Abstract
The authors compared effects of macronutrients on self-reported appetite and selected fasting hormone levels. The Optimal Macronutrient Intake Trial to Prevent Heart Disease (OMNI-Heart) (2003–2005) was a randomized, 3-period, crossover feeding trial (n = 164) comparing the effects of 3 diets, each rich in a different macronutrient. Percentages of kilocalories of carbohydrate, fat, and protein were 48, 27, and 25, respectively, for the protein-rich diet; 58, 27, and 15, for the carbohydrate-rich diet; and 48, 37, and 15 for the diet rich in unsaturated fat. Food and drink were provided for each isocaloric 6-week period. Appetite was measured by visual analog scales. Pairwise differences between diets were estimated using generalized estimating equations. Compared with the protein diet, premeal appetite was 14% higher on the carbohydrate (P = 0.01) and unsaturated-fat (P = 0.003) diets. Geometric mean leptin was 8% lower on the protein diet than on the carbohydrate diet (P = 0.003). Obestatin levels were 7% and 6% lower on the protein diet than on the carbohydrate (P = 0.02) and unsaturated-fat (P = 0.004) diets, respectively. There were no between-diet differences for ghrelin. A diet rich in protein from lean meat and vegetables reduces self-reported appetite compared with diets rich in carbohydrate and unsaturated fat and can be recommended in a weight-stable setting. The observed pattern of hormone changes does not explain the inverse association between protein intake and appetite.
Keywords: appetite, cross-over studies, diet, dietary carbohydrates, dietary fats, dietary proteins, ghrelin, leptin
The effects of macronutrients on appetite are controversial. Some research suggests a satiety hierarchy in which protein is more satiating than carbohydrate and carbohydrate is more satiating than fat (1). Short-term (<1 week) crossover trials, often single-meal studies, suggest that diets higher in protein decrease appetite and energy intake (2, 3). Trials examining the effect of protein on appetite over a longer period (>2 weeks) corroborate findings from shorter-term studies in settings of weight loss and maintenance of weight loss (4, 5). Other research suggests that there is no difference among macronutrients (6). Public perception is that fat is more satiating than protein and carbohydrate (7).
Although clinical trials provide some evidence that short-term exposure to diets rich in protein may reduce appetite (2–5), mechanisms are unclear. One possible explanation is that dietary protein affects concentrations of appetite hormones. Under this scenario, appetite hormones are mediating variables. Leptin purportedly decreases food intake by signaling the availability of energy reserves (8). Obestatin and ghrelin are products of the same gene but may have opposing effects on weight regulation, according to data from animal models (9); while obestatin may reduce energy intake, ghrelin probably promotes meal initiation (10).
Our purpose in this study was to compare the effects of 3 healthy diets, each emphasizing a different macronutrient, on self-reported appetite and fasting levels of selected appetite hormones. We hypothesized that a protein-rich diet, compared with a diet rich in carbohydrate and another diet rich in unsaturated fat, would decrease self-reported appetite, increase leptin and obestatin levels, and reduce ghrelin levels.
MATERIALS AND METHODS
OMNI-Heart
We used data from the Optimal Macronutrient Intake Trial to Prevent Heart Disease (OMNI-Heart). Detailed descriptions of OMNI-Heart and its main results have been published elsewhere (11, 12). Briefly, the primary objective of this National Heart, Lung, and Blood Institute-sponsored trial was to compare the effects of 3 diets, each rich in a different macronutrient, on blood pressure, lipid levels, and estimated cardiovascular disease risk. The study was conducted in 2 clinical centers located in Boston, Massachusetts, and Baltimore, Maryland, from April 2003 to June 2005. Participants were recruited through newspaper notices and mass mailings. Institutional review boards at each study center and an independent data and safety monitoring board approved the protocol and monitored the trial. Each participant provided written informed consent. Participants were aged 30 years or older and had prehypertension or stage 1 hypertension (systolic blood pressure 120–159 mm Hg or diastolic blood pressure 80–99 mm Hg). Exclusion criteria were cardiovascular disease, recent cancer, diabetes, a low density lipoprotein cholesterol level greater than 220 mg/dL, a fasting triacylglycerol (triglyceride) level greater than 750 mg/dL, weight greater than 350 pounds (159 kg), current use of medications that affect blood pressure or blood lipid levels, unwillingness to suspend vitamin and mineral supplementation, and alcohol consumption in excess of 14 drinks per week.
Design
Eligibility was ascertained and baseline data were collected over 3 screening visits. During a 6-day run-in period, participants were provided each diet for 2 days. The initial calorie level at the start of the run-in period was estimated using sex, height, weight, and physical activity level. Weight was measured each weekday, and caloric intake was adjusted by a dietitian as needed to maintain weight.
