Abstract
The authors conducted a cross-sectional study of the association of serum selenium with the prevalence of peripheral arterial disease among 2,062 US men and women 40 years of age or older participating in the National Health and Nutrition Examination Survey, 2003–2004. Serum selenium was measured by using inductively coupled plasma-dynamic reaction cell-mass spectrometry. Peripheral arterial disease was defined as an ankle-brachial blood pressure index <0.90. The age-, sex-, and race-adjusted prevalence of peripheral arterial disease decreased with increasing serum selenium (P for linear trend = 0.02), but there was an indication of an upturn in risk in the highest quartile of serum selenium. The fully adjusted odds ratios for peripheral arterial disease comparing selenium quartiles 2, 3, and 4 with the lowest quartile were 0.75 (95% confidence interval: 0.37, 1.52), 0.58 (95% confidence interval: 0.28, 1.19), and 0.67 (95% confidence interval: 0.34, 1.31), respectively. In spline regression models, peripheral arterial disease prevalence decreased with increasing serum selenium levels up to 150–160 ng/mL, followed by a gradual increase at higher selenium levels. The association between serum selenium levels and the prevalence of peripheral arterial disease was not statistically significant, although a U-shaped relation was suggested.
Keywords: antioxidants, cardiovascular diseases, cross-sectional studies, nutrition surveys, peripheral vascular diseases, selenium
Selenium, an essential micronutrient involved in antioxidant selenoenzymes such as glutathione peroxidases, has been hypothesized to prevent atherosclerotic disease (1–3). Glutathione peroxidase synthesis and activity, however, plateau at selenium levels above 70–90 ng/mL (4). In the United States compared with other countries, selenium intake is substantially higher (5), and most adults have serum selenium levels above 95 ng/mL (4). At these high concentrations, selenium is incorporated nonspecifically as selenomethionine in the synthesis of other plasma proteins, with unknown health effects (4). It is thus unclear whether increased selenium levels confer additional benefit for atherosclerosis prevention in the United States.
A recent meta-analysis of 14 prospective cohort studies found a modest, but statistically significant inverse association between selenium levels and coronary heart disease (3), but the 2 US studies in this meta-analysis found no association (3, 6, 7). Moreover, serum selenium levels were not associated with cardiovascular disease mortality in a prospective study in a representative US sample, although a U-shaped relation was suggested (8). Given the current interest in selenium supplements for chemoprevention of cancer (5), it is important to understand the overall impact of increased selenium intake on other health endpoints, including vascular disease.
Peripheral arterial disease, which affects about 8 million Americans, is characterized by flow-limiting atherosclerosis in the muscular arteries of the lower extremities and is an important marker of generalized atherosclerosis (9–11). Data on the association of selenium levels with peripheral arterial disease are very limited (12, 13). The objective of the present study was to assess the association between serum levels of selenium and reduced ankle-brachial blood pressure index (ABI), a specific subclinical marker for peripheral arterial disease, in the National Health and Nutrition Examination Survey (NHANES), 2003–2004. ABI values below 0.90 are considered diagnostic of peripheral arterial disease. Furthermore, a low ABI is an independent predictor of cardiovascular risk after adjusting for traditional cardiovascular risk factors (14, 15).
MATERIALS AND METHODS
NHANES is conducted by the National Center for Health Statistics (Hyattsville, Maryland) by using a complex multistage sampling design to obtain a representative sample of the civilian, noninstitutionalized US population. We used data from NHANES 2003–2004 (16) because it was the first NHANES survey to measure selenium and ABI levels simultaneously. In NHANES 2003–2004 interviews and physical examinations, the overall response rate was 76%. Serum selenium and ABI measurements were restricted to participants aged 40 years or older (N = 3,086). We excluded 2 pregnant women, 183 participants without selenium measurements, 514 participants without ABI measurements in both legs, and 314 participants with missing information on any adjustment covariate. We finally excluded 11 participants with left or right ABI measurements of more than 1.5, usually due to vessel stiffness. The final sample size was 2,062. The 2003–2004 NHANES study protocols were approved by the National Center for Health Statistics institutional review board. Oral and written informed consent was obtained from all participants.
