Decade) |
||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Educational level, years | ||||||
| <8 vs. 12–14 | −2.27*** | −2.56, −1.99 | 0.25 | −0.17, 0.66 | 0.58 | −0.12, 1.28 |
| 8–11 vs. 12–14 | −1.25*** | −1.47, −1.03 | 0.07 | −0.24, 0.38 | 0.42 | −0.07, 0.92 |
| >14 vs. 12–14 | 0.73*** | 0.47, 0.99 | −0.03 | −0.38, 0.32 | −0.06 | −0.62, 0.49 |
| Wealth | ||||||
| Baseline wealth, percentile | ||||||
| ≤20th vs. >80th | −1.53*** | −1.86, −1.20 | 0.26 | −0.26, 0.77 | −0.02 | −0.85, 0.81 |
| 21st–50th vs. >80th | −0.98*** | −1.25, −0.71 | 0.06 | −0.32, 0.44 | −0.21 | −0.82, 0.40 |
| 51st–80th vs. >80th | −0.37** | −0.62, -0.12 | −0.17 | −0.51, 0.16 | −0.07 | −0.58, 0.44 |
| Change in wealthc | ||||||
| Wealth increasing | 0.03 | −0.11, 0.17 | ||||
| Wealth decreasing | −0.15* | −0.30, −0.01 | ||||
| Annual income | ||||||
| Baseline income, percentile | ||||||
| ≤20th vs. >80th | −2.01*** | −2.39, -1.63 | −0.23 | −0.75, 0.27 | 1.04** | 0.26, 1.81 |
| 21st–50th vs. >80th | −1.21*** | −1.49, −0.92 | 0.21 | −0.18, 0.59 | −0.13 | −0.70, 0.44 |
| 51st–80th vs. >80th | −0.52*** | −0.77, −0.26 | −0.02 | −0.37, 0.34 | −0.09 | −0.63, 0.45 |
| Change in incomec | ||||||
| Income increasing | 0.12* | 0.00, 0.25 | ||||
| Income decreasing | −0.09 | −0.23, 0.04 | ||||
Abbreviation: CI, confidence interval.
P < 0.05;
P < 0.01;
P < 0.001. All tests were 2-sided.
All associations were adjusted for age, gender, ethnicity, marital status, change in marital status, survivorship, imputation of partially completed cognition tests, and baseline cognitive impairment, but not adjusted for other socioeconomic status (SES) variables (baseline or change).
For baseline SES, the association is with baseline cognition score; for change variables, the association is with cognition score at and after the time of the predictor change.
Change of at least 15% vs. smaller or no change, adjusted for baseline value.
Because there was a clear education gradient in cognition scores in the main-effects models (Tables 3–6), for the purpose of interaction testing, we replaced categorical education with a continuous education variable (years of schooling) to simplify interpretation of education interactions. Gender, marital status, and baseline low performance did not modify the association between education and cognition score slopes and practice effects. However, age and race did modify the education association with cognition score slopes. Thus, in the oldest age group (>89 years), each additional year of schooling was associated with an additional 0.65-point decline per decade (95% CI: 0.13, 1.18; P = 0.01) in total cognition score. In addition, among non-Mexican Hispanic Americans, each additional year of schooling was associated with a 0.35-point lower decline per decade (95% CI: 0.05, 0.65; P = 0.02) in recall score. We found no gender × marital status interaction.
DISCUSSION
Although there were large demographic and socioeconomic differences in older Americans’ cognition scores, we found few socioeconomic and demographic associations with their rates of cognitive decline over a 9-year period. Cognitive decline was faster in older cohorts, women, the widowed, and never-married individuals but was slower in non-Hispanic black Americans. However, at first assessment (mean age, 77.1 years), women had higher scores than men, and non-Hispanic black Americans had lower scores than non-Hispanic white Americans; thus, the gender and ethnic gaps in cognitive performance seen in the eighth decade diminished with further aging. This convergence of trajectories might explain why some previous studies have found gender and ethnic differences in cognitive performance (12, 13), while others have not (15, 44). Our findings are consistent with previous studies that have documented less cognitive decline in non-Hispanic black Americans (45, 46) and faster decline in older cohorts (13, 15, 30).
