Abstract
Cumulative exposure to socioeconomic disadvantage across the life course may be inversely associated with coronary heart disease (CHD); the mechanisms are not fully clear. An objective of this study was to determine whether cumulative life-course socioeconomic position (SEP) is associated with CHD incidence in a well-characterized US cohort that had directly assessed childhood and adulthood measures of SEP and prospectively measured CHD incidence. Furthermore, analyses aimed to evaluate whether adjustment for CHD risk factors reduces the association between cumulative life-course SEP and CHD. The authors examined 1,835 subjects who participated in the Framingham Heart Study Offspring Cohort from 1971 through 2003 (mean age, 35.0 years; 52.4% women). Childhood SEP was measured as father's education; adulthood SEP was assessed as own education and occupation. CHD incidence included myocardial infarction, coronary insufficiency, and coronary death. Cox proportional hazards analyses indicated that cumulative SEP was associated with incident CHD after adjustment for age and sex (hazard ratio = 1.82, 95% confidence interval: 1.17, 2.85 for low vs. high cumulative SEP score). Adjustment for CHD risk factors reduced that magnitude of association (hazard ratio = 1.29, 95% confidence interval: 0.78, 2.13). These findings underscore the potential importance of CHD prevention and treatment efforts for those whose backgrounds include low SEP throughout life.
Keywords: cohort studies, coronary disease, myocardial ischemia, social class, socioeconomic factors
Coronary heart disease (CHD) remains a major cause of mortality in the United States and worldwide, responsible for 10% of the disability-adjusted life years lost in developing countries and 18% in developed countries (1, 2). Strong inverse socioeconomic gradients in CHD exist in many developed countries, where adulthood socioeconomic position (SEP) is typically measured as participants’ own education, occupation, and income (3, 4). Evidence is fairly consistent that childhood SEP (often measured as parents’ occupation or education) is also inversely associated with CHD in developed countries (5, 6). There also tend to be socioeconomic gradients in the expected directions for CHD risk factors including smoking, diabetes, blood pressure, cholesterol, and, for women, obesity (3, 7–10).
To better understand how SEP may influence CHD, it is informative to conceptualize SEP across the life course (11–13). People experience a certain set of socioeconomic circumstances at every phase of their lives; each period may theoretically influence the course of chronic disease. The “accumulation-of-risk” SEP framework focuses on the total amount of (i.e., cumulative) exposure to socioeconomic disadvantage (11, 12). Initial evidence suggests that, in a number of studies in Europe using case-control designs (14) or nationally available death records (13, 15–18), cumulative SEP is inversely associated with cardiovascular disease. Less is known about the association of cumulative SEP with incident CHD in the United States. Furthermore, little is known about whether specific CHD risk factors may be particularly important in explaining life-course socioeconomic gradients in CHD.
An objective of this study was to determine whether cumulative life-course SEP is associated with CHD incidence in a well-characterized US cohort (The Framingham Offspring Study) that had directly assessed childhood and adulthood measures of SEP and prospectively measured CHD incidence. Furthermore, analyses aimed to evaluate whether adjustment for CHD risk factors reduces the association between cumulative life-course SEP and CHD. Exploratory analyses further evaluated whether any specific CHD risk factors (e.g., smoking, systolic blood pressure, cholesterol, fasting glucose, body mass index) may be particularly important explanatory mechanisms for the association of life-course SEP with CHD incidence.
MATERIALS AND METHODS
Study sample
The Framingham Heart Study is a community-based, longitudinal, observational cohort study initiated in 1948 to prospectively investigate risk factors for CHD. The Framingham Offspring Study began in 1971 with recruitment of 5,124 men and women who were offspring (or offspring's spouses) of the Original Cohort of the Framingham Heart Study. The design and selection criteria of the Framingham Offspring Study have been described elsewhere (19). At each examination visit, participants underwent medical history, physical examination, anthropometry, and laboratory assessment of CHD risk factors, as previously described (19). Framingham participants signed informed consent, and the Framingham Study is reviewed annually by the Boston University Medical Center Institutional Review Board.
There were 5,124 participants who completed Offspring Study examination 1 (during 1971–1975), and 4,989 agreed for their data to be in the open-access data set. Of these, 2,136 had no father in the Original Cohort of the Framingham Heart Study and hence were excluded from analyses, leaving 2,853 participants. Of these participants, 119 had fathers whose education variable was missing. A further 818 were missing their own education or occupation variables (150 died between examinations 1 and 2, 509 did not attend examination 2 or 3, and 159 did not answer the education/occupation question), leaving 1,916 participants. We further restricted participants to those aged ≥28 years at the time their own educational attainment and occupation were measured, and we excluded 21 participants with baseline CHD events, resulting in a final sample of 1,835 participants. Analyses on excluded (n = 3,154) versus included participants found that excluded participants were more likely to be older (age 36.9 vs. 35.0 years, respectively; P < 0.0001), to have slightly higher fasting glucose levels (103 mg/dL vs. 101 mg/dL, P < 0.0001), and to be taking antihypertensive medications (3.7% vs. 2.5% of participants, P = 0.03). Included and excluded participants were similar regarding other variables including sex, cigarette smoking, body mass index, systolic blood pressure, total:high density lipoprotein (HDL) cholesterol ratio, cholesterol-lowering medication use, and incident CHD events.