Serum selenium
Collection materials were screened for potential selenium contamination. After blood collection, serum aliquots were obtained, frozen at −20°C, and shipped to the Trace Elements Laboratory at the Wadsworth Center of the New York State Department of Health for analysis. Serum selenium levels were measured by using inductively coupled plasma-dynamic reaction cell-mass spectrometry. The laboratory procedures and quality control methods for serum selenium measurement have been described in detail elsewhere (17). The between-assay coefficients of variation for quality-control pooled samples analyzed throughout the duration of the survey ranged from 2.5% to 2.9%.
Peripheral arterial disease
A specific protocol was used to measure ABI in NHANES 2003–2004 (18). The measurements of blood pressure used for ABI were additional to and different from other measurements of blood pressure used to evaluate hypertension. Systolic blood pressure was measured on the right arm (brachial artery) and both ankles (posterior tibial arteries) with a Doppler device, the Parks Mini-Lab IV, model 3100 (Parks Medical Electronics, Inc., Aloha, Oregon). If the participant had a condition that would interfere with blood pressure reading in the right arm, the left arm was used. Systolic blood pressure was measured twice at each site for participants aged 40–59 years and once at each site for participants aged 60 years or older. The left and right ABI measurements were obtained by dividing the mean systolic blood pressure in each ankle by the mean systolic blood pressure in the arm. Peripheral arterial disease was defined as an ABI value of less than 0.90 in at least one leg (14, 15).
Other variables
Information about age, sex, race-ethnicity, education, family income, menopausal status for women, cigarette smoking, alcohol consumption, use of dietary supplements, and use of cholesterol- and blood-pressure-lowering medications was based on self-report. Body mass index was calculated by dividing measured weight in kilograms by measured height in meters squared. Three to 4 systolic blood pressure measurements were taken and were averaged by using standardized protocols. Diabetes was defined as a fasting serum glucose concentration of 126 mg/dL or higher, a nonfasting serum glucose concentration of 200 mg/dL or higher, a self-reported physician diagnosis, or current medication use. Glomerular filtration rate was estimated by using the Modification of Diet in Renal Disease Study equation with serum creatinine values (19).
Statistical methods
Participants were grouped in quartiles of serum selenium levels based on the weighted population distribution. Odds ratios and 95% confidence intervals for peripheral arterial disease prevalence comparing the 3 highest quartiles of serum selenium with the lowest quartile were estimated by using logistic regression. Tests for linear risk trend across serum selenium quartiles were performed by including an ordinal variable with the median selenium level of each quartile in the logistic regression models. To further explore the shape of the dose-response relation between serum selenium levels and peripheral arterial disease prevalence, we used restricted quadratic splines with knots at the 5th, 50th, and 95th percentiles of serum selenium distribution. These spline models require the same number of parameters as the quartile analysis, but they can accommodate a wide variety of smooth risk trends (20). Sensitivity analyses using different numbers and locations of the knots, with cubic instead of quadratic splines, and log-transforming serum selenium levels gave similar results (not shown). Statistical analyses were performed with the survey package in R software (to account for the complex sampling design in NHANES 2003–2004) (21, 22). Strata, primary sampling units, and examination sample weights were used to obtain unbiased point estimates and robust linearized standard errors.
RESULTS
The weighted prevalence of peripheral arterial disease in the study population was 4.9%. Compared with participants without peripheral arterial disease, those with disease were more likely to be older, black, ever smokers, nondrinkers, and diabetic; to have a lower educational level and family income; and to use cholesterol- and blood-pressure-lowering medications (Table 1). Participants with peripheral arterial disease, compared with those without disease, also had higher average levels of body mass index, systolic blood pressure, and C-reactive protein and lower high density lipoprotein cholesterol levels and glomerular filtration rate.
Table 1.