Higher SES bestowed relatively large advantages in cognition scores at the first assessment, so that high-SES adults aged 90 years or older performed as well as low-SES individuals aged 70–79 years; yet, SES appeared to have limited influence on rates of cognitive decline. Differences in learned test-taking strategies (47), comfort with testing staff, and cultural relevance of test items (48) can explain some of the SES and race/ethnicity differences in baseline test scores.
It has been speculated that higher education also has direct effects on brain structure (e.g., increase in synapses), which slows cognitive decline (47, 49, 50), and that greater psychosocial stress in lower-SES and minority ethnic communities, operating through glucocorticoid pathways, increases neuron loss (51). However, we did not find more rapid cognitive declines in these groups. Instead, the initial disadvantage for blacks and the lowest income group was partly offset by less rapid declines in both. Studies that have found otherwise may have been confounded by ceiling effects that mask declines in high performers (14, 52). Other potential confounders include 1) practice effect, which is larger in the better educated and reduces their apparent rate of decline (14, 52–55); and 2) differential dropout (14, 56). Dropout is often due to death, poor health, or severe cognitive impairment, and cognitive decline accelerates before these conditions (15, 56, 57) and therefore before dropout occurs (58, 59). In our study, we attempted to account for these sources of bias by using an instrument sensitive to early changes in cognitive functioning (60), using data from 5 waves of testing, explicitly modeling the practice effect, and adjusting for length of follow-up. Our findings are consistent with other studies with multiple waves of testing that have not found an association between SES and rate of cognitive decline in older adults (2, 12, 14, 15, 54, 61, 62).
The lack of an association between SES and rate of cognitive decline does not contradict the repeatedly demonstrated strong associations between SES and the incidence of dementia. Instead, our findings suggest that because low-SES adults start from a lower level of cognitive functioning than their high-SES peers but decline at the same rate, they are likely to reach levels that meet dementia criteria earlier, consistent with the brain reserve hypothesis (49, 50, 63) and with studies that found premorbid cognitive functioning to be a better predictor of dementia incidence than educational level (64).
The most consistent predictors of faster declines in cognitive functioning were being old and being single. Previous studies have also found that cognitive decline accelerates in later decades (13, 15, 16, 30, 54, 58, 65, 66) and is faster in never-married and widowed older adults (67), which may be related to less social support (68). Those in the bottom quartile of cognition scores at baseline did not decline any faster than others. Some previous studies have also not found faster declines in low performers (69, 70), but others have (71, 72); differential dropout may explain differences between studies.
Our findings are consistent with previous analyses of AHEAD data that reported similar ethnic differences before controlling for SES (46) and found no education association with cognition change scores (73), but they are in contrast to 2 previous AHEAD analyses that reported education associations with cognitive decline (74, 75). However, these studies did not separate cross-sectional (between-person) age differences in baseline cognition scores from longitudinal (within-person) declines in cognitive function with aging.
Some potential limitations of this study need to be noted. Although we found few socioeconomic associations with rate of decline in the AHEAD total cognition score, such associations might exist with cognition domains not tested. Secondly, although the AHEAD cognition tests avoid ceiling effects and detect early change, they may be susceptible to floor effects. However, except for a smaller practice effect for recall, the trajectories of those in the bottom quartile of baseline cognition scores were similar to those in the rest of the cohort, suggesting that floor effects were not significant in our study. In addition, older cohorts, who performed poorer than more recent cohorts at baseline, also declined faster, suggesting that the test was sensitive to declines even in low performers. Thirdly, there was greater loss to follow-up in low socioeconomic strata and in low-functioning individuals. We attempted to minimize bias from such differential attrition by controlling for length of study participation. Also note that the Telephone Interview for Cognitive Status is not sufficient for diagnosing dementia; thus, direct conclusions cannot be drawn regarding dementia incidence. Lastly, our findings were not controlled for physical health because of the possibility that poor health might mediate some of the socioeconomic and demographic associations with cognitive function.
