Abstract
Giardia intestinalis is a common gastrointestinal protozoan worldwide, but its effects on childhood growth in developing countries are not clearly understood. The authors aimed to describe its effects on child growth. They followed 220 Peruvian children daily for diarrhea, weekly for stool samples, and monthly for anthropometry. The authors modeled the effect of nutritional status on the risk of Giardia infection and the risk of diarrhea attributable to Giardia using negative binomial regression. They modeled the effects of Giardia infection on growth using linear regression, with 85% of children becoming infected with Giardia and 87% of these becoming reinfected. In multivariable analysis, the risk of Giardia infection did not vary with weight for age (relative risk = 1.00, 95% confidence interval: 0.89, 1.12) or height for age (relative risk = 0.92, 95% confidence interval: 0.82, 1.04). Giardiasis did not affect growth at 1 or 2 months following the first infection at any age interval. The longitudinal prevalence of Giardia between 6 and 24 months of age was not associated with height gain in that interval (p = 0.981). Giardia was not associated with an increased risk of diarrhea at any age interval. Study results question the importance of Giardia as a childhood pathogen in developing countries where giardiasis is hyperendemic.
Keywords: developing countries, diarrhea, Giardia lamblia, growth, natural history, Peru
Giardia intestinalis is a common gastrointestinal protozoan worldwide (1). Approximately 2.8 billion episodes are recorded each year (2). Infection varies from asymptomatic episodes to acute diarrhea, and it may persist as chronic diarrhea with concomitant malabsorption of nutrients (2).
In developed countries, giardiasis is associated with diarrheal outbreaks in children and adults and is a common cause of diarrhea in travelers (3). Infections with Giardia that become subacute or chronic may result in weight loss (4). In developing countries, the health effects of giardiasis are less well understood. Morbidity related to Giardia is limited to early childhood. It is also unclear whether the health effects of Giardia are transient or if they result in long-term health consequences as frequently documented in children with other parasitic infections (5).
Although several epidemiologic studies in developing countries have linked giardiasis with childhood growth retardation (6–10), other studies have failed to document any association (11–15). It is unclear if the inconsistency in results across studies is due to true differences in the study population, baseline childhood nutritional status, or average inoculum size or whether the inconsistency is due to study design issues, such as the use of a nonlongitudinal design, failure to appropriately control for confounding effects, or misclassification of Giardia exposure.
Our objective was to determine the health effects of Giardia in a birth cohort of Peruvian children.
MATERIALS AND METHODS
Study setting
We conducted the study in Pampas de San Juan de Miraflores, a periurban community of 40,000 inhabitants located 25 km south of Lima, Peru. This population has been described elsewhere (16–18).
Study participants and design
Our study sample consisted of children recruited at birth and followed between January 1995 and June 1998. We invited mothers in their last trimester of pregnancy to participate in this longitudinal study and then followed their children until 35 months of age or to the end of the study.
We obtained information on maternal height, per capita household income, household water supply and storage, and sanitation. We constructed a composite water and sanitation score based on the household's water supply, water storage, and sanitation facility, as previously described (17, 18).
The study design is described elsewhere (17, 18). We obtained daily records of diarrheal surveillance. On each field visit, field workers asked mothers to indicate if the child had had diarrhea and to specify the number of liquid or semiliquid stools that the child had passed during 24 hours.
Stool collection took place weekly. We provided plastic cups (5 ml) and spoons regularly to mothers. At the beginning of the study, we instructed mothers on how to fill the plastic cups with fresh stool samples. We examined all weekly stool samples for Giardia infection using light microscopy of direct wet-mounted and formalin ether-concentrated stools (19). Prior analyses reported results on 224 children (19). We excluded four children because they lacked information regarding Giardia status.
We recorded anthropometry monthly. We measured weight with Salter scales, recumbent length in children from birth to less than 2 years of age, and standing height in children 2 years of age or older. No child was treated for Giardia during the study.
This study was approved by the Committee of Human Research of the Johns Hopkins School of Public Health.
Cohort retention and missing data
Cohort retention has been described elsewhere (16–18). Three percent of mothers refused to participate in the study, and 4 percent of children did not have any anthropometric measurements. Of those with anthropometric measurements, 74 percent were followed for 6 months or longer, and 70 percent had complete information on diarrheal surveillance, anthropometery, and baseline information. Children in the study for 6 months or longer did not differ by gender (p = 0.787), per capita household income (p = 0.189), maternal education (p = 0.787), and water and sanitation score (p = 0.600) from those who were followed for less than 6 months. They were also more likely to live in a house owned by the parents than were children in the study for less than 6 months (p < 0.001).
There was a low rate of missing information on diarrheal surveillance and weekly stools. Through multiple revisits, we missed less than 5.5 percent of diarrheal surveillance visits (159,551 daily diarrheal records from a total of 168,843 possible follow-up days). In 10 percent of instances, we collected less than three weekly stools per month per child.
Outcomes
The goals of this study were to determine if 1) malnutrition was a risk factor for Giardia, 2) giardiasis was associated with diarrhea, and 3) children who become infected with Giardia for the first time grew at a slower rate than did noninfected children in the first or second month after the onset of infection.
The first outcome was Giardia infection. A Giardia episode began when we identified the first Giardia-positive stool and ended during the week of the first Giardia-negative stool when at least three consecutive weekly stools tested negative for Giardia over a 4-week period (6). We categorized a child-week as Giardia positive if we identified Giardia during that child-week. We defined the onset of a Giardia infection as the first child-week of a Giardia episode.
