Abstract
In epidemiologic studies and in studies of discordant twins, cigarette smoking has been consistently associated with a lower risk of Parkinson's disease, but whether this association is causal remains controversial. Alternatively, an infectious or toxic exposure in childhood or early adulthood could affect both the reward mechanisms that determine smoking behavior and the future risk of Parkinson's disease. If so, parental smoking, commonly established before the birth of the first child, would be unlikely to be related to Parkinson's disease risk. The authors assessed the association between Parkinson's disease and parental smoking during childhood in the Nurses’ Health Study and the Health Professionals Follow-up Study conducted in the United States. During 26 years and 18 years of follow-up, respectively, 455 newly diagnosed Parkinson's disease cases were documented among those who provided information on parental smoking. The age-adjusted, pooled relative rate of Parkinson's disease was 0.73 (95% confidence interval: 0.53, 1.00; P-trend = 0.04) comparing participants who reported that both parents smoked with those who reported that neither did. Adjustment for caffeine and alcohol intake did not materially change the results. If the inverse association between smoking and Parkinson's disease were due to confounding by an environmental factor or were the result of reverse causation, it is unlikely that parental smoking would predict Parkinson's disease.
Keywords: causality, Parkinson disease, smoking, tobacco smoke pollution
Despite the well-documented adverse health effects of cigarette smoking, cigarette smokers and users of other tobacco products have lower rates of Parkinson's disease than nonsmokers/nonusers do. Rates of Parkinson's disease decrease as dose of cigarettes per day and number of years of smoking increase, and they increase as number of years since quitting increases (1–5). In longitudinal studies, current smokers, compared with never smokers, were found to have one-third the risk of Parkinson's disease (5). Among men never smokers, use of smokeless tobacco was also associated with lower rates of Parkinson's disease (6, 7). In an ecologic study, country-specific variations in gender ratios of Parkinson's disease over time were found to be correlated with variations in gender ratios of smoking (8).
Whether the observed inverse associations between smoking and Parkinson's disease are causal remains controversial; the association could be explained by a still-unknown third factor that increases the risk of Parkinson's disease and also causes an aversion to smoking. This factor is unlikely to be genetic because, in monozygotic and dizygotic twin studies, which control tightly for genetics and shared environment, smoking remains associated with Parkinson's disease (9, 10). However, confounding by an environmental factor cannot be eliminated by twin studies. For example, a person could be exposed to a toxic chemical or an infectious agent that causes subclinical damage to the dopaminergic system, which is involved in novelty seeking and addiction, and this exposure could reduce his or her desire to smoke while independently increasing the risk of Parkinson's disease. This apparently intractable confounding could be circumvented by examining the relation between parental smoking and risk of Parkinson's disease because the hypothetical toxic chemical or infectious agent is less likely to affect parents’ smoking behavior, which is typically established before the birth of the first child (11). Children of smokers are exposed to environmental smoke and are more likely to become smokers themselves, so if smoking is truly protective, they would be expected to have a lower risk of Parkinson's disease than children of parents who do not smoke. On the other hand, if the association between smoking and Parkinson's disease were due to exposure to a toxic chemical or an infectious agent, no relation would be expected between parental smoking, which was established prior to the offspring being exposed, and Parkinson's disease risk. We examined the association between parental smoking and Parkinson's disease rates in the Nurses’ Health Study and Health Professionals Follow-up Study in the United States.
MATERIALS AND METHODS
Study population
The Nurses' Health Study enrolled 121,701 female nurses aged 35–55 years who returned a mailed questionnaire in 1976 regarding lifestyle and medical history (12). The Health Professionals Follow-up Study enrolled 51,529 males aged 40–75 years who returned a similar questionnaire in 1986 (13). Participants of both cohorts have received follow-up questionnaires biennially to record newly diagnosed illnesses and to update lifestyle and dietary information.