After successful completion of the run-in period, participants were randomly assigned to receive 1 of 6 sequences of the 3 diets. Randomization was stratified by clinic. Participants and all staff involved in data collection were masked with regard to diet sequence. Each feeding period lasted 6 weeks and was followed by a washout period of 2–4 weeks wherein participants ate their own food.
Intervention diets
Each diet complied with the US Department of Agriculture's Dietary Guidelines for Americans (13), having lower saturated fat, cholesterol, and sodium levels and higher potassium, calcium, and magnesium levels than the typical US diet. Isocaloric diets were provided to participants, and calorie level was individually assigned and monitored each weekday to maintain weight throughout the trial. The carbohydrate-rich diet provided 58% of energy as carbohydrate, 15% as protein, and 27% as fat (6% of energy as saturated fat, 13% as monounsaturated fat, and 8% as polyunsaturated fat). In the protein-rich diet, 10% of energy from carbohydrate was replaced with protein, with two-thirds of the increased protein being derived from plant-based foods. Similarly, in the unsaturated-fat-rich diet, 10% of energy from carbohydrate was replaced with unsaturated fats, predominantly monounsaturated fats.
Additional details on the composition of the intervention diets are provided elsewhere (14). Glycemic load for the 2,100-calorie level was 242 for the carbohydrate diet, 177 for the protein diet, and 183 for the unsaturated-fat diet, using the white bread index as the reference (15). Energy density, including all food, juice, and milk, was measured through composite analysis of a week of menus for the 3 2,100-calorie diets. Mean energy density (kcal/g) was 1.10 (standard deviation (SD), 0.08) for the carbohydrate diet, 1.07 (SD, 0.11) for the protein diet, and 1.13 (SD, 0.09) for the unsaturated-fat diet.
Appetite measurement
Self-reported appetite.
Participants consumed 1 meal per day at the study site each weekday. A self-reported appetite questionnaire was completed at the study site immediately before and after consumption of each meal every weekday during week 3 of each of the 3 diet periods. The appetite measure had 3 components: hunger, prospective consumption (amount of food that could be eaten in a designated time period), and fullness. Participants rated each component on a 100-mm visual analog scale adapted from questionnaires used by other investigators (16, 17). Words were placed at each end of the scale to denote extreme ratings. Each rating was scored independently by 2 reviewers, and differences were reconciled by an adjudicator if the scores disagreed by more than 2 mm. A summary score was calculated separately for the pre- and postmeal ratings by summing the average hunger and prospective consumption ratings and subtracting fullness ratings from the 5 questionnaires completed each weekday during week 3. Higher summary scores indicated greater appetite.
Appetite hormones.
Prior to randomization and at week 6 of each intervention period, blood was drawn after at least 8 hours of fasting. Median fasting time prior to blood drawing for each of the 3 diets was 13 hours (interquartile range, 12–14). Median time of blood drawing for the baseline and week 6 samples was 10:25 AM (interquartile range, 8:56 AM–11:45 AM). Samples were centrifuged, processed, and stored at −70°C within 2 hours of collection. Assays were performed in duplicate at the Bayview General Clinical Research Center (Johns Hopkins Bayview Medical Center, Baltimore, Maryland). Plasma samples were analyzed by radioimmunoassay for leptin (Linco Research, Inc., St. Charles, Missouri), obestatin (Phoenix Pharmaceuticals, Inc., Belmont, California), and total ghrelin (Linco). Intraassay coefficients of variation were 5.5% for leptin, 8.9% for obestatin, and 3.9% for ghrelin.
Statistical analysis
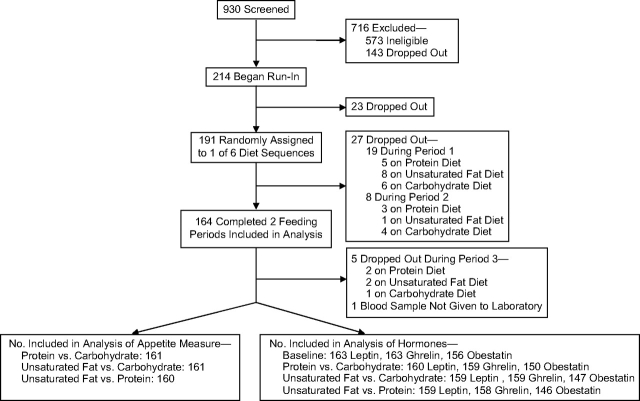
The sample size (n = 164) for the study was determined by the number of OMNI-Heart participants who had completed at least 2 of the 3 feeding periods (Figure 1). The data provided 90% power to detect a 3% change in the appetite measure between diets at a significance level of P < 0.05 (2-sided). Small changes in hormone concentrations were also detectable, as the study provided 90% power to detect a 2% change in ghrelin and a 14% change in leptin at a significance level of P < 0.05 (2-sided) (18, 19). No obestatin data had been published in humans at the time of study initiation; hence, minimum detectable differences were not estimated.