The study's limitations are outweighed by its strengths, which include the size and diversity of the AHEAD sample, the sensitivity of the test measure to early change, the modeling of practice effects, and control for differential lengths of follow-up. In conclusion, in this large, nationally representative study, age and marital status, but not SES, were associated with rates of cognitive decline. We submit that the risk of dementia in late life depends more on the peak level of cognitive functioning achieved earlier in the life course than on socioeconomic conditions in the later years of life. Decreasing socioeconomic and ethnic disparities in the risk of dementia may therefore require increased emphasis on reducing educational and health disparities at younger ages.
Acknowledgments
Author affiliations: Division of Geriatrics, David Geffen School of Medicine at UCLA, Los Angeles, California (Arun S. Karlamangla, Dana Miller-Martinez, Teresa E. Seeman, Joshua Chodosh); Department of Community Health Sciences, School of Public Health, UCLA, Los Angeles, California (Carol S. Aneshensel, Richard G. Wight); and Health Services Research, VA Greater Los Angeles Health System, Los Angeles, California (Joshua Chodosh).
This work was supported by a grant from the National Institute on Aging (R01 AG022537 to C. S. A., Principal Investigator).
Conflict of interest: none declared.
Glossary
Abbreviations
- AHEAD
The Study of Assets and Health Dynamics Among the Oldest Old
- CI
confidence interval
- SES
socioeconomic status
References
- 1.Kemper S, Greiner LH, Marquis JG, et al. Language decline across the life span: findings from the Nun Study. Psychol Aging. 2001;16(2):227–239. [PubMed] [Google Scholar]
- 2.Lamar M, Resnick SM, Zonderman AB. Longitudinal changes in verbal memory in older adults: distinguishing the effects of age from repeat testing. Neurology. 2003;60(1):82–86. doi: 10.1212/wnl.60.1.82. [DOI] [PubMed] [Google Scholar]
- 3.Federal Interagency Forum on Aging Related Statistics. Older Americans 2004: Key Indicators of Well-Being. Hyattsville, MD: National Center for Health Statistics; 2004. ( http://www.agingstats.gov/Agingstatsdotnet/Main_Site/Data/Data_2004.aspx) [Google Scholar]
- 4.Schaie KW. The Seattle Longitudinal Study: a thirty-five-year inquiry of adult intellectual development. Z Gerontol. 1993;26(3):129–137. [PubMed] [Google Scholar]
- 5.Zelinski EM, Stewart ST. Individual differences in 16-year memory changes. Psychol Aging. 1998;13(4):622–630. doi: 10.1037//0882-7974.13.4.622. [DOI] [PubMed] [Google Scholar]
- 6.Comijs HC, Dik MG, Deeg DJ, et al. The course of cognitive decline in older persons: results from the Longitudinal Aging Study Amsterdam. Dement Geriatr Cogn Disord. 2004;17(3):136–142. doi: 10.1159/000076346. [DOI] [PubMed] [Google Scholar]
- 7.Sachs-Ericsson N, Blazer DG. Racial differences in cognitive decline in a sample of community-dwelling older adults: the mediating role of education and literacy. Am J Geriatr Psychiatry. 2005;13(11):968–975. doi: 10.1176/appi.ajgp.13.11.968. [DOI] [PubMed] [Google Scholar]
- 8.Zelinksi EM, Gilewski MJ, Schaie KW. Individual differences in cross-sectional and 3-year longitudinal memory performance across the adult life span. Psychol Aging. 1993;8(2):176–186. doi: 10.1037//0882-7974.8.2.176. [DOI] [PubMed] [Google Scholar]
- 9.Zelinksi EM, Gilewski MJ. Effects of demographic and health variables on Rasch scaled cognitive scores. J Aging Health. 2003;15(3):435–464. doi: 10.1177/0898264303253499. [DOI] [PubMed] [Google Scholar]
- 10.Zelinksi EM, Crimmins A, Reynolds S, et al. Do medical conditions affect cognition in older adults? Health Psychol. 1998;17(6):504–512. doi: 10.1037//0278-6133.17.6.504. [DOI] [PubMed] [Google Scholar]