Childhood SEP: father's education
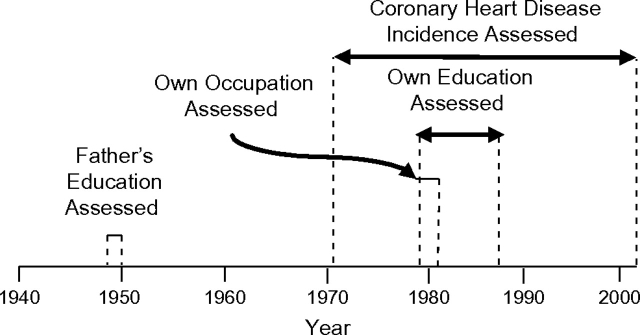
Childhood SEP was measured by father's educational attainment, obtained directly from Offspring Study cohort participants’ fathers who were enrolled in the Framingham Heart Study Original Cohort. Father's educational level was measured at enrollment between 1948 and 1950 when their mean age was 44 years (range: 28–62) (Figure 1). Father's education was ascertained directly from the father as a 6-category variable: ≤eighth grade, some high school (i.e., did not graduate from high school), high school graduate, some college (i.e., did not graduate from college), college graduate, and a final category including postgraduate school, business college, nursing school, music school, and art school. For analyses, father's education was categorized into 3 groups: <high school, high school, and >high school.
Figure 1.
Time line of assessments for exposures (father's education, own occupation, own education) and outcome (coronary heart disease incidence). Covariates were assessed at examination 1 of the Framingham Heart Study Offspring Cohort (1971–1975), Framingham, Massachusetts. Enrollment and initiation of examination 1 for the Original Cohort of the Framingham Heart Study took place during 1948–1950. Enrollment and initiation of examination 1 for the Offspring Cohort took place during 1971–1975. Initiation of Offspring Cohort examination 2 took place during 1979–1982. Initiation of Offspring Cohort examination 3 occurred during 1984–1987.
We explored the use of mother's education as a measure of childhood SEP. Mother's educational attainment was not associated with incident CHD in this cohort (hazard ratio (HR) = 1.25, 95% confidence interval (CI): 0.84, 1.90 for mother's education <high school vs. >high school, after adjusting for age and sex). The vast majority of mothers (84%) in this cohort had the same occupation (homemaker), likely because of the historical time period when Offspring Study participants were children (approximately during the 1930s–1950s), when it was less common for mothers to work outside the home. Consequently, father's education was used as a measure of childhood SEP.
Adulthood SEP: own education and occupation
Own education was measured directly from Framingham Offspring Study participants at examination 3 (1984–1987); if examination 3 education was missing, the examination 2 assessment (1979–1982) was used (Figure 1). Education was available in 6 categories of years of education: 0–4, 5–8, 9–11, 12, 13–16, ≥17. For analyses, own education was collapsed into 3 groups: ≤12, 13–16, and ≥17 years of education. Own occupation was measured at examination 2 (1979–1982) by asking what kind of work the participants do (or did), categorized as professional, executive, supervisory, technical, laborer, clerical, sales, and housewife. To obtain higher levels of education or occupation, participants were restricted to those aged ≥28 years when educational attainment and occupation were measured to allow 10 years from likely completion of high school (at age 18 years on average). Sensitivity analyses using data on participants aged ≥40 years were performed, as described below.
Accumulation-of-risk SEP framework
Analyses utilizing an accumulation-of-risk framework used a cumulative SEP score (range: 0–6) including father's education (<high school = 0, high school = 1, >high school = 2), own education (≤12 years = 0, 13–16 years = 1, ≥17 years = 2), and own occupation (laborer = 0, clerical/sales/homemaker = 1, executive/professional/supervisory/ technical = 2). Higher cutpoints were used for educational categories of Offspring Study compared with Original Cohort fathers to account for secular trends of increased normative levels of education across generations.
Coronary heart disease
At all examinations, participants underwent standardized physician-administered medical history assessments. All participants suspected of experiencing stroke were invited back for a detailed neurologic examination. Hospital and nursing home records as well as outside medical office records were routinely sought for all cardiovascular events and all deaths. In addition, Framingham Study personnel surveyed the only hospital in town daily for participant emergency room visits and hospitalizations. Suspected cardiovascular disease events and deaths were reviewed by a panel of 3 investigators, who examine all relevant available data (Framingham Study clinic data; outside medical, nursing home, and hospitalization records) and make event determinations by using previously published criteria (20).