Figure 1.
Participation in the appetite component of the Optimal Macronutrient Intake Trial to Prevent Heart Disease, 2003–2005. Ghrelin data were missing for 1 sample and obestatin data were missing for 29 samples because of insufficient plasma to conduct the assay.
Leptin data were loge-transformed to improve normality and back-transformed for interpretation. The mean change between baseline and each of the intervention diets was calculated for all hormones. Pairwise differences between diets were estimated using generalized estimating equations with an exchangeable correlation structure accounting for site and period. Tests for carryover were conducted by including indicator variables for diet assignment in the previous period for each of the outcome measures. There was no evidence of differential carryover by intervention diet.
Subgroup analyses were based on groups specified a priori: body mass index (weight (kg)/height (m)2), age, and sex. Effect modification of between-diet differences in appetite measures was estimated by including interaction terms for intervention diet by median age (53 years), sex, and obesity (modeling body mass index as both continuous and categorical (body mass index <30 vs. ≥30)) in generalized estimating equations models that accounted for period, site, age (continuous), sex, and body mass index (continuous and categorical separately). Tests for diet-subgroup interactions were performed by comparing −2 loge likelihood estimates from nested models with the appropriate chi-squared distribution.
The association between fasting hormone levels and self-reported appetite ratings was estimated using data from the first period to calculate crude and partial Spearman correlations with adjustment for intervention diet and site.
Significance was defined as P < 0.05 without adjustment for multiple comparisons. Analyses were performed using SAS (version 9.1; SAS Institute Inc., Cary, North Carolina).
RESULTS
The study population was diverse, with a mean age of 53.6 years (SD, 10.8); 55% were male, 55% were African-American, and 46% were obese (Table 1). Fewer than half of the participants reported ever drinking any alcohol.
Table 1.
Baseline Characteristics of Participants in the Optimal Macronutrient Intake Trial to Prevent Heart Disease, 2003–2005
| Characteristic (n = 164) | Mean (SD) | No. | % |
| Age, years | 53.6 (10.8) | ||
| Female sex | 73 | 45 | |
| Race | |||
| African-American | 90 | 55 | |
| Non-Hispanic white | 66 | 40 | |
| Other | 8 | 5 | |
| Weight, kg | 87.7 (18.6) | ||
| BMIa | 30.3 (6.1) | ||
| Obesity status | |||
| Normal weight (BMI < 25) | 34 | 21 | |
| Overweight (BMI 25–29.9) | 55 | 33 | |
| Obese (BMI ≥ 30) | 75 | 46 | |
| Alcohol intake | |||
| Ever consuming alcohol | 73 | 45 | |
| Servings/week (among drinkers) | 4.1 (3.5) | ||
| Education | |||
| High school or less | 33 | 20 | |
| Some college | 56 | 34 | |
| College graduate | 75 | 46 | |
| Smoking | |||
| Current smoker | 18 | 11 | |
| Former smoker | 46 | 28 | |
| Never smoker | 100 | 61 |
Abbreviations: BMI, body mass index; SD, standard deviation.
Weight (kg)/height (m)2.
As previously described (12), adherence to the intervention diets, as measured by self-report in daily diaries, was excellent—all study food and no nonstudy food was consumed on at least 95% of the person-days for each diet. Objective measurements (stable weights and similar fasting sodium and potassium concentrations on all 3 diets, and elevated urinary nitrogen levels on just the protein-rich diet) corroborated the self-reported adherence assessment (12). Over 98% of the appetite questionnaires were completed. Sixty percent of the appetite questionnaires were administered at lunch and 39% at dinner; fewer than 1% of the questionnaires were administered at breakfast.