The second outcome was diarrhea. A diarrheal episode began when the mother or caretaker indicated that a child had diarrhea, with the child having passed three or more liquid or semiliquid stools in a day, and ended when the child passed fewer than three liquid or semiliquid stools per day over 2 consecutive days (20). We categorized each Giardia-positive child-week as diarrhea positive or diarrhea negative according to the presence or absence of at least 1 day of diarrhea during the child-week of follow-up.
The third outcome was growth following the first onset of Giardia infection. We defined a growth interval as two consecutive weight (or height) measurements. Two growth intervals were considered: 30 days ± 10 days after the index measurement and 60 days ± 10 days after the index measurement. We categorized a growth interval as Giardia positive if we identified at least one Giardia-positive week during that interval. Any growth interval subsequent to the first Giardia-positive growth interval was excluded from our analysis.
The fourth outcome was height gain between 6 and 24 months of age. We calculated the longitudinal prevalence of Giardia and the longitudinal prevalence of diarrhea between 6 and 24 months of age and determined their contributions to height gain in this interval with linear regression.
Predictors
We classified breastfeeding as exclusive (breast milk as the sole source of nutrition), mixed (supplementation with other sources of nutrition), or none; age into less than 6 months, 6–11 months, 12–17 months, 18–23 months, and 24–35 months; calendar time into the seasonal quarters of summer (December 1 to February 29), fall (March 1 to May 31), winter (June 1 to August 31), and spring (September 1 to November 30); maternal height into quartiles; water and sanitation scores into the tertiles of worst (3), intermediate (4–8), and best (9); and per capita household income into quartiles.
We defined growth intervals as diarrhea positive if there was at least 1 day of diarrhea within that interval and as diarrhea negative if otherwise. We calculated weight-for-age (WAZ), height-for-age (HAZ), and weight-for-height (WHZ) z scores on the basis of the 2006 World Health Organization child growth standards (21).
Biostatistical analysis
We estimated the prevalence of Giardia as the proportion of Giardia-positive child-weeks and the incidence as the number of new Giardia episodes per 52 child-weeks or the number of new diarrheal episodes per 52 child-weeks. We assessed trends in Giardia infection using stratified categories of nutritional status and a chi-square test for trend.
We used multivariable negative binomial regression to model the number of Giardia-positive child-weeks by gender (reference: boys), age categories (reference: 0–5 months), seasonal quarters (reference: fall), water and sanitation categories (reference: intermediate level), breastfeeding status (reference: no breastfeeding), maternal height (reference: <145 cm), per capita household income (reference: <75 soles), and the logarithm of child-weeks as the offset. We also included the following nutritional status indicators in the model: WAZ, HAZ, and WHZ. To avoid collinearity between nutritional indicators, we ran three separate regression models for each continuous nutritional status indicator and two models for categorized measures—one model that included HAZ and WHZ and another model that included WAZ and WHZ. We measured nutritional status as the z-score value recorded during the 30 days prior to the child-week of interest. We adjusted for within-child correlation using a Huber-White estimator. This estimator allows for a robust adjustment of the variance-covariance matrix. We used negative binomial regression because our count data exhibited overdispersion. For each risk factor included in the regression model, we calculated the relative risk and its corresponding 95 percent confidence intervals based on the model estimates.
We used negative binomial regression to model the number of diarrhea-positive child-weeks as a function of Giardia infection, stratified by age and after adjustment for gender, seasonal quarters, WAZ, HAZ, water and sanitation score, maternal height, per capita household income, breastfeeding, and the logarithm of child-weeks as the offset.
We used a multivariate, multiple variable linear regression model to study the effect of giardiasis on growth. The reference group for this analysis consisted of Giardia-negative growth intervals. We regressed weight (or height) at the end of a growth interval on weight (or height) at the beginning of the interval. Other covariates included the presence or absence of Giardia during the growth interval, age, gender, the presence or absence of diarrhea during the growth interval, water and sanitation categories, maternal height, per capita household income, and breastfeeding. Diarrhea was also used as a surrogate for other pathogens. We used a continuous autoregressive error term to account for serial correlation between measurements from the same child and maximum likelihood for parameter estimation. We fitted two separate regression models: The first model examined monthly growth intervals, and the second model examined bimonthly growth intervals. We adjusted for the following covariates: Giardia infection during the growth interval stratified by age, gender, presence or absence of diarrhea during the growth interval, maternal height, water and sanitation categories, per capita household income, and breastfeeding. Given that there were only six monthly and 20 bimonthly growth intervals that were Giardia positive and had a recorded episode of diarrhea in the first week of infection with Giardia, we did not have sufficient data points to examine growth differences between diarrhea-associated giardiasis and asymptomatic giardiasis stratified by age.
We used linear regression to model the longitudinal prevalence of Giardia on height growth between 6 and 24 months of age in the subset of children with height measurements at these ages. We restricted this analysis to children who had a height measurement in the age interval 5.5–6.99 months, those who had a height measurement in the age interval 23.5–24.99 months, and children who had at least 186 days of diarrheal surveillance between these height measurements (108 children met these criteria). We calculated height gain as the difference in height divided by the difference in age; longitudinal prevalence of Giardia as the number of Giardia-positive weeks divided by the number of total weeks and multiplied by 100; and longitudinal diarrheal prevalence as the number of diarrhea days divided by the total number of surveillance days multiplied by 100. We fitted a linear model with height gain as the outcome and using the following predictors: longitudinal prevalence of Giardia between 6 and 24 months, longitudinal diarrheal prevalence between 6 and 24 months, gender, water and sanitation score, per capita household income, and maternal height. We conducted regression diagnostics to identify outliers and influential data points.