CHD incidence was identified as occurring in participants diagnosed since onset of the Framingham Offspring Study (1971–1975) until 2003 (Figure 1). Clinically validated CHD events included myocardial infarction, coronary insufficiency, and coronary death (sudden and nonsudden). Secondary analyses investigated the outcome cardiovascular disease, which included clinically validated measures of myocardial infarction, coronary insufficiency, cerebrovascular events (including cerebral embolism, intracerebral hemorrhage, subarachnoid hemorrhage, and other cerebrovascular accident), heart failure requiring hospitalization, and death due to the aforementioned outcomes.
Covariates
Risk factors were measured at baseline, Offspring Study examination 1 (1971–1975) (Figure 1). Cigarette smoking was determined by self-report and was defined as smoking regularly in the year prior to the examination. Systolic blood pressure was calculated as the average of the clinic physician's 2 seated systolic blood pressure measurements. Body mass index was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Body weight was measured to the nearest 1 pound with a standing beam balance and with subjects wearing examination robes and undergarments. This measurement was then converted to kilograms (1 pound = 0.454 kg). Height was measured with the use of a stadiometer (to the nearest 0.25 inch and was then converted to meters (1 inch = 0.025 m). Fasting glucose was measured with a hexokinase reagent kit (A-gent glucose test; Abbott, South Pasadena, California). Glucose assays were run in duplicate, and the intraassay coefficient of variation ranged from 2% to 3% depending on the assayed glucose concentration. HDL and total cholesterol concentrations were measured by automated enzymatic techniques (21). Medication use was self-reported.
Statistical analyses
Sex- and age-adjusted descriptive statistics (predicted means and percent prevalences) were generated for CHD and CHD risk factors (systolic blood pressure, total:HDL cholesterol ratio, fasting glucose, body mass index, cigarette smoking, and antihypertensive medication use) according to father's education, own education, and own occupation.
Cox proportional hazards analyses evaluated the association of SEP with incidence of CHD. Secondary analyses used cardiovascular disease instead of CHD as the outcome. Analyses were adjusted for potential confounders including age and sex, as well as for CHD risk factors (described above). Cholesterol medication use was not included in analyses because only 5 participants used these medications at baseline. Pearson correlation coefficients and variance inflation were used to evaluate collinearity, which found that systolic and diastolic blood pressure variables were highly collinear (r = 0.81). The 3 SEP variables (father's education, own education, and own occupation) were found to have minimal variance inflation and were not correlated highly enough to be of concern to simultaneously adjust for all 3 in a single multivariable model (correlation coefficients ranged from 0.27 to 0.51). Consequently, all 3 measures of SEP were simultaneously adjusted for in analyses to evaluate whether any specific SEP measures contributed more strongly to CHD risk.
Marginal hazards models were run by using the procedure PHREG in SAS version 9.1 software (SAS Institute, Inc., Cary, North Carolina) with option COVSANDWICH to account for clustering of outcomes by family. Sex-specific analyses were underpowered and could not be conducted because only 44 CHD events in females and 100 events in males occurred. Formal interaction tests did not show evidence of effect modification by sex. Consequently, data for males and females were pooled in analyses. Power analyses were performed by using the computer program PS: Power and Sample Size Calculations, version 2.1.31 (Vanderbilt Medical Center, Nashville, Tennessee) according to criteria reported by Dupont and Plummer (22).
Sensitivity analyses were performed on a sample further restricted to participants ≥40 years of age (rather than ≥28 years of age) at baseline to assess associations between occupation and CHD incidence among participants who had more time to attain higher occupational levels. Further sensitivity analyses investigated associations between cumulative life-course SEP and CHD incidence during the time frame after which all SEP measures were obtained (1988–2003 rather than 1971–2003) (Figure 1).
RESULTS
The Framingham Heart Study Offspring participants included in the present study were a mean age of 35.0 years at baseline, and 52.4% were women. The age range at examination 1 (1971–1975) was 19–62 years. Father's education was inversely associated with several CHD risk factors, including smoking, body mass index, systolic blood pressure, total:HDL cholesterol ratio, and fasting glucose. Own education was inversely associated with smoking, systolic blood pressure, and total:HDL cholesterol ratio. Own occupation was inversely related to smoking and body mass index. Furthermore, unequal proportions of females were represented in the own education and occupation categories (Table 1).
Table 1.
