Abstract
The authors compared self-reported occupational exposures with a workplace-specific job exposure matrix (JEM) in a 2004 survey of Texas health-care professionals (n = 3,650), by asthma status. Sensitivity, specificity, chance-corrected (κ) and chance-independent (φ) agreement, and associations of self-reported exposures with asthma were compared with those for the JEM. Among asthmatics, the median sensitivity of self-reported exposures was 74% (range, 53–90); specificity was 64% (range, 27–74). For nonasthmatics, median sensitivity was 67% (range, 40–88) and specificity was 70% (range, 33–82). Sensitivity was higher among asthmatics for exposures involving perceptible odors. Specificity was higher among nonasthmatics for instrument cleaning and exposure to adhesives/solvents. Asthmatics showed better agreement with the JEM for patient-care-related cleaning (φ = 0.51 vs. 0.40); there was little difference for other exposures. In all cases, confidence intervals overlapped. Prevalence ratios were higher with self-reported exposures than with the JEM; differences were greatest for cleaning products, adhesives/solvents, and gases/vapors. However, confidence intervals overlapped with those obtained using the JEM. In asthma studies, differential reporting bias by health status should be taken into consideration. Findings favor using externally developed methods of exposure classification, although information gleaned from examining distributions of exposure self-reports, particularly among nondiseased persons, can provide useful information for improving the reliability of exposure ascertainment.
Keywords: asthma, bias (epidemiology), epidemiologic methods, occupational exposure
In the 1990s, concerns were raised regarding respiratory hazards among health-care workers, partly because of reports of asthmatic reactions to airborne latex following implementation of the Occupational Safety and Health Administration's 1992 bloodborne pathogens standard, which led to marked increases in the use of powdered gloves (1, 2). Health-care settings harbor other potential asthmagenic exposures as well, including cleaning products and disinfectants, pharmaceutical products, sensitizing metals in dental alloys, methacrylates, solvents, and administered aerosolized medications (3). Studies of workplace asthma, however, have some important methodological limitations. Assessment of both current and past exposures remains a challenge in occupational epidemiologic studies, especially since direct quantitative measurements are rarely available (4).
In studies conducted in general populations, self-reported occupational exposures appear to be influenced by the individual asthma status of respondents (5). Whether this occurs in industry-specific or workforce-based studies of asthma, such as health-care settings, is less well established. We examined this issue by comparing self-reported occupational exposures, obtained through a postal survey of asthma in a representative sample of health-care professionals, with a recently developed asthma risk-factor job exposure matrix (JEM).
MATERIALS AND METHODS
Data for the present analysis came from a cross-sectional study conducted in a sample of 5,600 Texas health-care workers in 2004. The study was designed to assess the prevalence of new-onset asthma and associated occupational exposure risk; details have been previously reported (6, 7). In brief, the parent study consisted of the development and validation of a questionnaire, with sections on asthma and occupational and nonoccupational exposures, that was subsequently mailed to a statewide representative sample of physicians, nurses, respiratory therapists, and occupational therapists in Texas. In addition, an externally developed asthma risk factor JEM was created and has also been previously described (7).
The initial source of information for the JEM was a subset of the 1981–1983 National Occupational Exposure Survey, limited to known and suspected asthmagens in the health-care sector (8). This list was then updated through a series of walk-throughs conducted in 3 Houston hospitals accredited by the Joint Commission on Accreditation of Healthcare Organizations. The matrix structure consisted of an occupational axis with 139 job-practice setting combinations and an exposure axis with 5 main classes: 1) use of cleaning products/disinfectants, listed by task (patient-care-related, medical instrument cleaning and disinfection, or general cleaning of building surfaces); 2) use of powdered latex gloves (predominantly during the period of greatest use in the United States, 1992–2000); 3) administration of aerosolized medications to patients; 4) use of adhesives or solvents (subdivided into patient-care-related use and application to general surfaces); and 5) exposure to gases/vapors. Exposures were independently coded by a panel of 5 experts in occupational hygiene, occupational medicine, and/or hospital safety, with disagreements being resolved by consensus. Final JEM-based exposures were dichotomized as 0 (high probability that the majority of workers in a given cell had no exposure or were exposed less than once per week) or 1 (low or high probability of such exposures occurring at least once per week at work in the majority of workers in a given cell).