Between-diet differences
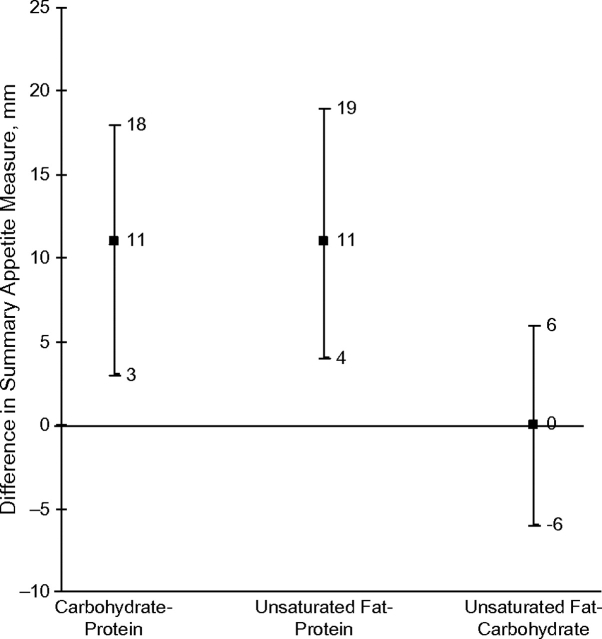
Appetite was lower on the protein-rich diet than on the carbohydrate-rich or unsaturated-fat-rich diet. Ratings were 11 points (14%) higher on both the carbohydrate and unsaturated-fat diets than on the protein diet (Table 2 and Figure 2). Differences in postmeal ratings were qualitatively similar to premeal estimates. There were no differences in appetite between the unsaturated-fat and carbohydrate diets as measured by the appetite measure.
Table 2.
Scores for Self-reported Appetite Measures and Fasting Hormone Levels on 3 Different Intervention Diets, Optimal Macronutrient Intake Trial to Prevent Heart Disease, 2003–2005a
| Protein-Rich Diet |
Carbohydrate-Rich Diet |
Unsaturated-Fat-Rich Diet |
P for Between-Diet Difference |
||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Carbohydrate-Protein | Unsaturated Fat-Protein | Carbohydrate-Unsaturated Fat | |
| Appetite measure summaryb | |||||||||
| Premeal | 78 | 69, 87 | 89 | 81, 96 | 89 | 81, 97 | 0.01 | 0.003 | 0.92 |
| Postmeal | −55 | −61, −50 | −48 | −54, −42 | −48 | −54, −42 | 0.005 | 0.007 | 0.99 |
| Appetite measure scale | |||||||||
| Hunger | |||||||||
| Premeal | 52 | 48, 56 | 57 | 54, 60 | 58 | 55, 61 | 0.002 | <0.001 | 0.54 |
| Postmeal | 13 | 11, 15 | 13 | 11, 15 | 15 | 13, 17 | 0.86 | 0.19 | 0.12 |
| Prospective consumptionc | |||||||||
| Premeal | 60 | 57, 63 | 64 | 61, 67 | 63 | 60, 66 | 0.004 | 0.03 | 0.41 |
| Postmeal | 13 | 11, 15 | 16 | 14, 19 | 16 | 13, 18 | 0.005 | 0.01 | 0.72 |
| Fullness | |||||||||
| Premeal | 34 | 31, 37 | 32 | 29, 35 | 32 | 29, 35 | 0.21 | 0.12 | 0.81 |
| Postmeal | 82 | 79, 84 | 77 | 74, 80 | 78 | 75, 81 | 0.002 | 0.006 | 0.38 |
| Appetite hormone level | |||||||||
| Leptin, ng/mLd | 9.7 | 8.3, 11.4 | 10.5 | 9.0, 12.3 | 9.9 | 8.5, 11.6 | 0.003 | 0.42 | 0.05 |
| Obestatin, pg/mL | 114.7 | 108.4, 121.0 | 122.6 | 114.6, 130.6 | 121.7 | 115.5, 128.0 | 0.02 | 0.004 | 0.80 |
| Ghrelin, pg/mL | 829.0 | 771.0, 886.9 | 839.0 | 783.5, 894.6 | 829.1 | 773.8, 884.4 | 0.42 | 0.48 | 0.83 |
Abbreviation: CI, confidence interval.
Least-square mean values and P values were estimated from models with an exchangeable correlation structure, with adjustment for site and period.
The appetite measure was defined as the sum of hunger and prospective consumption ratings minus fullness ratings as measured by the visual analog scale.
The prospective consumption scale measured the amount of food a subject could eat at a given time.
Geometric mean values. Estimates for leptin were back-transformed from loge-transformation.
Figure 2.
Adjusted mean between-diet differences in the premeal self-reported summary appetite measure, Optimal Macronutrient Intake Trial to Prevent Heart Disease, 2003–2005. The summary appetite measure was calculated as hunger plus prospective consumption minus fullness. Comparisons were adjusted for period and site in generalized estimating equations models with an exchangeable correlation structure. Results from pairwise comparisons: carbohydrate versus protein, P = 0.01; unsaturated fat versus protein, P = 0.003; unsaturated fat versus carbohydrate, P = 0.92. Bars, 95% confidence interval.
Although the effects of the diets on appetite were observed in both pre- and postmeal summary ratings, individual scale components (i.e., hunger, prospective consumption, fullness) varied by time point. Before the on-site study meal, hunger ratings were approximately 10% lower, prospective consumption was 6% lower, and fullness was 6% higher on the protein diet than on both the unsaturated-fat and carbohydrate diets (Table 2). Immediately following the meal, hunger ratings did not differ between diets, but prospective consumption ratings were approximately 23% lower and fullness ratings were 5% higher on the protein diet than on the unsaturated-fat and carbohydrate diets.