Age- and Sex-adjusted Baseline Characteristics of Participants According to Life-Course Socioeconomic Position, Framingham Heart Study Offspring Cohort, United States, 1971–1975a
| Father's Education |
|||
| <High School (n = 958) | High School (n = 433) | >High School (n = 444) | |
| Age, yearsb | 37.3 (36.8, 37.9) | 31.8 (31.0, 32.6) | 33.1 (32.3, 33.9) |
| Sex (% female)b | 52.3 | 55.0 | 50.2 |
| Body mass index, kg/m2 | 25.5 (25.2, 25.7) | 25.0 (24.6, 25.4) | 24.6 (24.2, 25.0) |
| Systolic blood pressure, mm Hg | 122 (121, 123) | 122 (121, 124) | 120 (119, 122) |
| Total:HDL cholesterol ratio | 4.3 (4.2, 4.4) | 4.1 (4.0, 4.2) | 4.0 (3.9, 4.1) |
| Fasting glucose, mg/dL | 102 (101, 102) | 101 (100, 102) | 100 (99, 102) |
| Taking antihypertensive medication, % | 1.6 (1.0, 2.7) | 2.2 (1.2, 4.2) | 0.5 (0.2, 1.6) |
| Current smoker, % | 45.7 (42.5, 49.0) | 44.9 (40.2, 49.7) | 36.7 (32.3, 41.4) |
| Own Education |
|||
| ≤12 years (n = 741) | 13–16 years (n = 777) | ≥17 years (n = 317) | |
| Age, yearsb | 37.0 (36.3, 37.6) | 34.1 (33.5, 34.7) | 32.5 (31.7, 33.4) |
| Sex (% female)b | 57.9 | 55.3 | 32.9 |
| Body mass index, kg/m2 | 25.3 (25.0, 25.6) | 25.1 (24.8, 25.3) | 25.0 (24.6, 25.4) |
| Systolic blood pressure, mm Hg | 122 (121, 123) | 122 (121, 123) | 120 (119, 122) |
| Total:HDL cholesterol ratio | 4.2 (4.1, 4.3) | 4.2 (4.1, 4.3) | 3.9 (3.8, 4.1) |
| Fasting glucose, mg/dL | 102 (101, 103) | 100 (100, 101) | 101 (100, 103) |
| Taking antihypertensive medication, % | 1.5 (0.9, 2.6) | 1.5 (0.9, 2.6) | 1.3 (0.5, 3.2) |
| Current smoker, % | 51.6 (47.9, 55.3) | 43.4 (40.0, 47.0) | 24.2 (19.8, 29.2) |
| Own Occupation |
|||
| Laborer (n = 401) | Housewife/Clerical/Sales (n = 775) | Supervisory/Technical/Professional/Executive (n = 659) | |
| Age, yearsb | 36.9 (36.0, 37.8) | 35.5 (34.8, 36.1) | 33.3 (32.6, 33.9) |
| Sex (% female)b | 21.7 | 85.2 | 32.6 |
| Body mass index, kg/m2 | 25.7 (25.3, 26.1) | 25.0 (24.7, 25.4) | 25.0 (24.7, 25.3) |
| Systolic blood pressure, mm Hg | 122 (120, 123) | 122 (121, 123) | 121 (120, 126) |
| Total:HDL cholesterol ratio | 4.2 (4.0, 4.3) | 4.2 (4.1, 4.3) | 4.1 (4.0, 4.2) |
| Fasting glucose, mg/dL | 101 (100, 103) | 101 (100, 102) | 102 (101, 103) |
| Taking antihypertensive medication, % | 1.8 (0.9, 3.4) | 1.2 (0.6, 2.2) | 1.7 (0.9, 3.0) |
| Current smoker, % | 52.3 (47.1, 57.5) | 44.1 (40.2, 48.1) | 36.9 (33.2, 40.9) |
Abbreviation: HDL, high density lipoprotein.
Data are expressed as predicted mean value or percent prevalence (95% confidence interval).
Calculated by using univariate analyses.
Age- and sex-adjusted Cox proportional hazards models showed that cumulative SEP across the life course was inversely associated with CHD incidence (HR = 1.82, 95% CI: 1.17, 2.85 for low vs. high cumulative SEP score) (Table 2). Further adjustment for CHD risk factors reduced the association (HR = 1.29, 95% CI: 0.78, 2.13). In exploratory analyses adjusting for individual CHD risk factors, smoking was associated with the greatest reduction in the point estimate (age-, sex-, and smoking-adjusted HR = 1.43, 95% CI: 0.90, 2.27). Adjusting for body mass index, systolic blood pressure, and HDL:total cholesterol ratio also somewhat reduced the effect size; adjusting for fasting glucose and antihypertensive medication use had little effect on the strength of association between cumulative SEP and CHD incidence (Table 2).
Table 2.