For the present analysis, anyone reporting a history of physician-diagnosed asthma or wheezing (aside from colds) in the previous 12 months was defined as asthmatic. Questionnaire-based self-reported occupational exposure items, centering on both general categories and specific products, were grouped into the same 5 categories as the JEM categories: use of cleaning products/disinfectants, by task (12 items); use of powdered latex gloves (1 item); administration of aerosolized medications to patients (3 items); use of adhesives or solvents (3 items); and exposure to gases/vapors (3 items). The same set of questions was asked with respect to both the job that respondents currently held and the job in which they had been employed the longest. The full questionnaire is available online at http://oem.bmj.com/cgi/content/full/63/3/173/DC1.
Self-reported occupational exposures were compared with the JEM-based exposure codings, which served as a putative gold standard. Sensitivity and specificity (with corresponding 95% confidence intervals) were computed and compared, by asthma status, for both the current and longest-held jobs. Agreement between self-reports and the JEM was examined, by asthma status, through calculation of both chance-corrected (Cohen's kappa (κ)) and chance-independent (phi (φ)) agreement. Measurement of κ allows assessment of agreement beyond chance and is a common way of determining agreement statistically. However, over the past few years, some limitations of its use have been identified (9–11). Among these is the tendency of κ to underestimate at times the level of agreement in instances where there are marked differences in the proportion of cases in a given cell (i.e., cells indicating either very low or very high prevalence of exposure). In contrast, the φ statistic is a measure of agreement that is independent of chance and is based on the odds ratio (9). It is measured on the same scale as κ (i.e., ranging from −1 to 1) and may provide a better gauge of agreement in these circumstances. Levels of agreement for both κ and φ were defined as follows: <0, poor; 0–0.2, slight; 0.2–0.4, fair; 0.4–0.6, moderate; 0.6–0.8, substantial; and 0.8–1.0, nearly perfect (12).
Since the prevalences of the 2 variables were expected to be high, associations between asthma and both exposure assessment methods (self-reports and JEM) were estimated and compared using Poisson regression with robust variance estimates (13, 14). All analyses were performed with Stata SE, version 9 (Stata Corporation, College Station, Texas).
RESULTS
Table 1 presents the characteristics of the 3,650 respondents (response rate, 65%) (7). On average, respondents were middle-aged, female, and non-Hispanic white, and 34% had ever smoked. They averaged more than 20 years on the job, and the distributions across the 4 professions were similar by design. Approximately 16% of respondents had been previously diagnosed by a physician as having asthma, and one-third reported a history of physician-diagnosed asthma or wheezing in the previous 12 months.
Table 1.
Characteristics of a Study Sample of Health-Care Workers (n = 3,650)a, by Asthma Status, Texas, 2004
| Asthmatics (n = 1,082)b |
Nonasthmatics (n = 2,568) |
|||
| Mean (SD) | Range | Mean (SD) | Range | |
| Age, years | 45.1 (11.4) | 21.6–87.6 | 45.4 (12.0) | 21.2–91.6 |
| Seniority, yearsc | 19.1 (11.5) | 0–64 | 19.2 (12.0) | 0–64 |
| No. | % | No. | % | |
| Gender | ||||
| Male | 308 | 29.1 | 837 | 33.8 |
| Female | 749 | 70.9 | 1,642 | 66.2 |
| Race/ethnicity | ||||
| Non-Hispanic white | 763 | 73.7 | 1,695 | 70.2 |
| Hispanic | 153 | 14.8 | 335 | 13.9 |
| Non-Hispanic black | 48 | 4.6 | 145 | 6.0 |
| Other | 72 | 7.0 | 241 | 10.0 |
| Ever smoking | 441 | 40.8 | 760 | 30.3 |
| Profession | ||||
| Physician | 178 | 16.5 | 674 | 26.8 |
| Occupational therapist | 273 | 25.2 | 674 | 26.8 |
| Nurse | 300 | 27.7 | 626 | 24.9 |
| Respiratory therapist | 331 | 30.6 | 538 | 21.4 |
Abbreviation: SD, standard deviation.