Geometric mean fasting leptin level was 8% lower on the protein diet than on the carbohydrate diet (Table 3). Compared with the protein diet, fasting obestatin was 8 pg/mL (7%) higher on the carbohydrate diet and 7.0 pg/mL (6%) higher on the unsaturated-fat diet (Tables 2 and 3). There were no between-diet differences in fasting ghrelin level.
Table 3.
Between-Diet Differencesa in Fasting Levels of Appetite Hormones, Optimal Macronutrient Intake Trial to Prevent Heart Disease, 2003–2005
| Appetite Hormone | Carbohydrate-Protein |
Unsaturated Fat-Protein |
Unsaturated Fat-Carbohydrate |
|||||||||
| No. of Subjects | Mean | 95% CI | P Value | No. of Subjects | Mean | 95% CI | P Value | No. of Subjects | Mean | 95% CI | P Value | |
| Leptin, ng/mLb | 160 | 1.08 | 1.00, 1.12 | 0.003 | 159 | 1.02 | 0.97, 1.08 | 0.42 | 159 | 0.95 | 0.90, 1.00 | 0.05 |
| Obestatin, pg/mL | 150 | 8.0 | 1.1, 14.8 | 0.02 | 146 | 7.0 | 1.6, 12.5 | 0.004 | 147 | −0.9 | −7.8, 6.0 | 0.80 |
| Ghrelin, pg/mL | 159 | 10.0 | −17.2, 37.3 | 0.42 | 158 | 0.2 | −25.6, 25.9 | 0.48 | 159 | −9.9 | −35.8, 16.0 | 0.83 |
Abbreviation: CI, confidence interval.
Comparisons were adjusted for period and site in generalized estimating equations models with an exchangeable correlation structure.
Between-diet differences in leptin were back-transformed from loge-transformation. Mean values for leptin represent the ratio of geometric means.
No appreciable differences were observed in effect estimates for self-reported appetite and appetite hormones in analyses stratified by sex, age, or obesity. Comparison of nested models provided no support for effect modification.
Changes from baseline
Compared with baseline levels, fasting leptin was significantly lower on all 3 intervention diets (Table 4). None of the intervention diets were associated with changes in fasting obestatin or ghrelin concentrations in comparison with baseline.
Table 4.
Fasting Hormone Concentrations at Baseline and Changes From Baseline Levels, Optimal Macronutrient Intake Trial to Prevent Heart Disease, 2003–2005
| Appetite Hormone | Baseline |
Change From Baseline to Intervention Dieta |
||||||||||||
| Carbohydrate-Rich Diet |
Protein-Rich Diet |
Unsaturated-Fat-Rich Diet |
||||||||||||
| No. of Subjects | Mean (SE) | No. of Subjects | Mean | 95% CI | P Value | No. of Subjects | Mean | 95% CI | P Value | No. of Subjects | Mean | 95% CI | P Value | |
| Leptin, ng/mLb | 163 | 11.5 (1.1) | 162 | 0.9 | 0.8, 0.9 | 0.02 | 160 | 0.8 | 0.8, 0.9 | <0.0001 | 160 | 0.9 | 0.8, 0.9 | 0.0002 |
| Obestatin, pg/mL | 156 | 120.6 (4.1) | 152 | 2.0 | −5.2, 9.2 | 0.58 | 150 | −6.2 | −14.2, 1.7 | 0.12 | 147 | 1.1 | −6.5, 8.8 | 0.77 |
| Ghrelin, pg/mL | 163 | 816.0 (31.1) | 162 | 21.4 | −19.4, 62.2 | 0.30 | 160 | 10.5 | −23.0, 43.9 | 0.54 | 160 | 18.6 | −22.1, 59.4 | 0.37 |
Abbreviations: CI, confidence interval; SE, standard error.
Generalized estimating equations model with an exchangeable correlation structure, with adjustment for site and period. Change from baseline was defined as follow-up value minus baseline value.
Leptin values were back-transformed from the loge scale. Measures shown are the baseline geometric mean value (SE) and the ratios of diet values to baseline values.
Associations between fasting hormone levels and self-reported appetite
Partial Spearman correlations between fasting ghrelin level and fasting obestatin level were 0.56 (P < 0.0001) after adjustment for intervention diet and site. No associations were observed between fasting ghrelin and fasting leptin (r = −0.09, P = 0.28) or between fasting obestatin and fasting leptin (r = −0.07, P = 0.37) after adjustment for intervention diet and site. Crude and adjusted estimates were similar.