Cox Proportional Hazards Ratios Demonstrating the Association of Cumulative Life-Course Socioeconomic Position Score With Incidence of Coronary Heart Disease, Framingham Offspring Study, United States, 1971–2003
| Cumulative SEP Scorea | No. | No. of Events | Model Adjustmentb |
|||||||||||||||
| Baseline |
Cigarette Smoking |
Body Mass Index |
Systolic Blood Pressure |
Total:HDL Cholesterol Ratio |
Fasting Glucose |
Antihypertensive Medication Use |
All CHD Risk Factorsc |
|||||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| 0 or 1 | 573 | 66 | 1.82 | 1.17, 2.85 | 1.43 | 0.90, 2.27 | 1.75 | 1.10, 2.79 | 1.71 | 1.09, 2.69 | 1.70 | 1.07, 2.71 | 1.85 | 1.17, 2.93 | 1.82 | 1.17, 2.85 | 1.29 | 0.78, 2.13 |
| 2 or 3 | 647 | 48 | 1.62 | 1.01, 2.61 | 1.45 | 0.90, 2.32 | 1.60 | 0.99, 2.59 | 1.56 | 0.97, 2.49 | 1.49 | 0.92, 2.42 | 1.67 | 1.03, 2.70 | 1.62 | 1.01, 2.61 | 1.38 | 0.85, 2.25 |
| 4 to 6 | 615 | 30 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
Abbreviations: CHD, coronary heart disease; CI, confidence interval; HDL, high density lipoprotein; HR, hazard ratio; SEP, socioeconomic position.
Analyses used a cumulative SEP score including father's education, own education, and own occupation. Scores were calculated for each SEP measure separately and then summed (range, 0–6): father's education: <high school = 0, high school = 1, >high school = 2; own education: ≤12 years = 0, 13–16 years = 1, ≥17 years = 2; own occupation: laborer = 0, clerical/sales/homemaker = 1, executive/professional/supervisory/technical = 2.
All models were adjusted for age and sex.
CHD risk factors include smoking, body mass index, systolic blood pressure, total:HDL cholesterol ratio, fasting glucose, and antihypertensive medication use.
In an effort to provide information regarding whether any of the 3 subcomponents of the cumulative SEP score may be contributing particularly strongly to the gradient between cumulative SEP and CHD incidence, we analyzed the individual association of father's education, own education, and own occupation with CHD incidence. Father's education and own education were inversely associated with CHD incidence after adjusting for age and sex (HR = 1.65, 95% CI: 1.02, 2.66 for father's education <high school vs. >high school, and HR = 1.85, 95% CI: 1.05, 3.27 for own education ≤12 years vs. ≥17 years; Table 3). Further adjustment for other SEP measures reduced the estimated effect sizes only a small amount for both father's education and own education (HR = 1.53, 95% CI: 0.92, 2.55 for participants whose father's education was <high school vs. >high school, and HR = 1.62, 95% CI: 0.85, 3.09 for participants whose own education was ≤12 years vs. ≥17 years). However, the 95% confidence intervals encompassed both a null effect (i.e., HR = 1.0) and a large effect (e.g., HR = 2.5), indicating that the statistical power was low (1 − β = 0.43 for a cumulative 30-year incidence of CHD of 7.5% vs. 5% for father's education <high school (n = 958) vs. father's education >high school (n = 444), with α = 0.05). Occupation was not associated with CHD incidence (Table 3).
Table 3.
Cox Proportional Hazards Ratios for the Association of Socioeconomic Position With Incidence of Coronary Heart Disease, Framingham Offspring Study, United States, 1971–2003
| SEP Measure and SEP Level | No. of Events | Model Adjustment |
|||||
| Age, Sex |
Age, Sex, Other SEP Measuresa |
Age, Sex, CHD Risk Factorsb |
|||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Father's education | |||||||
| <High school | 97 | 1.65 | 1.02, 2.66 | 1.53 | 0.92, 2.55 | 1.35 | 0.81, 2.23 |
| High school | 26 | 1.56 | 0.88, 2.77 | 1.50 | 0.84, 2.69 | 1.49 | 0.83, 2.69 |
| >High school | 21 | 1.00 | 1.00 | 1.00 | |||
| Own education | |||||||
| ≤12 years | 71 | 1.85 | 1.05, 3.27 | 1.63 | 0.86, 3.11 | 1.20 | 0.66, 2.12 |
| 13–16 years | 58 | 1.81 | 1.02, 3.21 | 1.76 | 0.98, 3.17 | 1.31 | 0.71, 2.40 |
| >16 years | 15 | 1.00 | 1.00 | 1.00 | |||
| Own occupation | |||||||
| Laborer | 50 | 1.20 | 0.80, 1.78 | 0.98 | 0.62, 1.55 | 0.90 | 0.59, 1.36 |
| Homemaker, clerical, or sales | 44 | 1.02 | 0.64, 1.64 | 0.92 | 0.57, 1.49 | 0.84 | 0.52, 1.36 |
| Professional, executive, supervisory, or technical | 50 | 1.00 | 1.00 | 1.00 | |||
Abbreviations: CHD, coronary heart disease; CI, confidence interval; HR, hazard ratio; SEP, socioeconomic position.
“Other SEP measures” refers to adjustment for measures of SEP other than the exposure of interest. For example, analyses of father's education were adjusted for own education and own occupation.