Statistical analyses were performed by using STATA, version 7 (StataCorp LP, College Station, Texas), S-Plus 2000 (MathSoft, Seattle, Washington), and SAS, version 8 (SAS Institute, Inc., Cary, North Carolina), software packages.
RESULTS
During the 4-year study period (1995–1998), we followed the 220 study children for 20,884 child-weeks, with a median time to follow-up of 23 months. We recorded 3,324 monthly and 2,826 bimonthly growth intervals, from which we excluded those intervals during which stools were not collected (12 monthly and 11 bimonthly intervals) and those intervals subsequent to first infection with Giardia (1,701 monthly and 1,763 bimonthly intervals). Data on 1,611 monthly growth intervals and 1,052 bimonthly growth intervals were used in the final analysis.
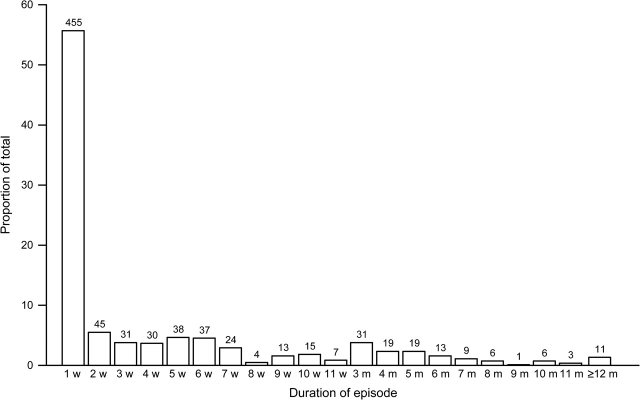
The overall prevalence of Giardia was 18.8 percent per child-week (3,911 Giardia-positive weeks/20,844 child-weeks of follow-up). Eighty-five percent (188/220) of the study children became infected with Giardia during their follow-up. The median age at first infection was 6 months (range: from 6 days to 23 months). Reinfection with Giardia was a common event: 87 percent (164/188) of children had a subsequent infection. We recorded a total of 817 Giardia episodes, yielding an incidence of 2.0 episodes per 52 child-weeks. The incidence of Giardia increased with age (table 1). Fifty-five percent (451/817) of Giardia episodes were 1 week in duration. The duration of Giardia episodes ranged from 1 week to 22 months (figure 1).
TABLE 1.
Incidence of Giardia intestinalis and diarrheal episodes per 52 child-weeks for 220 children living in Las Pampas de San Juan, Lima, Peru, between 1995 and 1998
| Age category (months) | Total no. of child-weeks (n = 20,884) |
Giardia |
Diarrhea |
||||||
| Overall |
Symptomatic |
Asymptomatic |
|||||||
| No. of Giardia episodes | Incidence | No. of Giardia episodes | Incidence | No. of Giardia episodes | Incidence | No. of diarrheal episodes | Incidence | ||
| 0–5 | 4,592 | 127 | 1.44 | 7 | 0.08 | 120 | 1.36 | 199 | 2.25 |
| 6–11 | 4,723 | 149 | 1.64 | 12 | 0.13 | 137 | 1.51 | 373 | 4.11 |
| 12–17 | 4,053 | 186 | 2.39 | 14 | 0.18 | 172 | 2.21 | 387 | 4.96 |
| 18–23 | 3,190 | 143 | 2.33 | 7 | 0.11 | 136 | 2.22 | 205 | 3.34 |
| 24–35 | 4,326 | 212 | 2.55 | 11 | 0.13 | 201 | 2.42 | 223 | 2.68 |
FIGURE 1.
Duration of Giardia episodes for children living in Las Pampas de San Juan, Lima, Peru, between 1995 and 1998. Duration is expressed in weeks (w) or months (m). Each bar represents the percentage of Giardia episodes that lasted a specific number of weeks or months. The numbers above the bars represent the number of episodes that lasted a specific number of weeks or months.
The prevalence of Giardia infection increased with age (table 2) and was similar between girls and boys (18.8 percent vs. 18.7 percent). Children were less likely to have giardiasis in the summer than in the fall (relative risk (RR) = 0.77, 95 percent confidence interval (CI): 0.66, 0.91). The water and sanitation score, breastfeeding status, maternal height, and gender were not significantly associated with the risk of giardiasis.
TABLE 2.