- 11.Verhaeghen P, Borchelt M, Smith J. Relation between cardiovascular and metabolic disease and cognition in very old age: cross-sectional and longitudinal findings from the Berlin Aging Study. Health Psychol. 2003;22(6):559–569. doi: 10.1037/0278-6133.22.6.559. [DOI] [PubMed] [Google Scholar]
- 12.Gerstorf D, Herlitz A, Smith J. Stability of sex differences in cognition in advanced old age: the role of education and attrition. J Gerontol B Psychol Sci Soc Sci. 2006;61(4):P245–P249. doi: 10.1093/geronb/61.4.p245. [DOI] [PubMed] [Google Scholar]
- 13.Finkel D, Reynolds CA, McArdle JJ, et al. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Dev Psychol. 2003;39(3):535–550. doi: 10.1037/0012-1649.39.3.535. [DOI] [PubMed] [Google Scholar]
- 14.Christensen H, Hofer SM, Mackinnon AJ, et al. Age is no kinder to the better educated: absence of an association investigated using latent growth techniques in a community sample. Psychol Med. 2001;31(1):15–28. doi: 10.1017/s0033291799002834. [DOI] [PubMed] [Google Scholar]
- 15.Singer T, Verhaeghen P, Ghisletta P, et al. The fate of cognition in very old age: six-year longitudinal findings in the Berlin Aging Study (BASE) Psychol Aging. 2003;18(2):318–331. doi: 10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- 16.Hultsch DF, Hertzog C, Small BJ, et al. Short-term longitudinal change in cognitive performance in later life. Psychol Aging. 1992;7(4):571–584. doi: 10.1037//0882-7974.7.4.571. [DOI] [PubMed] [Google Scholar]
- 17.Brayne C, Gill C, Paykel ES, et al. Cognitive decline in an elderly population—a two wave study of change. Psychol Med. 1995;25(4):673–683. doi: 10.1017/s0033291700034930. [DOI] [PubMed] [Google Scholar]
- 18.Deary IJ, MacLennan WJ, Starr JM. Is age kinder to the initially more able? Differential ageing of a verbal ability in the Healthy Old People in Edinburgh Study. Intelligence. 1998;26(4):357–375. [Google Scholar]
- 19.Butler SM, Ashford JW, Snowdon DA. Age, education, and changes in Mini-Mental State Exam scores of older women: findings from the Nun Study. J Am Geriatr Soc. 1996;4(6):675–681. doi: 10.1111/j.1532-5415.1996.tb01831.x. [DOI] [PubMed] [Google Scholar]
- 20.Evans DA, Beckett LA, Albert MS, et al. Level of education and change in cognitive function in a community population of older persons. Ann Epidemiol. 1993;3(1):71–77. doi: 10.1016/1047-2797(93)90012-s. [DOI] [PubMed] [Google Scholar]
- 21.Farmer ME, Kittner SJ, Rae DS, et al. Education and change in cognitive function: the Epidemiologic Catchment Area Study. Ann Epidemiol. 1995;5(1):1–7. doi: 10.1016/1047-2797(94)00047-w. [DOI] [PubMed] [Google Scholar]
- 22.Albert MS, Jones K, Savage CR, et al. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging. 1995;10(4):578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- 23.Lyketsos CG, Chen LS, Anthony JC. Cognitive decline in adulthood: an 11.5 year follow up of the Baltimore Epidemiologic Catchment Area Study. Am J Psychiatry. 1999;156(1):58–65. doi: 10.1176/ajp.156.1.58. [DOI] [PubMed] [Google Scholar]
- 24.van Gelder BM, Tijhuis M, Kalijn S, et al. Marital status and living situation during a 5-year period are associated with a subsequent 10-year cognitive decline in older men. J Gerontol B Psychol Sci Soc Sci. 2006;61(4):P213–P219. doi: 10.1093/geronb/61.4.p213. [DOI] [PubMed] [Google Scholar]
- 25.Jacqmin-Gadda H, Fabrigoule C, Commenges D, et al. A 5-year longitudinal study of the Mini-Mental State Examination in normal aging. Am J Epidemiol. 1997;145(6):498–506. doi: 10.1093/oxfordjournals.aje.a009137. [DOI] [PubMed] [Google Scholar]