Sample size varies by category because of missing values.
Health-care workers with a prior physician diagnosis of asthma or wheezing in the previous 12 months.
Years in a health-care profession.
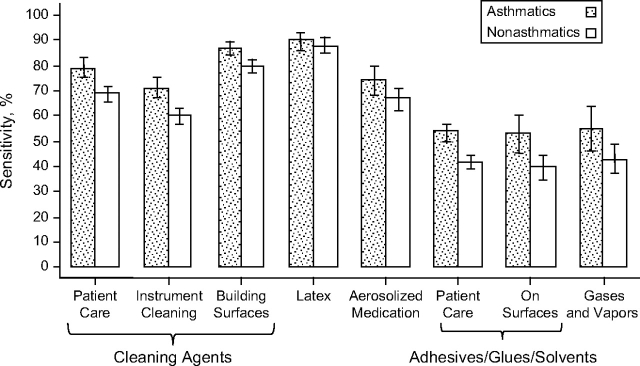
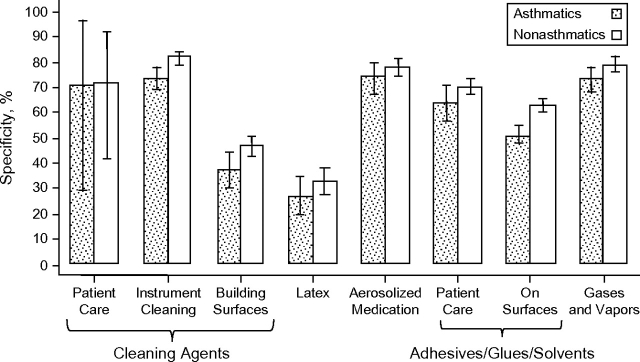
Figures 1 and 2 present results for sensitivity and specificity. Overall, the sensitivity of self-reported exposures, for the longest-held job, was higher among asthmatics than among nonasthmatics (median values, 74% (range, 53–90) and 67% (range, 40–88), respectively), while specificity was lower among asthmatics (median values, 64% (range, 27–74) and 70% (range, 33–82), respectively). By exposure category, sensitivity was significantly higher among asthmatics for exposures to cleaning products/disinfectants and adhesives/solvents, whether applied in patient care or on general surfaces (Figure 1), as evidenced by the nonoverlapping 95% confidence intervals. In contrast, specificity was significantly higher among nonasthmatics only for medical instrument cleaning and use of adhesives/solvents on building surfaces (Figure 2).
Figure 1.
Sensitivity of self-reported occupational exposures for the longest-held job among asthmatic and nonasthmatic health-care workers (n = 3,650), Texas, 2004. Bars, 95% confidence interval.
Figure 2.
Specificity of self-reported occupational exposures for the longest-held job among asthmatic and nonasthmatic health-care workers (n = 3,650), Texas, 2004. Bars, 95% confidence interval.
Table 2 presents findings with respect to agreement by exposure category, for the longest-held job. The κ and φ values ranged from slight agreement to moderate agreement. In all cases, confidence intervals for agreement among asthmatics and nonasthmatics overlapped. Overall, asthmatics tended to show slightly stronger concordance with the JEM coding than nonasthmatics for patient-care-related cleaning (φ = 0.51 vs. 0.40), administration of aerosolized medications (φ = 0.48 vs. 0.45), and use of adhesives/solvents in patient care (φ = 0.18 vs. 0.13), but there was little difference with respect to other exposures. Agreement with the JEM was poorest for self-reported exposure to adhesives/solvents on general surfaces (φ = 0.04 and φ = 0.03 for asthmatics and nonasthmatics, respectively).
Table 2.