Relative risk of Giardia intestinalis infection for 220 children living in Las Pampas de San Juan, Lima, Peru, 1995–1998
| Characteristic | No. of child-weeks |
Crude relative risk | Adjusted relative risk | 95% confidence interval | |
| Total (n = 20,884) | Giardia positive (n = 3,911) | ||||
| Gender | |||||
| Boys | 11,433 | 2,136 | 1.00 | 1.00 | |
| Girls | 9,451 | 1,775 | 1.01 | 0.91 | 0.71, 1.16 |
| Age (months) | |||||
| 0–5 | 4,592 | 188 | 1.00 | 1.00 | |
| 6–11 | 4,723 | 402 | 2.02 | 1.95 | 1.46, 2.61 |
| 12–17 | 4,053 | 762 | 4.53 | 4.29 | 3.28, 5.61 |
| 18–23 | 3,190 | 901 | 6.93 | 6.93 | 5.24, 9.18 |
| 24–35 | 4,326 | 1,658 | 9.25 | 9.56 | 7.22, 12.66 |
| Season | |||||
| Fall | 5,057 | 971 | 1.00 | 1.00 | |
| Winter | 5,616 | 1,074 | 1.01 | 1.02 | 0.88, 1.18 |
| Spring | 5,836 | 1,193 | 1.05 | 1.06 | 0.90, 1.26 |
| Summer | 4,375 | 673 | 0.83 | 0.77 | 0.66, 0.91 |
| Water and sanitation | |||||
| Worst | 5,690 | 1,099 | 1.14 | 1.10 | 0.84, 1.45 |
| Intermediate | 12,655 | 2,171 | 1.00 | 1.00 | |
| Best | 2,539 | 641 | 1.45 | 1.27 | 0.92, 1.77 |
| Per capita household income (soles) | |||||
| 0–74 | 4,387 | 1,054 | 1.00 | 1.00 | |
| 75–119 | 5,699 | 1,140 | 0.83 | 0.84 | 0.62, 1.15 |
| 120–176 | 4,701 | 798 | 0.71 | 0.70 | 0.47, 1.04 |
| ≥177 | 6,097 | 919 | 0.63 | 0.58 | 0.42, 0.82 |
| Breastfeeding status | |||||
| None | 10,822 | 2,379 | 1.00 | 1.00 | |
| Mixed | 9,284 | 1,506 | 0.75 | 1.12 | 0.89, 1.40 |
| Exclusive | 778 | 26 | 0.15 | 0.81 | 0.50, 1.31 |
| Maternal height (cm) | |||||
| <145 | 4,654 | 806 | 1.00 | 1.00 | |
| 145–148 | 5,331 | 1,077 | 1.16 | 1.05 | 0.75, 1.48 |
| 149–155 | 7,294 | 1,351 | 1.07 | 1.07 | 0.80, 1.45 |
| ≥156 | 3,605 | 677 | 1.08 | 1.29 | 0.85, 1.97 |
| Weight-for-height z score | |||||
| Wasted | 195 | 32 | 1.13 | 1.02 | 0.82, 1.27 |
| Normal | 16,358 | 3,216 | 1.00 | 1.00 | |
| Above normal | 4,331 | 663 | 0.91 | 0.94 | 0.76, 1.17 |
| Height-for-age z score | |||||
| Stunted | 2,683 | 632 | 1.29 | 1.78 | 0.82, 3.89 |
| Normal | 14,439 | 2,655 | 1.00 | 1.00 | |
| Above normal | 3,762 | 624 | 0.90 | 1.08 | 0.86, 1.35 |
Nutritional status
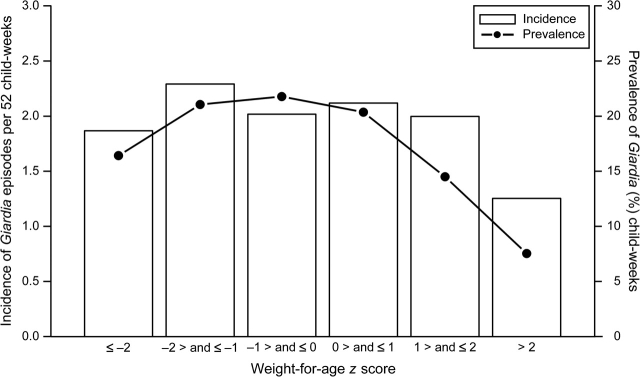
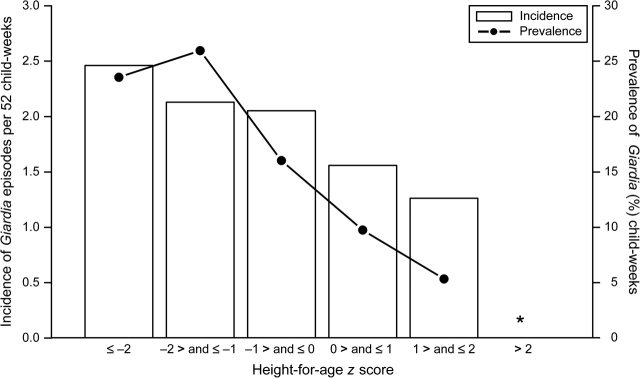
Twenty-six children (12 percent) were underweight, 97 children (44 percent) were stunted, and 14 children were wasted (6 percent) during at least 1 child-month of follow-up. Eighteen children (8 percent) were both underweight and stunted, and four children (2 percent) were both stunted and wasted during at least 1 child-month of follow-up. There was no significant trend in the incidence of Giardia episodes per 52 child-weeks and in the prevalence of Giardia-positive child-weeks, by WAZ (figure 2) and HAZ (figure 3). In single-variable analysis, the weekly risk of Giardia infection decreased by 15 percent for each unit of z-score increase in WAZ (RR = 0.85, 95 percent CI: 0.76, 0.95); decreased by 27 percent for each unit of z-score increase in HAZ (RR = 0.73, 95 percent CI: 0.65, 0.82), and remained unchanged by each unit of z-score increase in WHZ (RR = 1.00, 95 percent CI: 0.91, 1.11). The adjusted model, however, revealed that the risk of Giardia infection was not associated with WAZ (RR = 1.00, 95 percent CI: 0.89, 1.12), HAZ (RR = 0.92, 95 percent CI: 0.82, 1.04), or WHZ (RR = 1.04, 95 percent CI: 0.94, 1.14). Age at the time of Giardia infection was the strongest confounder of the relation between nutritional status and risk of Giardia.