In cross-sectional analyses, higher fasting leptin was associated with lower appetite at both premeal (r = −0.2, P = 0.0001) and postmeal (r = −0.3, P < 0.0001) assessments. Fasting obestatin and ghrelin were not correlated with the appetite measure. Crude and adjusted associations were similar.
DISCUSSION
In this randomized, isocaloric, crossover feeding study of adults with prehypertension or stage 1 hypertension, a healthy diet rich in protein lowered appetite in comparison with healthy diets rich in carbohydrate or unsaturated fat. Contrary to our hypotheses, the protein-rich diet reduced fasting leptin levels compared with the carbohydrate-rich diet, reduced fasting obestatin levels compared with both other diets, and had no effect on fasting ghrelin levels. We expected that both fasting leptin and fasting obestatin levels would increase and fasting ghrelin levels would decrease on the protein diet as compared with the carbohydrate and unsaturated-fat diets. These findings suggest that fasting levels of leptin, obestatin, and ghrelin do not explain the satiating effect of protein.
Our finding that self-reported appetite was 10%–15% lower on the protein diet than on the unsaturated-fat and carbohydrate diets is consistent with conclusions from 2 systematic reviews from smaller (<20 participants), short-term (single-meal) trials assessing the effects of very high protein (median level, 58.5% kcal) diets on thermogenesis, satiety, and weight loss (2, 3). Our trial provides evidence that the satiating effect of protein applies to a large, diverse study population, does not attenuate after several weeks, and applies to diets having a protein content (25% kcal) that could be adopted as a healthy dietary regimen. We cannot rule out the possibility of residual confounding (i.e., that another component of the plant-rich protein sources was responsible for the satiating effect), since nutrient levels are highly correlated. However, the 3 OMNI-Heart diets were similar with regard to characteristics that may affect appetite, including fiber, glycemic index, and energy density.
Fasting leptin was modestly correlated with the self-reported appetite measure. This corroborates data in obese women of a positive association between leptin and satiety (20). Investigators conducting short-term (<1 day) studies have reported a greater leptin response immediately following a meal (21, 22) and higher 24-hour circulating leptin levels (23) after exposure to a high-carbohydrate diet as compared with a high-fat diet. Results from longer-term studies (3 days–7 weeks) comparing fasting leptin levels after carbohydrate-rich diets versus fat-rich diets (24, 25) also corroborate those of the current study. Few data on the effects of protein on leptin levels were available prior to this study (26), and our data suggest that protein intake does not increase leptin levels. These findings suggest that fasting leptin does not mediate the satiating effect of protein.
The role of fasting obestatin in appetite regulation is uncertain. Initial reports of fasting obestatin activity demonstrated reduced food intake after administration of obestatin to fasting rodents (9), and these data were replicated by some other investigators (27), but not all (28). Contrary to expectation, fasting obestatin was significantly reduced, rather than increased, on the protein-rich diet. Therefore, fasting obestatin may be related to satiety, but the relation may be more complex in humans than in animals. Perhaps this represents a counterregulatory response, in that obestatin levels are lower when energy intake is perceived to be adequate.
One possible explanation for the null findings regarding fasting ghrelin is that the etiologically relevant concentration was postmeal, rather than premeal. Single-meal studies investigating the postprandial response of fasting ghrelin levels to manipulation of macronutrient intake suggest that fasting ghrelin level decreases more quickly and dramatically after carbohydrate consumption than after fat consumption (29–31). However, data from the current study and from other crossover trials comparing fasting ghrelin levels after longer-term exposure (>1 day) to diets differing in macronutrient composition show no effect of macronutrient composition on fasting ghrelin levels (32–34).
One limitation of our appetite hormone measurements was that postmeal blood was not collected. This limited our ability to assess the effect of macronutrient intake on levels of ghrelin, obestatin, and leptin, as well as levels of hormones released immediately after eating, such as cholecystokinin and glucagon-like peptide 1. Another limitation is that self-reported appetite was measured twice each day, immediately before and after the main meal; additional assessments at predefined intervals may provide a better measure of satiety.
Strengths of this trial include the diverse study population, high adherence, and completeness of data collection. The crossover design of this trial provided us with the opportunity to study appetite, an outcome with high interindividual variability. Appetite is not well-suited to assessment in parallel-arm trials unless the sample size is large (17). Dietary intervention studies are often limited by measurement error, but provision of all food and drink for 19 weeks of controlled feeding increased internal validity. While most other appetite studies are single-meal studies, we were able to measure appetite over an entire week of meals on each of the intervention diets after participants had been on the diet for 3 weeks. Hence, our measurements were more likely to reflect enduring physiologic effects of macronutrient intakes and were less likely to be influenced by outliers than measurements made after a single meal.