Comparison of Self-reported Exposures in the Longest-held Job With Occupational Exposures Assessed by a Job Exposure Matrix Among Health-Care Workers (n = 3,650)a, by Asthma Status, Texas, 2004
| Exposure Category | Self-reported Exposure Prevalence, % | Asthmaticsb |
Nonasthmaticsc |
Association With Asthmad |
|||||||
| κe | 95% CI | φf | κe | 95% CI | φf | Job Exposure Matrix |
Self-Reports |
||||
| PR | 95% CI | PR | 95% CI | ||||||||
| Cleaning products | |||||||||||
| Patient care | 64.4 | 0.07 | 0.00, 0.14 | 0.51 | 0.04 | 0.01, 0.07 | 0.40 | 0.89 | 0.52, 1.52 | 1.26 | 1.00, 1.59 |
| Instrument cleaning | 42.2 | 0.44 | 0.39, 0.50 | 0.45 | 0.42 | 0.38, 0.46 | 0.44 | 1.19 | 1.02, 1.39 | 1.24 | 1.06, 1.44 |
| Building surfaces | 78.3 | 0.25 | 0.18, 0.33 | 0.32 | 0.27 | 0.23, 0.32 | 0.30 | 1.32 | 1.11, 1.56 | 1.35 | 1.11, 1.65 |
| Powdered latex gloves | 70.7 | 0.19 | 0.10, 0.28 | 0.29 | 0.23 | 0.17, 0.29 | 0.31 | 1.11 | 0.95, 1.32 | 1.18 | 0.91, 1.53 |
| Aerosolized medications | 39.0 | 0.48 | 0.39, 0.56 | 0.48 | 0.45 | 0.39, 0.51 | 0.45 | 1.22 | 1.04, 1.43 | 1.21 | 0.99, 1.48 |
| Adhesives/solvents | |||||||||||
| Patient care | 31.1 | 0.13 | 0.07, 0.18 | 0.18 | 0.10 | 0.06, 0.13 | 0.13 | 1.26 | 1.06, 1.51 | 1.34 | 1.15, 1.56 |
| On surfaces | 31.1 | 0.03 | −0.03, 0.08 | 0.04 | 0.02 | −0.02, 0.06 | 0.03 | 0.78 | 0.59, 1.02 | 1.34 | 1.15, 1.56 |
| Gases/vapors | 27.5 | 0.28 | 0.18, 0.37 | 0.30 | 0.23 | 0.16, 0.30 | 0.26 | 0.85 | 0.72, 0.99 | 1.18 | 0.96, 1.45 |
Abbreviations: CI, confidence interval; PR, prevalence ratio.
Actual sample size varies by exposure.
Health-care workers with a prior physician diagnosis of asthma or wheezing in the previous 12 months.
Health-care workers without a prior physician diagnosis of asthma or wheezing in the previous 12 months.
Multivariate Poisson prevalence ratios with robust variance, adjusted for seniority (quartiles), race/ethnicity, body mass index, and atopy; weighted survey samples.
Cohen's unweighted kappa.
Chance-independent agreement.
Table 2 also presents the estimates of association between occupational exposures, both as assessed by the JEM and as self-reported, and asthma. Prevalence ratio point estimates were higher for self-reports in all categories but 1 (administration of aerosolized medications); differences were greatest for use of cleaning products in patient care, application of adhesives/solvents to general surfaces, and exposure to gases/vapors. However, in all cases but 1 (application of adhesives/solvents to general surfaces), the 95% confidence intervals overlapped with those obtained using the JEM as the metric of occupational exposure. In 60% of respondents, the current job was also the longest-held job. Results for current job status were very similar to results for the longest-held job (data not shown).
DISCUSSION
In this study of health-care workers, there were differences in sensitivity, specificity, and agreement for certain self-reported occupational exposures when they were examined by respondents’ asthma status. This suggests that differential misclassification bias in occupational exposures should be taken into account when designing studies of workplace asthma in health-care settings. Some idea of the general directionality of the bias can be gleaned by examining the prevalence ratios for associations between asthma and self-reported occupational exposures, which tended to be higher than the corresponding estimates when the JEM was used as the source for exposure estimates. The observed differences in prevalence ratio estimates between self-reports and the JEM (such as those for use of adhesives/solvents on general surfaces) were consistent with the differences found in both sensitivity and specificity. However, the fact that confidence intervals overlapped in virtually all instances suggests that the effect is slight.