FIGURE 2.
Unadjusted incidence and prevalence of Giardia by categories of weight for age, Lima, Peru, 1995–1998. We estimated incidence as the number of new Giardia episodes per 52 child-weeks and prevalence of Giardia as the proportion of Giardia-positive child-weeks. The bars represent the incidence of Giardia, and the filled circles connected by lines represent the prevalence of Giardia. Although single-variable analysis suggests that the risk of giardiasis varies with weight for age, in the adjusted model we did not find an association between infection with Giardia and weight for age (relative risk = 1.00, 95% confidence interval: 0.89, 1.12).
FIGURE 3.
Unadjusted incidence and prevalence of Giardia by categories of height for age, Lima. Peru, 1995–1998. We estimated incidence as the number of new Giardia episodes per 52 child-weeks and prevalence of Giardia as the proportion of Giardia-positive child-weeks. The bars represent the incidence of Giardia, and the filled circles connected by lines represent the prevalence of Giardia. “*” indicates that there were insufficient data points in the category of height-for-age z score greater than 2 to accurately estimate the incidence or prevalence of Giardia. Although single-variable analysis suggests that the risk of giardiasis varies with height for age, in the adjusted model we did not find an association between infection with Giardia and height for age (relative risk = 0.92, 95% confidence interval: 0.82, 1.04).
Diarrhea
Diarrheal incidence was previously estimated at 3.2 episodes per child-year for this cohort (15). We recorded an episode of diarrhea in 6 percent (235/3,911) of Giardia-positive child-weeks and in 6.2 percent (1,055/16,973) of Giardia-negative child-weeks. The median duration of a diarrheal episode was 2 days (range: 1–47 days). Giardia was not associated with diarrhea (RR = 0.95, 95 percent CI: 0.79, 1.13), after adjustment for age, gender, weight for age, height for age, water and sanitation score, seasonal quarters, and breastfeeding status. Giardia was not associated with diarrhea even after age stratification (table 3).
TABLE 3.
Relative risk of diarrhea from Giardia intestinalis, by age in months, for children living in Las Pampas de San Juan, Lima, Peru, between 1995 and 1998, with and without adjustment for gender, season, nutritional status, and breastfeeding status*
| Age (months) | No. of child-weeks |
Crude relative risk | Adjusted relative risk | 95% confidence interval | |
| G+D+/G+ (n = 235/3,911) | G−D+/G− (n = 1,055/16,973) | ||||
| 0–5 | 10/188 | 157/4,404 | 1.57 | 1.56 | 0.7, 3.3 |
| 6–11 | 25/402 | 319/4,321 | 0.88 | 0.86 | 0.6, 1.2 |
| 12–17 | 70/762 | 298/3,291 | 1.01 | 1.02 | 0.8, 1.4 |
| 18–23 | 49/901 | 146/2,289 | 0.84 | 1.00 | 0.7, 1.4 |
| 24–35 | 81/1,658 | 135/2,668 | 1.00 | 0.94 | 0.7, 1.4 |
The risk of developing Giardia-associated diarrhea was calculated by dividing the number of Giardia-positive and diarrhea-positive (G+D+) child-weeks by the number of Giardia-positive (G+) child-weeks. The risk of developing diarrhea due to other causes was calculated by dividing the number of Giardia-negative and diarrhea-positive (G−D+) child-weeks by the number of Giardia-negative (G−) child-weeks.
Growth
Giardiasis did not affect child growth at 1 or 2 months following the onset of first infection with Giardia, despite age stratification (table 4). Overall, children who became infected with Giardia for the first time gained, 1 month after infection, 0.03 kg less (95 percent CI: −0.14, 0.07) and grew 0.01 cm less (95 percent CI: −0.24, 0.21) than noninfected children, after adjustment for age, gender, diarrhea, water and sanitation, maternal height, and per capita household income. This lack of effect persisted in the second month following infection with Giardia. Two months after infection, children who became infected with Giardia for the first time gained 0.08 kg more (95 percent CI: −0.03, 0.18) and grew 0.09 cm more (95 percent CI: −0.14, 0.32) than noninfected children.
TABLE 4.