In conclusion, for reduced appetite, people should consume a healthy diet rich in protein. The pattern of hormone changes suggests that fasting levels of leptin, obestatin, and ghrelin do not explain the inverse association between protein intake and appetite. In future long-term studies, investigators should measure appetite hormones at multiple time points, including postmeal; measure other appetite hormones (e.g., glucagon-like peptide 1); and evaluate neurobehavioral mechanisms in order to better understand how protein intake affects appetite.
Acknowledgments
Author affiliations: Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Jeannette M. Beasley, Brett A. Ange, Cheryl A. M. Anderson, Edgar R. Miller, Janet T. Holbrook, Lawrence J. Appel); Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins Medical Institutions, Baltimore, Maryland (Brett A. Ange, Cheryl A. M. Anderson, Edgar R. Miller, Lawrence J. Appel); Department of Internal Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Edgar R. Miller, Lawrence J. Appel); Department of Internal Medicine, University of Texas Medical Branch, Austin, Texas (Tate P. Erlinger); and Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Frank M. Sacks).
This work was supported by the National Institutes of Health under the National Eye Institute Clinical Trials Training Program in Vision Research (training grant EY 07127), a Jean Hankin Nutritional Epidemiology Research Grant, and the Optimal Macronutrient Intake Trial to Prevent Heart Disease (grants HL67098, DK63214, HL68712, and RR02635).
This paper was presented during the Jeremiah and Rose Stamler Research Award for New Investigators session at the 48th Cardiovascular Disease Epidemiology and Prevention Conference, Colorado Springs, Colorado, March 13–15, 2008.
Conflict of interest: none declared.
Glossary
Abbreviations
- OMNI-Heart
Optimal Macronutrient Intake Trial to Prevent Heart Disease
- SD
standard deviation
References
- 1.Poppitt SD, McCormack D, Buffenstein R. Short-term effects of macronutrient preloads on appetite and energy intake in lean women. Physiol Behav. 1998;64(3):279–285. doi: 10.1016/s0031-9384(98)00061-4. [DOI] [PubMed] [Google Scholar]
- 2.Eisenstein J, Roberts SB, Dallal G, et al. High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr Rev. 2002;60(7):189–200. doi: 10.1301/00296640260184264. [DOI] [PubMed] [Google Scholar]
- 3.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23(5):373–385. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]
- 4.Apolzan JW, Carnell NS, Mattes RD, et al. Inadequate dietary protein increases hunger and desire to eat in younger and older men. J Nutr. 2007;137(6):1478–1482. doi: 10.1093/jn/137.6.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leidy HJ, Carnell NS, Mattes RD, et al. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007;15(2):421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 6.Raben A, Agerholm-Larsen L, Flint A, et al. Meals with similar energy densities but rich in protein, fat, carbohydrate, or alcohol have different effects on energy expenditure and substrate metabolism but not on appetite and energy intake. Am J Clin Nutr. 2003;77(1):91–100. doi: 10.1093/ajcn/77.1.91. [DOI] [PubMed] [Google Scholar]
- 7.Oakes ME. Filling yet fattening: stereotypical beliefs about the weight gain potential and satiation of foods. Appetite. 2006;46(2):224–233. doi: 10.1016/j.appet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Margetic S, Gazzola C, Pegg GG, et al. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26(11):1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JV, Ren PG, Avsian-Kretchmer O, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science. 2005;310(5750):996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 10.Cummings DE, Shannon MH. Roles for ghrelin in the regulation of appetite and body weight. Arch Surg. 2003;138(4):389–396. doi: 10.1001/archsurg.138.4.389. [DOI] [PubMed] [Google Scholar]
- 11.Carey VJ, Bishop L, Charleston J, et al. Rationale and design of the Optimal Macro-Nutrient Intake Heart Trial to Prevent Heart Disease (OMNI-Heart) Clin Trials. 2005;2(6):529–537. doi: 10.1191/1740774505cn123oa. [DOI] [PubMed] [Google Scholar]
- 12.Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Agriculture. Dietary Guidelines for Americans. 6th ed. Washington, DC: US GPO; 2005. [Google Scholar]