Asthmatics showed a higher sensitivity for reporting exposures, which suggests that false-negative answers for certain occupational exposures would be fewer in comparison with nonasthmatics. In contrast, there was little difference in specificity between asthmatics and nonasthmatics, with the exception of lower values among asthmatics for reported exposure to medical instrument cleaning and/or use of adhesives/solvents on general surfaces. For these 2 categories, the data suggest that false-positive responses would probably be higher among asthmatics. However, for most categories, there are not likely to be major differences in false-positivity rates based on health status. Thus, one may argue that the “differential misclassification” features 2 sides of the coin, with asthmatics having a tendency toward higher sensitivity but slightly lower specificity when describing exposure to certain workplace agents. De Vocht et al. (5) noticed a similar pattern in their community-based study, with sensitivity of self-reported exposure to vapors, gases, dust, and fumes being around 48% for asthmatics versus 42% for nonasthmatics; specificity was much higher and also differed between the 2 groups (83% and 87% for asthmatics and nonasthmatics, respectively). In another general community study conducted in Norway, the sensitivity of reported exposure to dusts, gases, asbestos, or quartz was higher among asthmatics, who also showed a lower specificity than nonasthmatics in that population (15).
Sensitivity was higher among asthmatics with respect to exposure to cleaning products/disinfectants and adhesives/solvents. One characteristic of both of these exposure groups is that they house compounds likely to have fairly perceptible and obvious odors. This, combined with probable heightened awareness of airborne exposures among asthmatics, may explain the greater perception of their presence in this group as compared with nonasthmatics. On the other hand, the use of powdered latex gloves, the use of most aerosolized medications, and the presence of nonspecific gases/vapors in health-care settings are less noticeable or less likely to be detected by a characteristic odor. Higher sensitivity and/or agreement for exposures that are easier to sense have been previously reported for solvents (16) and vibration (17), although subgroup analysis by health status was not performed in either of those studies.
In most instances, the level of agreement between respondents, regardless of health status, and the JEM was only fair to moderate and was similar to that reported by de Vocht et al. (5) in a general community study of asthma. In the latter study, however, exposures were limited to a single question on “vapor, gas, dust, [or] fumes,” which is more subject to perception than inquiries about specific tasks or products. The number of exposure/task categories in our study allowed a more detailed evaluation. Teschke et al. (4), in a review of occupational exposure assessment in case-control studies, reported a wide range of κ values when comparing self-reports with expert assessment, ranging from −0.05 to 0.94 (median, 0.6). However, prevalence of exposure was generally not factored into the assessment of agreement in these prior studies, which may have influenced assessment of agreement based on κ alone. In our study, examination of agreement through both chance-corrected and chance-independent approaches permitted more insight by allowing us to consider situations in which there were either very large or very small proportions of exposed workers in a cell. This was best observed with respect to application of cleaning products/disinfectants in patient care, where in the JEM over 90% of participants were coded as being exposed occupationally at least once per week. For the remaining exposure categories, however, κ and φ values differed relatively little. The usefulness of calculating both κ and φ has also been observed in other contexts, such as measurement of interrater agreement in interpretation of chest radiographs for detection of abnormalities consistent with adult respiratory distress syndrome, where the underlying prevalence of an abnormality was very low (10, 11).
The presence of reporting bias for occupational exposures by health status has been evaluated in other studies and, generally, little to no effect or differences have been found (18–20). However, in most of these studies, health status was usually based on other conditions, including cancer and carpal tunnel syndrome. There is reason to believe that asthmatics would behave differently. Asthma symptoms are typically triggered by exposure to various airborne stimuli. Among the recognized triggers are compounds associated with strong, sometimes characteristic fragrances and/or odors, including perfumes, cleaning products/disinfectants, and environmental tobacco smoke, to name a few. In addition, current asthma management principles include counseling patients to recognize the triggers of their symptoms so that either avoidance or environmental controls can be implemented where possible (21). All this would probably make asthmatics more attuned to their immediate external environment, regardless of whether or not the specific airborne compound was an actual trigger of their symptoms; hence, this might make it more likely for them to recall these kinds of exposures more readily. Baldwin et al. (22) found that asthmatics in Arizona were more likely to report feeling ill in the presence of compounds with strong, noticeable odors, including drying paint, new carpet, perfume, and cleaning agents, when compared with a control group. It is possible that persons who had wheezing due to other causes, such as chronic obstructive pulmonary disease, but did not have a prior physician diagnosis of asthma could have been misclassified as asthmatics in our study. However, reanalysis of the data using a stricter definition of asthma (e.g., limiting cases to only those persons with physician-diagnosed asthma, which would have “missed” as-yet-undiagnosed asthma in persons who wheezed) did not significantly alter either the magnitude or the directionality of the point estimates (data not shown).