Monthly and bimonthly weight and height gain difference, by age in months, between Giardia-infected and noninfected children living in Las Pampas de San Juan, Lima, Peru, between 1995 and 1998, with and without adjustment for gender, diarrhea, sanitation score, maternal height, and per capita household income
| Age category (months) | No. of growth intervals |
Crude weight gain difference | Adjusted weight gain difference | 95% confidence interval | Crude height gain difference | Adjusted height gain difference | 95% confidence interval | |
| Total | Giardia infected | |||||||
| Monthly growth | ||||||||
| 0–5 | 446 | 18 | 0.09 | 0.09 | −0.18, 0.36 | −0.37 | −0.37 | −1.04, 0.31 |
| 6–11 | 550 | 31 | −0.12 | −0.11 | −0.30, 0.09 | 0.12 | 0.04 | −0.37, 0.45 |
| 12–17 | 356 | 41 | −0.05 | −0.04 | −0.22, 0.14 | 0.09 | 0.10 | −0.25, 0.45 |
| 18–23 | 169 | 23 | 0.06 | 0.08 | −0.15, 0.32 | 0.01 | 0.00 | −0.48, 0.47 |
| 24–35 | 90 | 11 | −0.15 | −0.24 | −0.59, 0.12 | −0.05 | −0.08 | −0.72, 0.56 |
| Bimonthly growth | ||||||||
| 0–5 | 299 | 33 | 0.10 | 0.11 | −0.15, 0.37 | 0.06 | 0.11 | −0.45, 0.67 |
| 6–11 | 425 | 59 | −0.03 | −0.01 | −0.17, 0.16 | 0.07 | 0.07 | −0.28, 0.43 |
| 12–17 | 209 | 41 | 0.15 | 0.19 | −0.02, 0.39 | 0.02 | 0.05 | −0.34, 0.45 |
| 18–23 | 77 | 12 | 0.04 | 0.02 | −0.42, 0.45 | 0.37 | 0.11 | −0.78, 1.01 |
| 24–35 | 42 | 11 | 0.35 | 0.24 | −0.14, 0.61 | −0.02 | 0.25 | −0.47, 0.96 |
In the subset analysis of 108 children with height measurements between 6 and 24 months of age, the longitudinal prevalence of Giardia infection did not explain height gain (0 cm per percent increase in longitudinal prevalence of Giardia infection; p = 0.981). Regression diagnostics did not reveal outliers or influential data points.
DISCUSSION
In our study population, Giardia infection did not adversely affect child health. Giardiasis was ubiquitous: 85 percent of children acquired Giardia, and 87 percent of these became reinfected. Malnutrition was not a risk factor for Giardia infection. Moreover, children infected with Giardia were not at greater risk of developing diarrhea or at greater risk of slower weight or height gain than were noninfected children. Repeated Giardia infections between 6 and 24 months of age did not adversely affect height gain in this interval.
Our finding that giardiasis did not affect childhood growth is consistent with results from other studies that also did not find any growth effects (11, 14) or showed positive growth effects (12) following Giardia infection. Although one clinical trial found that Giardia-positive children who received monthly treatment with metranidazole grew better than those who did not receive metrodinazole (9), it is unclear whether this effect was due to the elimination of Giardia or due to the elimination of some other enteric pathogen. The proportion of malnourished children in our study was in the range of that found in other Giardia studies conducted in different countries (13, 15, 22, 23). These studies reported that 0.6–23 percent of children were underweight, and 2.4–40 percent of children were stunted.
Nutrient malabsorption has been previously reported in at least 50 percent of patients with symptomatic giardiasis (13, 22, 24). Cross-sectional (13, 22, 24) and longitudinal (6, 7) studies have found that symptomatic giardiasis delayed childhood growth. Although diarrhea can cause growth delays up to 4 months after a diarrheal episode (17, 18, 25, 26), the longitudinal prevalence of Giardia between 6 and 24 months was not associated with height gain between 6 and 24 months of age. Our study also did not identify many subjects who experienced giardiasis with diarrhea. This finding is consistent with those from a previous study on Giardia infection in Peru (19).
Our analytical approach avoids reverse causality bias by including only growth intervals up to 2 months following the onset of first infection with Giardia. We controlled for the effect of other diarrheal pathogens that may affect growth by accounting for diarrhea. Furthermore, a subset analysis of children with height measurements between 6 and 24 months of age failed to show a relation between longitudinal prevalence of Giardia and height gain. One possible explanation for the lack of growth effects due to giardiasis is that some Giardia strains may be more pathogenic than others (27). Geographic differences in Giardia genotypes could also account for the inconsistency of results seen in the literature.
Although Giardia is a well-recognized cause of diarrhea in developed countries, we did not find an association between infection with Giardia and diarrhea. Our observations support findings in other countries (1, 6, 8, 28, 29) that found no link between Giardia and diarrhea in regions where Giardia is hyperendemic. It is unclear why differences in giardiasis between developed and developing countries may exist. One possibility is that multiple infections during early childhood may have primed cell-mediated immune responses or may have resulted in tolerance due to early exposure to Giardia antigens, enabling infections with Giardia later in life to be asymptomatic. Previous Peruvian studies have documented that children experience high rates of Giardia reinfection (19) and that children attain adult levels of seropositivity against Giardia by 6 months of age (30). Another reason why we may have not found an association between Giardia and diarrhea is that we did not examine stool samples obtained during a diarrheal episode. Our definition, however, allowed for a match of up to 7 days between a Giardia-positive child-week and a diarrheal episode. Additionally, we may have missed symptomatic Giardia cases associated with soft, greasy stools rather than with diarrhea. Finally, light microscopy is a commonly used method of detection for Giardia (31), but it primarily detects cysts as trophozoites may die by the time stool samples arrive and are examined in the laboratory. Given that trophozoite detection is strongly correlated with symptomatic giardiasis (32), fewer trophozoites could have diluted the risk of Giardia-associated diarrhea toward the null hypothesis. As with Cryptosporidium, detection of Giardia in the stool is further complicated if the parasitic load in stool falls below the microscope's minimal level of detection (33). This may give the appearance that Giardia is shed intermittently (31). We therefore minimized nondifferential misclassification of exposure status by defining Giardia infection using episodes, rather than monthly or periodic parasite excretion, as previous studies had done (6, 8, 12–14, 22, 24). Although fluorescent microscopy is the “gold standard” for detection of Giardia, in our laboratory we have recently found that formal ether stool concentration followed by light microscopy in the hands of very experienced microscopists has sensitivity similar to that of fluorescent microscopy for detection of Giardia (Vitaliano Cama, Johns Hopkins Bloomberg School of Public Health, personal communication, 2007). A previous study also reported that microscopic analysis of stools had equal sensitivity in diagnosing infection as did an enzyme-linked immunosorbent antigen assay (34).