Case ascertainment
Health Professionals Follow-up Study and Nurses’ Health Study participants were first asked about a lifetime diagnosis of Parkinson's disease on the 1988 and 1994 questionnaires, respectively, and on every follow-up questionnaire thereafter. Confirmation of self-reported Parkinson's disease has been described elsewhere (14). Briefly, permission to contact the treating neurologist was sought from participants who reported a new diagnosis of Parkinson's disease. Neurologists (or internists if the neurologists did not respond) were then asked to complete a questionnaire to provide their judgment on the certainty of the diagnosis, the date on which symptoms were first noticed, and the date of diagnosis; copies of medical records were also sought. Confirmed cases of Parkinson's disease included those for which 1) the level of certainty of the diagnosis reported by the treating neurologist or internist was definite or probable, 2) the medical record indicated a final diagnosis of Parkinson's disease by a neurologist, or 3) there was evidence at a neurologic examination of at least 2 of the 3 cardinal signs of Parkinson's disease (rest tremor, rigidity, or bradykinesia) in the absence of features suggesting other diagnoses. A movement disorder specialist, blinded to exposure status, reviewed the medical records. Overall, the diagnosis was confirmed by the treating neurologist in 84.6% and by the treating internist in 9.7% of the cases and by review of the medical records in the remainder.
Exposure assessment
In both cohorts (women in 1982 and men in 2004), participants reported whether their parents (neither, mother only, father only, both) smoked while living with them during childhood. To gain statistical power, the categories “mother only” and “father only” were collapsed to “one parent smoked.”
Statistical methods
Participants contributed person-time of follow-up from the date of return of the baseline questionnaire to the date of Parkinson's disease diagnosis, death from any cause, or end of follow-up (June 2002 for the Nurses’ Health Study and January 2004 for the Health Professionals Follow-up Study). Mantel-Haenzel age-adjusted incidence relative rates and 95% confidence intervals were obtained relative to the incidence rates for those whose parents did not smoke. To adjust for other risk factors for Parkinson's disease, we used Cox proportional hazards analysis. A Wald test determined whether the relative rate from each study was heterogeneous. A pooled relative rate was calculated by weighting the study-specific log relative rate by the inverse of their variances using a random-effects model (15). All reported P values are 2-sided.
RESULTS
During 26 years of follow-up in the Nurses’ Health Study, 328 newly diagnosed Parkinson's disease cases were documented. The 1982 questionnaire was completed by 110,163 eligible women; of these, 92,921 gave information on parental smoking (287 cases). During 18 years of follow-up in the Health Professionals Follow-up Study, 384 newly diagnosed Parkinson's disease cases were documented. The 2004 questionnaire was completed by 34,884 eligible men; of these, 30,668 gave information on parental smoking (168 cases). The average age at diagnosis of Parkinson's disease was 68.8 years (range: 46.6–87.8) for the men and 69.3 (range: 46.3–81.3) for the women.
As expected, participants’ baseline smoking status was predicted by parental smoking. Ever smokers constituted 69% of the women and 57% of the men whose parents were both smokers but only 46% of the women (P < 0.0001) and 41% of the men (P < 0.0001) whose parents were both nonsmokers. We calculated expected relative rates of Parkinson's disease for each level of parental smoking status as the averages of published study-specific relative rates for pack-years (2) weighted by the distribution of pack-years of smoking at baseline within each category of parental smoking. We conservatively predicted a reduction in risk of Parkinson's disease of 11% for men and 13% for women who reported that both parents smoked compared with those who reported that neither parent smoked. Daily caffeine and alcohol intake was higher among those whose parents smoked (Table 1).
Table 1.