- 26.Unger JM, van Belle G, Heyman A. Cross-sectional versus longitudinal estimates of cognitive change in nondemented older people: a CERAD Study. J Am Geriatr Soc. 1997;47(5):559–563. doi: 10.1111/j.1532-5415.1999.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 27.Carmelli D, Swan GE, LaRue A, et al. Correlates of change in cognitive function in survivors from the Western Collaborative Group Study. Neuroepidemiology. 1997;16(6):285–295. doi: 10.1159/000109699. [DOI] [PubMed] [Google Scholar]
- 28.Alvarado BE, Zunzunegui MV, Del Ser T, et al. Cognitive decline is related to education and occupation in a Spanish elderly cohort. Aging Clin Exp Res. 2002;14(2):132–142. doi: 10.1007/BF03324426. [DOI] [PubMed] [Google Scholar]
- 29.Soldo BJ, Hurd MD, Rodgers WL, et al. Asset and Health Dynamics Among the Oldest Old: an overview of the AHEAD study. J Gerontol B Sci Soc Sci. 1997;52(spec no):S1–S20. doi: 10.1093/geronb/52b.special_issue.1. [DOI] [PubMed] [Google Scholar]
- 30.Sliwinski M, Buschke H. Modeling intraindividual cognitive change in aging adults: results from the Eisnstein Aging Studies. Aging Neuropsychol Cogn. 2004;11(2–3):196–211. [Google Scholar]
- 31.Ferrucci L, Del Lungo I, Guralnik JM, et al. Is the telephone interview for cognitive status a valid alternative in persons who cannot be evaluated by the Mini Mental State Examination? Aging (Milano) 1998;10(4):332–338. doi: 10.1007/BF03339796. [DOI] [PubMed] [Google Scholar]
- 32.Herzog AR, Wallace RB. Measures of cognitive functioning in the AHEAD Study. J Gerontol B Psychol Sci Soc Sci. 1997;52(spec no):37–48. doi: 10.1093/geronb/52b.special_issue.37. [DOI] [PubMed] [Google Scholar]
- 33.Petersen RC, Smith GE, Ivnik RJ, et al. Memory function in very early Alzheimer's disease. Neurology. 1994;44(5):867–872. doi: 10.1212/wnl.44.5.867. [DOI] [PubMed] [Google Scholar]
- 34.Karrasch M, Sinervä E, Grönholm P, et al. CERAD test performances in amnestic mild cognitive impairment and Alzheimer's disease. Acta Neurol Scand. 2005;111(3):172–179. doi: 10.1111/j.1600-0404.2005.00380.x. [DOI] [PubMed] [Google Scholar]
- 35.Herzog AR, Rodgers WL. Cognitive performance measures in survey research on older adults. In: Schwarz N, Park DC, Knauper B, et al., editors. Cognition, Aging, and Self-Reports. Philadelphia, PA: Psychology Press; 1999. [Google Scholar]
- 36.Monteiro IM, Boksay I, Auer SR, et al. Reliability of routine clinical instruments for the assessment of Alzheimer's disease administered by telephone. J Geriatr Psychiatry Neurol. 1998;11(1):18–24. doi: 10.1177/089198879801100105. [DOI] [PubMed] [Google Scholar]
- 37.Raudenbush SW, Bryk AS, Cheong YF, et al. Hierarchical Linear and Nonlinear Modeling. Lincolnwood, IL: Scientific Software International; 2000. [Google Scholar]
- 38.Scherr PA, Albert MS, Funkenstein HH, et al. Correlates of cognitive function in an elderly community population. Am J Epidemiol. 1988;128(5):1084–1101. doi: 10.1093/oxfordjournals.aje.a115051. [DOI] [PubMed] [Google Scholar]
- 39.Kittner SJ, White LR, Farmer ME, et al. Methodological issues in screening for dementia: the problem of education adjustment. J Chronic Dis. 1986;39(3):163–170. doi: 10.1016/0021-9681(86)90019-6. [DOI] [PubMed] [Google Scholar]
- 40.Anstery KJ, Luszcz MA. Selective non-response to clinical assessment in the longitudinal study of aging: implications for estimating population levels of cognitive function and dementia. Int J Geriatr Psychiatry. 2002;17(8):704–709. doi: 10.1002/gps.651. [DOI] [PubMed] [Google Scholar]
- 41.Cagney KA, Lauderdale DS. Education, wealth, and cognitive function in later life. J Gerontol B Psychol Sci Soc Sci. 2002;57(2):P163–P172. doi: 10.1093/geronb/57.2.p163. [DOI] [PubMed] [Google Scholar]