CHD risk factors include smoking, body mass index, systolic blood pressure, total:HDL cholesterol ratio, fasting glucose, and antihypertensive medication use.
We conducted a series of secondary analyses. Analyses were repeated by using cardiovascular disease instead of CHD as an outcome (Web Tables 1 and 2; this information is described in the first 2 of 3 supplementary tables, all of which are posted on the Journal’s website (http://aje.oupjournals.org/)). Findings were generally similar when either outcome was used. Sensitivity analyses were performed to assess the association of occupation with CHD and cardiovascular disease incidence among participants aged ≥40 years rather than aged ≥28 years, as used in the analyses described above. Effect sizes were similar in both sets of analyses (Web Table 3). Because own education (1979–1987) and occupation (1979–1982) were assessed after commencement of CHD incidence measurements (1971–2003), we performed sensitivity analyses restricted to the years after completion of all SEP measures (1988–2003) to evaluate whether the timing of SEP and CHD measures had an impact on the findings. Analyses showed similar socioeconomic gradients in CHD (HR for high vs. low cumulative SEP score = 1.94, 95% CI: 1.19, 3.17 after adjusting for age and sex).
DISCUSSION
Evidence from our study supported an inverse association of cumulative life-course SEP with CHD incidence. Further adjustment for CHD risk factors substantially attenuated the strength of association.
Prior literature
With regard to adulthood socioeconomic disparities in CHD, a systematic review showed consistent inverse associations between adulthood SEP and CHD in developed nations since the 1970s (4). A separate systematic review reported inverse associations of childhood SEP with risk of cardiovascular disease in 31 of 40 studies (23). The accumulation-of-risk SEP framework suggests that as the duration and severity of socioeconomic disadvantage increase, resulting cumulative damage could place individuals at higher risk of CHD (12). Our study provides evidence to support this hypothesis, in that higher life-time exposure to socioeconomic deprivation was associated with increased risk of CHD. Adjusting for CHD risk factors reduced the strength of association and rendered it nonsignificant, which was not unexpected because CHD risk factors are candidate pathways by which SEP may influence CHD. For life-course SEP, a systematic review found consistently inverse associations between the accumulation-of-risk SEP framework and risk of cardiovascular disease (13), which is in agreement with our findings. In the latter review, the associations of early-life SEP (independent of adulthood SEP) and social mobility were less consistently associated with measures of cardiovascular disease (13).
Potential mechanisms
Cumulative life-course SEP may influence CHD through a number of mechanisms. Low adulthood SEP is typically inversely associated with many risk factors for CHD, such as smoking (7), blood pressure (8), diabetes (9), and, for women, obesity (10). Childhood SEP has been shown to be inversely associated with several CHD risk factors in adulthood, including smoking, blood pressure, cholesterol, and adiposity (24–26). Other potential mechanisms not measured in this study, including depression and stress, were not able to be evaluated.
Few if any studies on the association of cumulative SEP with CHD adjusted for CHD risk factors separately (13–18); consequently, little is known about which risk factors may be particularly important in explaining socioeconomic gradient in CHD. Note that methodological biases can be induced by adjusting for mediators; therefore, these mechanistic findings should be interpreted with caution (27).
In our study, adjusting for smoking reduced the strength of association between life-course SEP and CHD. Point estimates for the association of cumulative SEP with CHD incidence were lower after adjusting for smoking than for other CHD risk factors. However, given the wide 95% confidence intervals for these point estimates, it was not possible to ascertain whether smoking was more important than other CHD risk factors in reducing the association. We found strong inverse socioeconomic gradients in smoking in our study. Some studies on socioeconomic gradients in CHD, using other measures of SEP (only adulthood SEP or only childhood SEP), adjusted individually for smoking. For example, in a study on male physicians (who consequently had similar adulthood SEP), low childhood SEP was associated with a 2.40 (95% CI: 1.21, 4.74) higher relative risk of developing CHD before the age of 50 years. Adjustment for smoking only slightly and nonsignificantly reduced the association (relative risk = 2.24, 95% CI: 1.11, 4.51) (28). In the Whitehall II study, the magnitude of association of occupational class with CHD was nonsignificantly reduced by 18% after adjusting for smoking. Adjusting individually for hypertension, high cholesterol, and diabetes reduced the effect sizes by 14%, 3%, and 6%, respectively (29).
In a recent study on adulthood (that did not include childhood) socioeconomic gradients in mortality in 22 European countries, smoking-related conditions accounted for 22% and 6% of the socioeconomic gradient in the all-cause death rate among men and women, respectively (30). In another study in the United States and 11 European countries, adulthood (the study did not include childhood) socioeconomic gradients in cardiovascular disease mortality were highly associated with socioeconomic gradients in cigarette smoking and excessive alcohol consumption, unlike overweight, moderate alcohol consumption, and lack of fresh vegetables, which were not strongly associated with socioeconomic gradients in cardiovascular disease mortality (31). Overall, studies using SEP measures other than cumulative SEP provide limited evidence to suggest that smoking is a particularly important risk factor in explaining the socioeconomic gradients in CHD. Replication of findings using cumulative SEP is needed in other study samples to better ascertain the role of smoking in explaining cumulative socioeconomic gradients in CHD.