In summary, our data suggest that malnutrition was not a risk factor for giardiasis and that giardiasis did not affect child growth. Our findings question the importance of Giardia as a childhood pathogen in developing countries where giardiasis is hyperendemic.
Acknowledgments
Work for this study was provided in part by a National Research Service Award of the National Institutes of Health (F31-HD08488) to Dr. William Checkley and in part by an International Centers for Tropical Disease Research project grant of the National Institute of Allergy and Infectious Diseases awarded to the Johns Hopkins Bloomberg School of Public Health (U01-A135894). Financial support was also provided by the RG-ER Fund, an anonymous organization that is deeply concerned with childhood health in developing countries.
The authors thank Maria-Victoria Zunzunegui, Lise Seguin, and Pierre Philippe for comments on an earlier version of this manuscript and Paula Maguiña and Yung-Chieh Yen for administrative assistance.
This work was presented in part at the 19th Annual Meeting of the Society for Pediatric and Perinatal Epidemiology, Seattle, Washington, June 20–21, 2006 (abstract B62), and at the Second North American Congress of Epidemiology, Seattle, Washington, June 21–24, 2006 (abstract 127-S), where it was awarded a first place prize for a poster presentation.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- HAZ
height-for-age z score
- RR
relative risk
- WAZ
weight-for-age z score
- WHZ
weight-for-height z score
References
- 1.Fraser D, Dagan R, Naggan L, et al. Natural history of Giardia lamblia and Cryptosporidium infections in a cohort of Israeli Bedouin infants: a study of a population in transition. Am J Trop Med Hyg. 1997;57:544–9. doi: 10.4269/ajtmh.1997.57.544. [DOI] [PubMed] [Google Scholar]
- 2.Ali S, Hill D. Giardia intestinalis. Curr Opin Infect Dis. 2003;16:453–60. doi: 10.1097/00001432-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Moreira-Lima AA. Tropical diarrhea: new developments in traveller's diarrhea. Curr Opin Infect Dis. 2001;14:547–52. doi: 10.1097/00001432-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RC, Reynoldson JA, Mendis AH. Giardia and giardiasis. Adv Parasitol. 1993;32:71–160. doi: 10.1016/s0065-308x(08)60207-9. [DOI] [PubMed] [Google Scholar]
- 5.Black RE, Brown KH, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73:799–805. [PubMed] [Google Scholar]
- 6.Farthing MJ, Mata L, Urrutia JJ, et al. Natural history of Giardia infection of infants and children in rural Guatemala and its impact on physical growth. Am J Clin Nutr. 1986;43:395–405. doi: 10.1093/ajcn/43.3.395. [DOI] [PubMed] [Google Scholar]
- 7.Cole TJ, Parkin JM. Infection and its effect on the growth of young children: a comparison of The Gambia and Uganda. Trans R Soc Trop Med Hyg. 1977;71:196–8. doi: 10.1016/0035-9203(77)90005-0. [DOI] [PubMed] [Google Scholar]
- 8.Fraser D, Bilenko N, Deckelbaum RJ, et al. Giardia lamblia carriage in Israeli Bedouin infants: risk factors and consequences. Clin Infect Dis. 2000;30:419–24. doi: 10.1086/313722. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Srivastava G. Drug therapy for Giardia infestation. Indian Pediatr. 1978;15:687–9. [PubMed] [Google Scholar]
- 10.Prado M, Cairncross S, Strina A, et al. Asymptomatic giardiasis and growth in young children; a longitudinal study in Salvador, Brazil. Parasitology. 2005;131:51–6. doi: 10.1017/s0031182005007353. [DOI] [PubMed] [Google Scholar]
- 11.Lunn PG, Erinoso HO, Northrop-Clewes CA, et al. Giardia intestinalis is unlikely to be a major cause of the poor growth of rural Gambian infants. J Nutr. 1999;129:872–7. doi: 10.1093/jn/129.4.872. [DOI] [PubMed] [Google Scholar]
- 12.Ish-Horowicz M, Korman SH, Shapiro M, et al. Asymptomatic giardiasis in children. Pediatr Infect Dis J. 1989;8:773–9. doi: 10.1097/00006454-198911000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Sackey ME, Weigel MN, Armijos RX. Predictors and nutritional consequences of intestinal parasitic infections in rural Ecuadorian children. J Trop Pediatr. 2003;49:17–23. doi: 10.1093/tropej/49.1.17. [DOI] [PubMed] [Google Scholar]
- 14.Campbell DI, McPhail G, Lunn PG, et al. Intestinal inflammation measured by fecal neopterin in Gambian children with enteropathy: association with growth failure, Giardia lamblia, and intestinal permeability. J Pediatr Gastroenterol Nutr. 2004;39:153–7. doi: 10.1097/00005176-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Muniz PT, Ferreira MU, Ferreira CS, et al. Intestinal parasitic infections in young children in Sao Paulo, Brazil: prevalences, temporal trends and associations with physical growth. Ann Trop Med Parasitol. 2002;96:503–12. doi: 10.1179/000349802125001311. [DOI] [PubMed] [Google Scholar]