Age-adjusted Baseline Risk Factors for Parkinson's Disease by Parental Smoking Status in the Nurses’ Health Study (1976) and the Health Professionals Follow-up Study (1986), United States
| Parental Smoking Status |
||||||
| Nurses' Health Study |
Health Professionals Follow-up Study |
|||||
| Neither Parent | 1 Parent | Both Parents | Neither Parent | 1 Parent | Both Parents | |
| Total no. (no. of cases) | 30,182 (104) | 45,562 (154) | 17,177 (29) | 11,907 (81) | 13,633 (66) | 5,128 (21) |
| Mean (range) age, years | 44 (30–63) | 44 (30–57) | 39 (30–60) | 53 (39–77) | 53 (39–77) | 47 (39–77) |
| Caffeine intake, g/day | 365 | 397 | 407 | 214 | 243 | 248 |
| Alcohol intake, g/day | 5.4 | 6.4 | 8.3 | 9.6 | 11.8 | 14.1 |
| Smoking, % | ||||||
| Never | 54.0 | 43.0 | 31.4 | 58.8 | 47.0 | 42.7 |
| <10 pack-years | 17.6 | 19.2 | 18.5 | 10.7 | 10.8 | 10.6 |
| 10–24 pack-years | 16.0 | 20.2 | 24.6 | 17.2 | 21.2 | 21.7 |
| 25–44 pack-years | 8.8 | 12.5 | 18.0 | 9.6 | 14.9 | 17.5 |
| ≥45 pack-years | 2.5 | 3.7 | 6.2 | 3.7 | 6.1 | 7.5 |
| Predicted relative rate based on pack-yearsa | Reference | 0.95 | 0.89 | Reference | 0.90 | 0.87 |
Predicted relative rates of Parkinson's disease for parental smoking were calculated as an average of the relative rates for pack-years published in the study by Hernán et al. (2) weighted by the distribution of smoking history at baseline within each level of parental smoking. (Compared with never smoking, the published relative rates for 1–9, 10–24, 25–44, and ≥45 pack-years were 1.0, 0.8, 0.4, and 0.4 in the Nurses’ Health Study and 0.6, 0.5, 0.5, and 0.3 in the Health Professionals Follow-up Study, respectively.)
Among both men and women, the risk of Parkinson's disease was lower for individuals who reported that both parents were smokers than for those who reported that neither parent smoked (Table 2). In a pooled analysis, those reporting that both parents were smokers had a 27% lower risk of Parkinson's disease than those reporting that neither parent smoked (relative rate = 0.73, 95% confidence interval: 0.53, 1.00; P-trend = 0.04) (Table 2). We further adjusted the pooled data for caffeine and alcohol intake at baseline and found that the effect estimate did not materially change but, as expected, that confidence intervals were wider (pooled relative rate = 0.76, 95% confidence interval: 0.55, 1.05).
Table 2.
Parental Smoking Status and Risk of Parkinson's Disease in the Nurses' Health Study (1976–2002) and the Health Professionals Follow-up Study (1986–2004), United States
| Parental Smoking Status | No. of Person-Years | No. of Cases | Age-adjusted RR | 95% CI | P for Heterogeneity |
| Health Professionals Follow-up Study | |||||
| Neither parent smoked | 208,563 | 81 | Reference | ||
| At least 1 parent smoked | 328,860 | 87 | 0.73 | 0.54, 0.99 | |
| 1 Parent smoked | 238,655 | 66 | 0.72 | 0.52, 0.99 | |
| Both parents smoked | 90,205 | 21 | 0.80 | 0.49, 1.30 | |
| Total | 537,423 | 168 | |||
| Nurses' Health Study | |||||
| Neither parent smoked | 755,123 | 104 | Reference | ||
| At least 1 parent smoked | 1,569,776 | 183 | 0.94 | 0.74, 1.20 | |
| 1 Parent smoked | 1,137,480 | 154 | 1.01 | 0.79, 1.30 | |
| Both parents smoked | 432,296 | 29 | 0.68 | 0.45, 1.03 | |
| Total | 2,324,899 | 287 | |||
| Pooled Analysis | |||||
| Neither parent smoked | 963,686 | 185 | Reference | ||
| At least 1 parent smoked | 1,898,636 | 270 | 0.85 | 0.67, 1.07 | 0.2 |
| 1 Parent smoked | 1,376,135 | 220 | 0.87 | 0.64, 1.20 | 0.1 |
| Both parents smoked | 522,501 | 50 | 0.73 | 0.53, 1.00 | 0.6 |
| Total | 2,862,322 | 455 | |||
| P for trend | 0.04 | 0.6 | |||
Abbreviations: CI, confidence interval; RR, relative rate.
We then restricted the multivariable pooled analysis to never smokers at baseline. There was no association between parental smoking and Parkinson's disease, as would be expected if the effect of parental smoking is primarily mediated through smoking by the offspring.