Differences in wording and timing between the questionnaire and the JEM may have led to underestimates when calculating κ and φ agreement. Although in the majority of cases the terminology was very similar, there were a few comparisons that were not made on the basis of identically worded exposure categories or time frames. For example, the questionnaire asked about exposure to “adhesives/remover/glues,” but there was lack of clarity as to where these were applied (i.e., in patient care or on surfaces). This may account for the lowest agreement values having been in this category, as well as for the only prevalence ratios that were significantly different from each other in the regression models. On the other hand, the questionnaire asked about glutaraldehyde, whereas in the JEM the closest category was “medical instrument cleaning,” yet agreement here was among the highest. Exposure time frames also varied between the JEM and the questionnaire. Whereas the JEM coding centered on the probability of exposure of the majority of workers in a given job exposure cell at least once per week, exposures in the questionnaire were dichotomized around daily exposures. However, in this case, using a once-per-week time frame in the questionnaire, if anything, would probably have increased the sensitivity of self-reported exposures even further than reported here.
Use of a carefully constructed JEM can be a less expensive method of assigning exposures than expert review by occupational hygienists or chemists, and since it is constructed externally—that is, “blinded” to health outcome status—differential misclassification of exposure is less likely to be present (23). However, limitations associated with the use of JEMs have also been identified, including the lack of variability of exposure estimates within jobs, the potential for nondifferential misclassification of exposure, the loss of statistical power, and the lack of formal validation of most JEMs (23–25).
For exposure classification in epidemiologic studies of asthma, whether by JEM or by self-report, enhancing specificity (i.e., reducing false-positive responses) is the more relevant issue (26). General community-based studies have shown the value of favoring specificity of exposure assignment over sensitivity, mainly because of lower exposure prevalences in general populations (26–28). When combined with increasingly specific disease definitions, the associations found between JEM-assigned exposures and disease outcomes may gain strength; this has been found in recent studies of work-related asthma as well (26, 27, 29). It is therefore possible that a more stringent and specific definition of self-reported exposure in our study could have yielded stronger associations with this exposure metric.
However, one should not infer from the presence of differences between self-reports and the JEM that information gleaned from self-reported occupational exposures should be disregarded (23). In fact, given the broad criterion for classification as “exposed” by the JEM (i.e., “low or high probability”), it is possible that some JEM-“exposed” subjects might not actually have been exposed in that particular category, raising the possibility that self-reports might be a more reliable indicator. Therefore, one way of possibly enhancing the specificity and accuracy of the population-specific JEM developed for the present study could be to incorporate information derived from self-reported exposures by nonasthmatics and apply it to the entire study population. For instance, cells in which a preset percentage of nonasthmatics (20%, 30%, 40%, etc.) reported exposure could be coded as exposed, whereas persons in cells below this percentage cutpoint would be considered nonexposed. By giving greater weight to exposures reported by a group tending to have fewer false-positive responses, specificity of the JEM might be improved. Incorporation of information from self-reports into coding of a JEM has been done previously (30, 31).
Overall, in this study, differences between self-reported exposures and the JEM were slight, except for exposures associated with perceptible odors. This mostly pertained to cleaning products/disinfectants and adhesives/solvents, constituting the main category for which reporting bias is most important. In epidemiologic studies, this bias can affect estimates of associations between these exposures and asthma, and hence should be taken into consideration. The findings tend to favor using externally developed methods of exposure classification in studies of workplace asthma—although information gleaned from examining distributions of exposure self-reports, particularly among nondiseased persons, can provide valuable information that could help to improve the reliability of exposure ascertainment.