- 16.Checkley W, Gilman RH, Black RE, et al. Effect of water and sanitation on childhood health in a poor Peruvian peri-urban community. Lancet. 2004;363:112–18. doi: 10.1016/S0140-6736(03)15261-0. [DOI] [PubMed] [Google Scholar]
- 17.Checkley W, Gilman R, Black R, et al. Effects of nutritional status on diarrhea in Peruvian children. J Pediatr. 2002;140:210–18. doi: 10.1067/mpd.2002.121820. [DOI] [PubMed] [Google Scholar]
- 18.Checkley W, Epstein LD, Gilman RH, et al. Effects of acute diarrhea on linear growth in Peruvian children. Am J Epidemiol. 2003;157:166–75. doi: 10.1093/aje/kwf179. [DOI] [PubMed] [Google Scholar]
- 19.Gilman RH, Marquis GS, Miranda E, et al. Rapid reinfection by Giardia lamblia after treatment in a hyperendemic Third World community. Lancet. 1988;1:343–5. doi: 10.1016/s0140-6736(88)91131-2. [DOI] [PubMed] [Google Scholar]
- 20.Black RE, Lanata CF. Epidemiology of diarrheal diseases in developing countries. Infections of the gastrointestinal tract. In: Blaser MJ, Smith PD, Greenberg HB, et al., editors. New York, NY: Raven Press; 1995. pp. 17–35. [Google Scholar]
- 21.WHO. Geneva, Switzerland: World Health Organization; 2006. child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age methods and development. [Google Scholar]
- 22.Simsek Z, Zeyrek FY, Kurcer MA. Effect of Giardia infections on growth and psychomotor development of children aged 0–5 years. J Trop Pediatr. 2004;50:90–3. doi: 10.1093/tropej/50.2.90. [DOI] [PubMed] [Google Scholar]
- 23.Newman RD, Moore SR, Lima AA, et al. A longitudinal study of Giardia lamblia infection in north-east Brazilian children. Trop Med Int Health. 2001;6:624–34. doi: 10.1046/j.1365-3156.2001.00757.x. [DOI] [PubMed] [Google Scholar]
- 24.Celiksoz A, Acioz M, Degereli S, et al. Effects of giardiasis on school success, weight and height indices of primary school children in Turkey. Pediatr Int. 2005;47:567–71. doi: 10.1111/j.1442-200x.2005.02110.x. [DOI] [PubMed] [Google Scholar]
- 25.Morrow AL, Reves RR, West MS, et al. Protection against infection with Giardia lamblia by breast-feeding in a cohort of Mexican infants. J Pediatr. 1992;121:363–70. doi: 10.1016/s0022-3476(05)81787-1. [DOI] [PubMed] [Google Scholar]
- 26.Alam DS, Marks GC, Baqui AH, et al. Association between clinical type of diarrhoea and growth of children under 5 years in rural Bangladesh. Int J Epidemiol. 2000;29:916–21. doi: 10.1093/ije/29.5.916. [DOI] [PubMed] [Google Scholar]
- 27.Read C, Walters J, Robertson ID, et al. Correlation between genotype of Giardia duodenalis and diarrhoea. Int J Parasitol. 2002;32:229–31. doi: 10.1016/s0020-7519(01)00340-x. [DOI] [PubMed] [Google Scholar]
- 28.Gilman RH, Brown KH, Visvesvara GS, et al. Epidemiology and serology of Giardia lamblia in a developing country: Bangladesh. Trans R Soc Trop Med Hyg. 1985;79:469–73. doi: 10.1016/0035-9203(85)90068-9. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan PS, DuPont HL, Arafat RR, et al. Illness and reservoirs associated with Giardia lamblia infection in rural Egypt: the case against treatment in developing world environments of high endemicity. Am J Epidemiol. 1988;127:1272–81. doi: 10.1093/oxfordjournals.aje.a114919. [DOI] [PubMed] [Google Scholar]
- 30.Miotti PG, Gilman RH, Santosham M, et al. Age-related rate of sero-positivity of antibody to Giardia lamblia in four diverse populations. J Clin Microbiol. 1986;24:972–5. doi: 10.1128/jcm.24.6.972-975.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janoff EN, Craft JC, Pickering LK, et al. Diagnosis of Giardia lamblia infections by detection of parasite-specific antigens. J Clin Microbiol. 1989;27:431–5. doi: 10.1128/jcm.27.3.431-435.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortega YR, Adam RD. Giardia: overview and update. Clin Infect Dis. 1997;25:545–9. doi: 10.1086/513745. [DOI] [PubMed] [Google Scholar]
- 33.Priest JW, Bern C, Roberts JM, et al. Changes in serum immunoglobulin G levels as a marker for Cryptosporidium sp. infection in Peruvian children. J Clin Microbiol. 2005;43:5298–300. doi: 10.1128/JCM.43.10.5298-5300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nash TE, Herrington DA, Levine MM. Usefulness of an enzyme-linked immunosorbent assay for detection of Giardia antigen in feces. J Clin Microbiol. 1987;25:1169–71. doi: 10.1128/jcm.25.7.1169-1171.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]