DISCUSSION
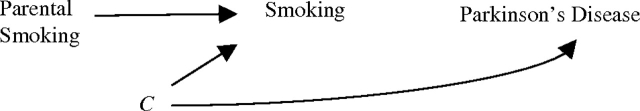
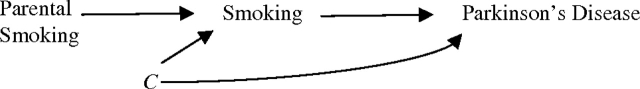
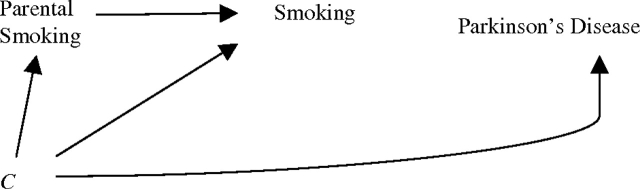
In this pooled analysis of 2 large cohorts, risk of Parkinson's disease was 27% lower for individuals whose parents smoked than for individuals whose parents were nonsmokers. An inverse association between smoking and Parkinson's disease has been found in numerous epidemiologic studies, but consensus is lacking as to whether the relation is causal, due to confounding, or a result of reverse causation. If the desire to initiate or continue smoking and Parkinson's disease were both independently affected by a third factor, then parental smoking should not be related to Parkinson's disease risk. This causal structure is illustrated with directed acyclic graphs (Figure 1) (16, 17). Under the paradigm of reverse causation, where subclinical disease influences smoking behavior, parental smoking would also not predict Parkinson's disease in offspring (Figure 2). Were smoking truly protective against Parkinson's disease, and parental smoking predicted smoking, then parental smoking would be expected to be inversely associated with Parkinson's disease, as we found (Figure 3).
Figure 1.
Paradigm I: The inverse relation between smoking and Parkinson's disease is not causal. The observed association is due to confounding. C represents one or more factors causally related to both Parkinson's disease and smoking status that, given it exists, would confound the association between smoking and Parkinson's disease. Under this paradigm, parental smoking would not be associated with Parkinson's disease risk because there is no open path between parental smoking and Parkinson's disease. Such a path could be provided by an influence of parental smoking on caffeine intake under the assumption that caffeine is causally related to Parkinson's disease (not shown in figure) (5, 14). Because adjustment for caffeine intake did not attenuate the relation between parental smoking and Parkinson's disease, such an explanation is unlikely.
Figure 2.
Paradigm II: Parkinson's disease prevents smoking. The observed association is due to reverse causation. Because there is no open path between parental smoking and Parkinson's disease, no association would be expected.
Figure 3.
Paradigm III: The inverse relation between smoking and Parkinson's disease is causal. C represents one or more factors causally related to Parkinson's disease and smoking status that, given it exists, could confound the association between smoking and Parkinson's disease. The effect of parental smoking on Parkinson's disease is probably mediated through its effects on individuals’ smoking status because there are no alternative open paths between parental smoking and Parkinson's disease even in the presence of C; the association, as illustrated, is causal.
An inverse association between parental smoking and Parkinson's disease could exist under other scenarios. The possibility cannot be ruled out, for example, that an exposure such as a toxic or infectious agent in a family could affect parental smoking behavior as well as the future risk of Parkinson's disease for both parents and children (Figure 4). Alternatively, a toxic or infectious agent may be more common in a household where there is parental smoking (Figure 5). Such an exposure would have to be transgenerational, affecting both parent and child in early life, and to be present in all of the settings in which the association between smoking and Parkinson's disease has been found.
Figure 4.
Paradigm IV: Parental smoking and Parkinson's disease are confounded. C represents a factor causally related to both parental smoking and Parkinson's disease. An assumption underlying the conclusion that smoking could be causally related to Parkinson's disease is the nonexistence of C.
Figure 5.
Paradigm V: Parental smoking is associated with an independent cause of Parkinson's disease. C represents a factor that is causally related to Parkinson's disease and is associated with parental smoking, although not causally. U represents a predictor of both parental smoking and C. Under this paradigm, the inverse association between parental smoking and Parkinson's disease would not be a result of a causal relation between smoking and Parkinson's disease.