- 42.Davis MA, Neuhaus JM, Moritz DJ, et al. Living arrangements and survival among middle-aged and older adults in the NHANES I epidemiologic follow-up study. Am J Public Health. 1992;82(3):401–406. doi: 10.2105/ajph.82.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chipperfield JG, Havens B. Gender differences in the relationship between marital status transitions and life satisfaction in later life. J Gerontol B Psychol Sci Soc Sci. 2001;56(3):P176–P186. doi: 10.1093/geronb/56.3.p176. [DOI] [PubMed] [Google Scholar]
- 44.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52(2):195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 45.Whitfield KE, Seeman TE, Miles TP, et al. Health indices as predictors of cognition among older African Americans: MacArthur studies of successful aging. Ethn Dis. 1997;7(2):127–136. [PubMed] [Google Scholar]
- 46.Sloan FA, Wang J. Disparities among older adults in measures of cognitive function by race or ethnicity. J Gerontol B Psychol Sci Soc Sci. 2005;60(5):P242–P250. doi: 10.1093/geronb/60.5.p242. [DOI] [PubMed] [Google Scholar]
- 47.Albert MS. How does education affect cognitive function? Ann Epidemiol. 1995;5(1):76–78. doi: 10.1016/1047-2797(94)00044-t. [DOI] [PubMed] [Google Scholar]
- 48.Cauce AM, Coronado N, Watson J. Conceptual, methodological, and statistical issues in culturally competent research. In: Hernandez M, Isaacs M, editors. Promoting Cultural Competence in Children's Mental Health Services. Baltimore, MD: Paul H Brookes; 1998. pp. 305–329. [Google Scholar]
- 49.Liao YC, Liu RS, Teng EL, et al. Cognitive reserve: a SPECT study of 132 Alzheimer's disease patients with an education range of 0–19 years. Dement Geriatr Cogn Disord. 2005;20(1):8–14. doi: 10.1159/000085068. [DOI] [PubMed] [Google Scholar]
- 50.Perneczky R, Drzega A, Diehl-Schmid J, et al. Schooling mediates brain reserve in Alzheimer's disease: findings of fluoro-deoxy-glucose-positron emission tomography. J Neurol Neurosurg Psychiatry. 2006;77(9):1060–1063. doi: 10.1136/jnnp.2006.094714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999;34(6):721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 52.Morris MC, Evans DA, Hebert LE, et al. Methodological issues in the study of cognitive decline. Am J Epidemiol. 1999;149(9):789–793. doi: 10.1093/oxfordjournals.aje.a009893. [DOI] [PubMed] [Google Scholar]
- 53.Lovden M, Ghisletta P, Lindenberger U. Cognition in the Berlin Aging Study (BASE): the first 10 years. Aging Neuropsychol Cogn. 2004;11(2–3):104–133. [Google Scholar]
- 54.Rabbitt P, Diggle P, Holland F, et al. Practice and drop-out effects during a 17-year longitudinal study of cognitive aging. J Gerontol B Psychol Sci Soc Sci. 2004;59(2):P84–P97. doi: 10.1093/geronb/59.2.p84. [DOI] [PubMed] [Google Scholar]
- 55.Rabbitt P, Lunn M, Wong D, et al. Age and ability affect practice gains in longitudinal studies of cognitive change. J Gerontol B Psychol Sci Soc Sci. 2008;63(4):P235–P240. doi: 10.1093/geronb/63.4.p235. [DOI] [PubMed] [Google Scholar]
- 56.Sliwinski MJ, Hofer SM, Hall C, et al. Modeling memory decline in older adults: the importance of preclinical dementia, attrition, and chronological age. Psychol Aging. 2003;18(4):658–671. doi: 10.1037/0882-7974.18.4.658. [DOI] [PubMed] [Google Scholar]
- 57.Bosworth HB, Schaie KW, Willis SL, et al. Age and distance to death in the Seattle Longitudinal Study. Res Aging. 1999;21(6):723–738. [Google Scholar]