Strengths and limitations
Strengths of our study include that childhood SEP was directly assessed from parents. A review found that those studies that measured SEP in childhood showed stronger associations between childhood SEP and disease outcomes compared with studies that measured adult recall of childhood SEP, probably because of reductions in measurement error (32). Furthermore, the measures of CHD used only clinically validated outcomes. CHD risk factors were routinely and directly assessed by using measures with good validity and reliability.
Weaknesses of this study include the relatively small sample size (n = 1,835) compared with larger studies; consequently, we had lower statistical power. Furthermore, this study included a community-based population of individuals of European descent (representing the demographics of the city of Framingham, Massachusetts, at study onset) residing in the northeastern United States; thus, generalizability of results to other communities, races, and ethnicities is uncertain. A limitation of the accumulation-of-risk SEP framework, as described by Pollitt et al. (13), is that cumulative life-course SEP measurements conflate SEP measures at specific times in the life course (e.g., SEP in early, middle, and late life); therefore, it is unknown which time period may be particularly important in influencing health. Furthermore, cumulative SEP measures typically give equal weighting to each subcomponent of SEP, which may not reflect true contributions of SEP to health. To provide information on relative contributions of each subcomponent of the cumulative SEP index to CHD incidence, we provided point estimates for each subcomponent. These analyses suggested that measures of father's education and own education were more important risk factors for CHD incidence than own occupation, at least as measured in this cohort.
In summary, this study found that directly assessed cumulative SEP across the life course was inversely associated with incident CHD in Framingham Offspring Study participants. Adjustment for CHD risk factors reduced the magnitude of association. These findings underscore the potential importance of CHD prevention and treatment efforts for those whose backgrounds include low SEP throughout their life course.
Supplementary Material
Acknowledgments
Author affiliations: Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Canada (Eric B. Loucks, John W. Lynch, Rebecca Fuhrer, Nisha D. Almeida, Golareh Agha); Department of Psychiatry, Douglas Mental Health Institute, McGill University, Montreal, Canada (Eric B. Loucks, Nisha D. Almeida); School of Health Sciences, University of South Australia, South Australia, Australia (John W. Lynch); Department of Medicine, McGill University, Montreal, Canada (Louise Pilote, Hugues Richard); Boston University and National Heart, Lung, and Blood Institute’s Framingham Heart Study, Framingham, Massachusetts (Joanne M. Murabito, Emelia J. Benjamin); Departments of Medicine and Preventive Medicine, Section of General Internal Medicine, Boston University School of Medicine, Boston, Massachusetts (Joanne M. Murabito, Emelia J. Benjamin); Cardiology Department and Whitaker Cardiovascular Institute, School of Medicine, Boston University, Boston, Massachusetts (Emelia J. Benjamin); and Department of Epidemiology, School of Public Health, Boston University, Boston, Massachusetts (Emelia J. Benjamin). Joanne M. Murabito and Emelia J. Benjamin contributed equally to the study.
The Framingham Heart Study is supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) contract N01-HC-25195. The research was supported by Canadian Institutes of Health operating grant MOP81239 (E. B. L.); Canadian Institutes of Health Interdisciplinary Capacity Enhancement grant HOA80072 (J. W. L.); NIH grants HL064753, HL076784, and AG028321 (E. J. B.); and a Canadian Institutes of Health New Investigator Award (E. B. L.).
The authors are grateful to the NHLBI for providing them with the limited access data set, in conjunction with Framingham Heart Study investigators who provided other important variables for analyses.
Conflict of interest: none declared.