Our analysis provides a novel approach to assessing causality, and, when taken with the results of previous longitudinal (1–5) and ecologic (8) studies, suggests a causal interpretation of the relation between smoking and Parkinson's disease over confounding or reverse causation. However, causality cannot be proven until a mechanism is elucidated (18).
Our interest in smoking and Parkinson's disease is etiologic. Smoking is hugely damaging to health; any benefit derived from a reduction in risk of Parkinson's disease is outweighed by the increased risks of cancer and cardiovascular disease. This, however, should not be an impediment to evaluating tobacco components for possible neuroprotective effects.
Acknowledgments
Author affiliations: Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Éilis J. O’Reilly, Alberto Ascherio); Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Éilis J. O’Reilly, Xiang Gao, Alberto Ascherio); Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Honglei Chen); Department of Neurology, Miller School of Medicine, University of Miami, Miami, Florida (Hannah Gardener); Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts (Michael A. Schwarzschild); and Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (Alberto Ascherio).
The study was supported by a National Institutes of Health/National Institute of Neurological Diseases and Stroke grant (to A. A.) to study Parkinson’s disease in the Health Professionals Follow-up Study and Nurses’ Health Study cohorts and by the intramural program of the National Institutes of Health, the National Institute of Environmental Health Sciences (to H. C.).
Conflict of interest: none declared.
References
- 1.Grandinetti A, Morens DM, Reed D, et al. Prospective study of cigarette smoking and the risk of developing idiopathic Parkinson's disease. Am J Epidemiol. 1994;139(12):1129–1138. doi: 10.1093/oxfordjournals.aje.a116960. [DOI] [PubMed] [Google Scholar]
- 2.Hernán MA, Zhang SM, Rueda-deCastro AM, et al. Cigarette smoking and the incidence of Parkinson's disease in two prospective studies. Ann Neurol. 2001;50(6):780–786. doi: 10.1002/ana.10028. [DOI] [PubMed] [Google Scholar]
- 3.Thacker EL, O'Reilly EJ, Weisskopf MG, et al. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68(10):764–768. doi: 10.1212/01.wnl.0000256374.50227.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritz B, Ascherio A, Checkoway H, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64(7):990–997. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- 5.Hernán MA, Takkouche B, Caamaño-Isorna F, et al. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol. 2002;52(3):276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 6.O'Reilly EJ, McCullough ML, Chao A, et al. Smokeless tobacco use and the risk of Parkinson's disease mortality. Mov Disord. 2005;20(10):1383–1384. doi: 10.1002/mds.20587. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti MD, Bower JH, Maraganore DM, et al. Smoking, alcohol, and coffee consumption preceding Parkinson's disease: a case-control study. Neurology. 2000;55(9):1350–1358. doi: 10.1212/wnl.55.9.1350. [DOI] [PubMed] [Google Scholar]
- 8.Morozova N, O'Reilly EJ, Ascherio A. Variations in gender ratios support the connection between smoking and Parkinson's disease. Mov Disord. 2008;23(10):1414–1419. doi: 10.1002/mds.22045. [DOI] [PubMed] [Google Scholar]
- 9.Tanner CM, Goldman SM, Aston DA, et al. Smoking and Parkinson's disease in twins. Neurology. 2002;58(4):581–588. doi: 10.1212/wnl.58.4.581. [DOI] [PubMed] [Google Scholar]
- 10.Wirdefeldt K, Gatz M, Pawitan Y, et al. Risk and protective factors for Parkinson's disease: a study in Swedish twins. Ann Neurol. 2005;57(1):27–33. doi: 10.1002/ana.20307. [DOI] [PubMed] [Google Scholar]
- 11.Goddard E. Why children start smoking. Br J Addict. 1992;87(1):17–25. doi: 10.1111/j.1360-0443.1992.tb01893.x. [DOI] [PubMed] [Google Scholar]
- 12.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 13.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 14.Ascherio A, Zhang SM, Hernán MA, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol. 2001;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 17.Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60(7):578–586. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quik M. Smoking, nicotine and Parkinson's disease. Trends Neurosci. 2004;27(9):561–568. doi: 10.1016/j.tins.2004.06.008. [DOI] [PubMed] [Google Scholar]