- 58.Rabbitt P, Diggle P, Smith D, et al. Identifying and separating the effects of practice and of cognitive ageing during a large longitudinal study of elderly community residents. Neuropsychologica. 2001;39(5):532–543. doi: 10.1016/s0028-3932(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 59.Rabbitt P, Lunn M, Wong D. Death, dropout, and longitudinal measurements of cognitive change in old age. J Gerontol B Psychol Sci Soc Sci. 2008;63(5):P271–P278. doi: 10.1093/geronb/63.5.p271. [DOI] [PubMed] [Google Scholar]
- 60.Järvenpää T, Rinne JO, Räihä I, et al. Characteristics of two telephone screens for cognitive impairment. Dement Geriatr Cogn Disord. 2002;13(3):149–155. doi: 10.1159/000048646. [DOI] [PubMed] [Google Scholar]
- 61.Wilson RS, Scher PA, Bienias JL. Socioeconomic characteristics of the community in childhood and cognition in old age. Exp Aging Res. 2005;31(4):393–407. doi: 10.1080/03610730500206683. [DOI] [PubMed] [Google Scholar]
- 62.Albert M, Blacker D, Moss MB, et al. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21(2):158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- 63.Stern Y, Gurland B, Tatemichi T, et al. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA. 1994;271(13):1004–1010. [PubMed] [Google Scholar]
- 64.Schmand B, Smit JH, Geerlings MI, et al. The effects of intelligence and education on the development of dementia. A test of the brain reserve hypothesis. Psychol Med. 1997;27(6):1337–1344. doi: 10.1017/s0033291797005461. [DOI] [PubMed] [Google Scholar]
- 65.Arbuckle TY, Maag U, Pushkar D, et al. Individual differences in trajectory of intellectual development over 45 years of adulthood. Psychol Aging. 1998;13(4):663–675. doi: 10.1037//0882-7974.13.4.663. [DOI] [PubMed] [Google Scholar]
- 66.Finkel D, Reynolds CA, McArdle JJ, et al. Cohort differences in trajectories of cognitive aging. J Gerontol B Psychol Sci Soc Sci. 2007;62(5):P286–P294. doi: 10.1093/geronb/62.5.p286. [DOI] [PubMed] [Google Scholar]
- 67.Helmer C, Damon D, Letenneur L, et al. Marital status and risk of Alzheimer's disease: a French population-based cohort study. Neurology. 1999;53(9):1953–1958. doi: 10.1212/wnl.53.9.1953. [DOI] [PubMed] [Google Scholar]
- 68.Seeman TE, Lusignolo TM, Albert M, et al. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20(4):243–255. doi: 10.1037//0278-6133.20.4.243. [DOI] [PubMed] [Google Scholar]
- 69.Barnes LL, Wilson RS, Schneider JA, et al. Gender, cognitive decline, and risk of AD in older persons. Neurology. 2003;60(11):1777–1781. doi: 10.1212/01.wnl.0000065892.67099.2a. [DOI] [PubMed] [Google Scholar]
- 70.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- 71.Chodosh J, Reuben DB, Albert MS, et al. Predicting cognitive impairment in high functioning community-dwelling older persons. MacArthur Successful Aging Study. J Am Geriatr Soc. 2002;50(6):1051–1060. doi: 10.1046/j.1532-5415.2002.50260.x. [DOI] [PubMed] [Google Scholar]
- 72.Srikanth VK, Quinn SJ, Donnan GA, et al. Long-term cognitive transitions, rates of cognitive change, and predictors of incident dementia in a population-based first-ever stroke cohort. Stroke. 2006;37(10):2479–2483. doi: 10.1161/01.STR.0000239666.46828.d7. [DOI] [PubMed] [Google Scholar]
- 73.Glymour MM, Weuve J, Berkman LF, et al. When is baseline adjustment useful in analysis of change? An example with education and cognitive change. Am J Epidemiol. 2005;162(3):267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 74.Alley D, Suthers K, Crimmins E. Education and cognitive decline in older Americans. Results from the AHEAD sample. Res Aging. 2007;29(1):1–22. doi: 10.1177/0164027506294245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McArdle JJ, Fisher GG, Kadlec KM. Latent variable analyses of age trends of cognition in the Health and Retirement Study, 1992–2004. Psychol Aging. 2007;22(3):525–545. doi: 10.1037/0882-7974.22.3.525. [DOI] [PubMed] [Google Scholar]