Acknowledgments
Author affiliations: School of Public Health, University of Texas, Houston, Texas (George L. Delclos, David Gimeno); Occupational Health Research Unit, Department of Experimental and Health Sciences, Faculty of Health and Life Sciences, Universitat Pompeu Fabra, Barcelona, Spain (George L. Delclos, Fernando G. Benavides); CIBER Epidemiología y Salud Pública, Barcelona, Spain (George L. Delclos, David Gimeno, Fernando G. Benavides, Jan-Paul Zock); Department of Epidemiology and Public Health and International Institute for Society and Health, Division of Population Health, University College London, London, United Kingdom (David Gimeno); Department of Public Health Sciences, College of Health and Human Services, University of North Carolina at Charlotte, Charlotte, North Carolina (Ahmed A. Arif); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Jan-Paul Zock); and Municipal Institute of Medical Research (IMIM-Hospital del Mar), Barcelona, Spain (Jan-Paul Zock).
This work was supported in part by the National Institute for Occupational Safety and Health (grants 5R01OH03945-01A1 and T42CCT610417) and the National Institute on Aging (grant AG13196 to D. G.).
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- JEM
job exposure matrix
References
- 1.Occupational Safety and Health Administration. Occupational Safety and Health Standards. Bloodborne Pathogens. US Department of Labor. (29 CFR Part 1910.1030). Washington, DC: Occupational Safety and Health Administration; 1992. ( http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10051). (Accessed July 12, 2008) [Google Scholar]
- 2.Tomazic-Jezic VJ. Latex allergy: recent developments in glove use and safety. User Facility Reporting. 2002:39. ( http://www.fda.gov/cdrh/fusenews/ufb39.html#4). (Accessed July 12, 2008) [Google Scholar]
- 3.Delclos GL. Respiratory hazards of health-care workers. Pulm Crit Care Update. 1996;11:1–8. [Google Scholar]
- 4.Teschke K, Olshan AF, Daniels JL, et al. Occupational exposure assessment in case-control studies: opportunities for improvement. Occup Environ Med. 2002;59(9):575–594. doi: 10.1136/oem.59.9.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vocht F, Zock JP, Kromhout H, et al. Comparison of self-reported occupational exposure with a job exposure matrix in an international community-based study on asthma. Am J Ind Med. 2005;47(5):434–442. doi: 10.1002/ajim.20154. [DOI] [PubMed] [Google Scholar]
- 6.Delclos GL, Arif AA, Aday L, et al. Validation of an asthma questionnaire for use in healthcare workers. Occup Environ Med. 2006;63(3):173–179. doi: 10.1136/oem.2005.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delclos GL, Gimeno D, Arif AA, et al. Occupational risk factors and asthma among health care professionals. Am J Respir Crit Care Med. 2007;175(7):667–675. doi: 10.1164/rccm.200609-1331OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seta JA, Sundin DS, Pedersen DH. National Occupational Exposure Survey Field Guidelines. Cincinnati, OH: National Institute of Occupational Safety and Health; 1988. (DHHS (NIOSH) publication no. 88-106) [Google Scholar]
- 9.Cook RJ, Farewell VT. Conditional inference for subject specific and marginal agreement: two families of agreement measures. Can J Stat. 1995;23(4):333–344. [Google Scholar]
- 10.Meade MO, Cook RJ, Guyatt GH, et al. Interobserver variation in interpreting chest radiographs for the diagnosis of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161(1):85–90. doi: 10.1164/ajrccm.161.1.9809003. [DOI] [PubMed] [Google Scholar]
- 11.Meade MO, Guyatt GH, Cook RJ, et al. Agreement between alternative classifications of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(2):490–493. doi: 10.1164/ajrccm.163.2.2006067. [DOI] [PubMed] [Google Scholar]
- 12.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 13.Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio [electronic article] BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 15.Bakke PS, Hanoa R, Gulsvik A. Relation of occupational exposure to respiratory symptoms and asthma in a general population sample: self-reported versus interview-based exposure data. Am J Epidemiol. 2001;154(5):477–483. doi: 10.1093/aje/154.5.477. [DOI] [PubMed] [Google Scholar]