Glossary
Abbreviations
- CHD
coronary heart disease
- CI
confidence interval
- HDL
high density lipoprotein
- HR
hazard ratio
- SEP
socioeconomic position
References
- 1.Kung HC, Hoyert DL, Xu J, et al. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56(10):1–120. [PubMed] [Google Scholar]
- 2.Mackay J, Mensah G. The Atlas of Heart Disease and Stroke. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 3.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88(4 pt 1):1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 4.González MA, Rodríguez Artalejo F, Calero JR. Relationship between socioeconomic status and ischaemic heart disease in cohort and case-control studies: 1960–1993. Int J Epidemiol. 1998;27(3):350–358. doi: 10.1093/ije/27.3.350. [DOI] [PubMed] [Google Scholar]
- 5.Galobardes B, Lynch JW, Davey Smith G. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiol Rev. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- 6.Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J Epidemiol Community Health. 2008;62(5):387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- 7.Gilman SE, Martin LT, Abrams DB, et al. Educational attainment and cigarette smoking: a causal association? Int J Epidemiol. 2008;37(3):615–624. doi: 10.1093/ije/dym250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colhoun HM, Hemingway H, Poulter NR. Socio-economic status and blood pressure: an overview analysis. J Hum Hypertens. 1998;12(2):91–110. doi: 10.1038/sj.jhh.1000558. [DOI] [PubMed] [Google Scholar]
- 9.Maty SC, Everson-Rose SA, Haan MN, et al. Education, income, occupation, and the 34-year incidence (1965–99) of type 2 diabetes in the Alameda County Study. Int J Epidemiol. 2005;34(6):1274–1281. doi: 10.1093/ije/dyi167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaren L. Socioeconomic status and obesity. Epidemiol Rev. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- 11.Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu Rev Public Health. 2005;26:1–35. doi: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- 12.Kuh D, Ben-Shlomo Y. Introduction: a life course approach to the aetiology of adult chronic disease. In: Kuh D, Ben-Shlomo Y, editors. A Life Course Approach to Chronic Disease Epidemiology. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2004. pp. 3–14. [Google Scholar]
- 13.Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review [electronic article] BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wamala SP, Lynch J, Kaplan GA. Women's exposure to early and later life socioeconomic disadvantage and coronary heart disease risk: the Stockholm Female Coronary Risk Study. Int J Epidemiol. 2001;30(2):275–284. doi: 10.1093/ije/30.2.275. [DOI] [PubMed] [Google Scholar]
- 15.Davey Smith G, Hart C, Blane D, et al. Lifetime socioeconomic position and mortality: prospective observational study. BMJ. 1997;314(7080):547–552. doi: 10.1136/bmj.314.7080.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davey Smith G, Hart C. Life-course socioeconomic and behavioral influences on cardiovascular disease mortality: the collaborative study. Am J Public Health. 2002;92(8):1295–1298. doi: 10.2105/ajph.92.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claussen B, Davey Smith G, Thelle D. Impact of childhood and adulthood socioeconomic position on cause specific mortality: the Oslo Mortality Study. J Epidemiol Community Health. 2003;57(1):40–45. doi: 10.1136/jech.57.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pensola TH, Martikainen P. Cumulative social class and mortality from various causes of adult men. J Epidemiol Community Health. 2003;57(9):745–751. doi: 10.1136/jech.57.9.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannel WB, Feinleib M, McNamara PM, et al. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 20.Kannel W, Wolf P, Garrison R. The Framingham Study: An Epidemiological Investigation of Cardiovascular Disease, Section 34. Bethesda, MD: National Heart, Lung, and Blood Institute; 1987. ((NIH) 87–2703) [Google Scholar]
- 21.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166(1):1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 22.Dupont WD, Plummer WD., Jr Power and sample size calculations: a review and computer program. Control Clin Trials. 1990;11(2):116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 23.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16(2):91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 24.Blane D, Hart CL, Smith GD, et al. Association of cardiovascular disease risk factors with socioeconomic position during childhood and during adulthood. BMJ. 1996;313(7070):1434–1438. doi: 10.1136/bmj.313.7070.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poulton R, Caspi A, Milne BJ, et al. Association between children's experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360(9346):1640–1645. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilman SE, Abrams DB, Buka SL. Socioeconomic status over the life course and stages of cigarette use: initiation, regular use, and cessation. J Epidemiol Community Health. 2003;57(10):802–808. doi: 10.1136/jech.57.10.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole SR, Hernán MA. Fallibility in estimating direct effects [comment] Int J Epidemiol. 2002;31(1):163–165. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- 28.Kittleson MM, Meoni LA, Wang NY, et al. Association of childhood socioeconomic status with subsequent coronary heart disease in physicians. Arch Intern Med. 2006;166(21):2356–2361. doi: 10.1001/archinte.166.21.2356. [DOI] [PubMed] [Google Scholar]
- 29.Singh-Manoux A, Nabi H, Shipley M, et al. The role of conventional risk factors in explaining social inequalities in coronary heart disease: the relative and absolute approaches to risk. Epidemiology. 2008;19(4):599–605. doi: 10.1097/EDE.0b013e3181761cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackenbach JP, Stirbu I, Roskam AJ, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358(23):2468–2481. doi: 10.1056/NEJMsa0707519. [DOI] [PubMed] [Google Scholar]
- 31.Mackenbach JP, Cavelaars AE, Kunst AE, et al. Socioeconomic inequalities in cardiovascular disease mortality; an international study. Eur Heart J. 2000;21(14):1141–1151. doi: 10.1053/euhj.1999.1990. [DOI] [PubMed] [Google Scholar]
- 32.Davey Smith G, Lynch JW. Socioeconomic differentials. In: Kuh D, Ben-Shlomo Y, editors. A Life Course Approach to Chronic Disease Epidemiology. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.