- 14.Swain JF, McCarron PB, Hamilton EF, et al. Characteristics of the diet patterns tested in the Optimal Macronutrient Intake Trial to Prevent Heart Disease (OmniHeart): options for a heart-healthy diet. J Am Diet Assoc. 2008;108(2):257–265. doi: 10.1016/j.jada.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34(3):362–6. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 16.Rogers PJ, Blundell JE. Effect of anorexic drugs on food intake and the micro-structure of eating in human subjects. Psychopharmacology (Berl) 1979;66(2):159–165. doi: 10.1007/BF00427624. [DOI] [PubMed] [Google Scholar]
- 17.Stubbs RJ, Hughes DA, Johnstone AM, et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr. 2000;84(4):405–415. doi: 10.1017/s0007114500001719. [DOI] [PubMed] [Google Scholar]
- 18.Stubbs RJ, van Wyk MC, Johnstone AM, et al. Breakfasts high in protein, fat or carbohydrate: effect on within-day appetite and energy balance. Eur J Clin Nutr. 1996;50(7):409–417. [PubMed] [Google Scholar]
- 19.Yildiz BO, Suchard MA, Wong ML, et al. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci U S A. 2004;101(28):10434–10439. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heini AF, Lara-Castro C, Kirk KA, et al. Association of leptin and hunger-satiety ratings in obese women. Int J Obes Relat Metab Disord. 1998;22(11):1084–1087. doi: 10.1038/sj.ijo.0800731. [DOI] [PubMed] [Google Scholar]
- 21.Romon M, Lebel P, Fruchart JC, et al. Postprandial leptin response to carbohydrate and fat meals in obese women. J Am Coll Nutr. 2003;22(3):247–251. doi: 10.1080/07315724.2003.10719300. [DOI] [PubMed] [Google Scholar]
- 22.Romon M, Lebel P, Velly C, et al. Leptin response to carbohydrate or fat meal and association with subsequent satiety and energy intake. Am J Physiol. 1999;277(5):E855–E861. doi: 10.1152/ajpendo.1999.277.5.E855. [DOI] [PubMed] [Google Scholar]
- 23.Havel PJ, Townsend R, Chaump L, et al. High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes. 1999;48(2):334–341. doi: 10.2337/diabetes.48.2.334. [DOI] [PubMed] [Google Scholar]
- 24.Schrauwen P, van Marken Lichtenbelt WD, Westerterp KR, et al. Effect of diet composition on leptin concentration in lean subjects. Metabolism. 1997;46(4):420–424. doi: 10.1016/s0026-0495(97)90059-7. [DOI] [PubMed] [Google Scholar]
- 25.Weigle DS, Duell PB, Connor WE, et al. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J Clin Endocrinol Metab. 1997;82(2):561–565. doi: 10.1210/jcem.82.2.3757. [DOI] [PubMed] [Google Scholar]
- 26.Weigle DS, Breen PA, Matthys CC, et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82(1):41–48. doi: 10.1093/ajcn.82.1.41. [DOI] [PubMed] [Google Scholar]
- 27.Lagaud GJ, Young A, Acena A, et al. Obestatin reduces food intake and suppresses body weight gain in rodents. Biochem Biophys Res Commun. 2007;357(1):264–269. doi: 10.1016/j.bbrc.2007.03.138. [DOI] [PubMed] [Google Scholar]
- 28.Gourcerol G, St-Pierre DH, Taché Y. Lack of obestatin effects on food intake: should obestatin be renamed ghrelin-associated peptide (GAP)? Regul Pept. 2007;141(1-3):1–7. doi: 10.1016/j.regpep.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Vicennati V, Genghini S, De Iasio R, et al. Circulating obestatin levels and the ghrelin/obestatin ratio in obese women. Eur J Endocrinol. 2007;157(3):295–301. doi: 10.1530/EJE-07-0059. [DOI] [PubMed] [Google Scholar]
- 30.Greenman Y, Golani N, Gilad S, et al. Ghrelin secretion is modulated in a nutrient- and gender-specific manner. Clin Endocrinol (Oxf) 2004;60(3):382–388. doi: 10.1111/j.1365-2265.2004.01993.x. [DOI] [PubMed] [Google Scholar]
- 31.Monteleone P, Bencivenga R, Longobardi N, et al. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. J Clin Endocrinol Metab. 2003;88(11):5510–5514. doi: 10.1210/jc.2003-030797. [DOI] [PubMed] [Google Scholar]
- 32.Erdmann J, Lippl F, Schusdziarra V. Differential effect of protein and fat on plasma ghrelin levels in man. Regul Pept. 2003;116(1-3):101–107. doi: 10.1016/s0167-0115(03)00195-2. [DOI] [PubMed] [Google Scholar]
- 33.Weigle DS, Cummings DE, Newby PD, et al. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J Clin Endocrinol Metab. 2003;88(4):1577–1586. doi: 10.1210/jc.2002-021262. [DOI] [PubMed] [Google Scholar]
- 34.Paul DR, Kramer M, Rhodes DG, et al. Preprandial ghrelin is not affected by macronutrient intake, energy intake or energy expenditure [electronic article] J Negat Results Biomed. 2005;4:2. doi: 10.1186/1477-5751-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]