- 16.Kromhout H, Oostendorp Y, Heederik D, et al. Agreement between qualitative exposure estimates and quantitative exposure measurements. Am J Ind Med. 1987;12(5):551–562. doi: 10.1002/ajim.4700120509. [DOI] [PubMed] [Google Scholar]
- 17.Wiktorin C, Hjelm E, Winkel J, et al. Reproducibility of a questionnaire for assessment of physical load during work and leisure time. J Occup Environ Med. 1996;38(2):190–201. doi: 10.1097/00043764-199602000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Blair A, Zahm SH. Patterns of pesticide use among farmers: implications for epidemiologic research. Epidemiology. 1993;4(1):55–62. doi: 10.1097/00001648-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Fritschi L, Siemiatycki J, Richardson L. Self-assessed versus expert-assessed occupational exposures. Am J Epidemiol. 1996;144(5):521–527. doi: 10.1093/oxfordjournals.aje.a008959. [DOI] [PubMed] [Google Scholar]
- 20.Nordstrom DL, Vierkant RA, Layde PM, et al. Comparison of self-reported and expert-observed physical activities at work in a general population. Am J Ind Med. 1998;34(1):29–35. doi: 10.1002/(sici)1097-0274(199807)34:1<29::aid-ajim5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.National Heart, Lung, and Blood Institute. Guidelines for the Diagnosis and Management of Asthma (EPR-3) Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. ( http://www.nhlbi.nih.gov/guidelines/asthma/index.htm). (Accessed July 12, 2008) [Google Scholar]
- 22.Baldwin CM, Bell IR, O'Rourke MK. Odor sensitivity and respiratory complaint profiles in a community-based sample with asthma, hay fever, and chemical odor intolerance. Toxicol Ind Health. 1999;15(3-4):403–409. doi: 10.1177/074823379901500314. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg M, Kromhout H, Guénel P, et al. Job exposure matrices in industry. Int J Epidemiol. 1993;22(suppl 2):S10–S15. doi: 10.1093/ije/22.supplement_2.s10. [DOI] [PubMed] [Google Scholar]
- 24.Louik C, Frumkin H, Ellenbecker MJ, et al. Use of a job-exposure matrix to assess occupational exposures in relation to birth defects. J Occup Environ Med. 2000;42(7):693–703. doi: 10.1097/00043764-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Stewart PA, Herrick RF, Blair A, et al. Highlights of the 1990 Leesburg, Virginia, international workshop on retrospective exposure assessment for occupational epidemiology studies. Scand J Work Environ Health. 1991;17(4):281–285. doi: 10.5271/sjweh.1701. [DOI] [PubMed] [Google Scholar]
- 26.Le Moual N, Kennedy SM, Kauffmann F. Occupational exposures and asthma in 14,000 adults from the general population. Am J Epidemiol. 2004;160(11):1108–1116. doi: 10.1093/aje/kwh316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zock JP, Cavallé N, Kromhout H, et al. Evaluation of specific occupational asthma risks in a community-based study with special reference to single and multiple exposures. J Expo Anal Environ Epidemiol. 2004;14(5):397–403. doi: 10.1038/sj.jea.7500337. [DOI] [PubMed] [Google Scholar]
- 28.Kromhout H, Heedrik D, Dalderup LM, et al. Performance of two general job-exposure matrices in a study of lung cancer morbidity in the Zutphen cohort. Am J Epidemiol. 1992;136(6):698–711. doi: 10.1093/oxfordjournals.aje.a116549. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy SM, Le Moual N, Choudat D, et al. Development of an asthma specific job exposure matrix and its application in the Epidemiological Study of Genetics and Environment in Asthma (EGEA) Occup Environ Med. 2000;57(9):635–641. doi: 10.1136/oem.57.9.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Post WK, Heederik D, Kromhout H, et al. Occupational exposures estimated by a population specific job exposure matrix and 25 year incidence rate of chronic nonspecific lung disease (CNSLD): The Zutphen Study. Eur Respir J. 1994;7(6):1048–1055. [PubMed] [Google Scholar]
- 31.Le Moual N, Bakke P, Orlowski E, et al. Performance of population specific job exposure matrices (JEMs): European collaborative analyses on occupational risk factors for chronic obstructive pulmonary disease with job exposure matrices (ECOJEM) Occup Environ Med. 2000;57(2):126–132. doi: 10.1136/oem.57.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]