Characteristics of the Study Population by the Presence or Absence of Peripheral Arterial Disease, National Health and Nutrition Examination Survey, 2003–2004a
| Peripheral Arterial Disease (n = 169) | No Peripheral Arterial Disease (n = 1,893) | P value | |
| Age, years | 67.2 (1.2) | 55.6 (0.4) | <0.001 |
| Sex: men | 48.3 (3.6) | 49.6 (1.4) | 0.75 |
| Race-ethnicity | 0.001 | ||
| White | 76.2 (4.8) | 80.4 (3.3) | |
| Black | 17.0 (3.8) | 8.5 (1.3) | |
| Mexican American | 4.4 (2.9) | 4.7 (1.7) | |
| Education <high school | 24.9 (4.5) | 15.8 (1.6) | 0.01 |
| Family income <$20,000 | 33.3 (4.6) | 19.6 (2.0) | 0.006 |
| Postmenopausal women | 44.1 (4.6) | 32.7 (1.4) | 0.02 |
| Cigarette smoking | 0.02 | ||
| Former | 48.0 (6.6) | 33.8 (1.2) | |
| Current | 23.6 (3.7) | 20.7 (1.1) | |
| Serum cotinine, ng/mL | 0.45 (1.5) | 0.40 (1.2) | 0.74 |
| Current alcohol drinking | 21.1 (3.6) | 32.8 (2.7) | 0.03 |
| Body mass index, kg/m2 | 29.9 (0.5) | 28.3 (0.2) | 0.01 |
| Dietary supplement use | 61.9 (3.7) | 63.1 (1.6) | 0.79 |
| C-reactive protein, mg/L | 3.4 (1.1) | 2.0 (1.0) | 0.001 |
| Total cholesterol, mg/dL | 201.9 (4.5) | 209.3 (1.4) | 0.13 |
| High density lipoprotein cholesterol, mg/dL | 51.8 (1.1) | 54.8 (0.5) | 0.03 |
| Cholesterol-lowering-medication use | 39.3 (4.7) | 19.2 (1.3) | <0.001 |
| Systolic blood pressure, mm Hg | 134.5 (1.4) | 127.1 (0.8) | <0.001 |
| Blood-pressure-lowering-medication use | 55.5 (5.2) | 29.2 (1.8) | <0.001 |
| Diabetes | 31.5 (6.8) | 11.0 (1.1) | 0.003 |
| Glomerular filtration rate <60 mL/minute per 1.73 m2 | 26.1 (4.5) | 10.3 (0.8) | <0.001 |
| Serum selenium, ng/mL | 134.5 (2.0) | 137.7 (1.2) | 0.11 |
Values are expressed as percentages (standard errors) for categorical variables or means (standard errors) for continuous variables; for serum cotinine and C-reactive protein, geometric means (geometric standard errors) are reported.
Participants in the highest quartile of serum selenium levels, compared with those in the lowest quartile, were more likely to be older, men, white, and nonsmokers and to use dietary supplements and cholesterol-lowering medications (Table 2). Serum selenium levels were also positively associated with total cholesterol, high density lipoprotein cholesterol, and systolic blood pressure and were inversely associated with body mass index and serum cotinine levels.
Table 2.
Characteristics of the Study Population by Quartile of Serum Selenium Level, National Health and Nutrition Examination Survey, 2003–2004a
| Quartile of Serum Selenium (ng/mL) |
P Value for Linear Trendb | ||||
| Quartile 1 (<125) | Quartile 2 (125–134) | Quartile 3 (135–147) | Quartile 4 (≥148) | ||
| Median serum selenium, ng/mL | 118 | 131 | 142 | 157 | |
| Age, years | 55.9 | 55.6 | 56.1 | 57.1 | 0.04 |
| Sex: men | 36.0 | 47.1 | 56.9 | 56.0 | <0.001 |
| Race-ethnicity | |||||
| White | 77.3 | 77.9 | 82.4 | 83.6 | 0.02 |
| Black | 14.2 | 8.9 | 7.7 | 5.1 | <0.001 |
| Mexican American | 3.2 | 4.8 | 4.4 | 5.3 | 0.05 |
| Education <high school | 15.8 | 13.3 | 12.3 | 13.2 | 0.54 |
| Family income <$20,000 | 25.5 | 14.3 | 16.8 | 19.8 | 0.33 |
| Postmenopausal women | 36.8 | 30.3 | 30.3 | 28.9 | 0.05 |
| Cigarette smoking | |||||
| Former | 28.0 | 35.4 | 31.9 | 38.7 | 0.03 |
| Current | 29.4 | 19.8 | 15.9 | 12.6 | <0.001 |
| Serum cotinine, ng/mL | 0.96 | 0.44 | 0.33 | 0.21 | <0.001 |
| Current alcohol drinking | 27.0 | 32.2 | 33.5 | 31.9 | 0.47 |
| Body mass index, kg/m2 | 28.8 | 28.2 | 28.8 | 27.6 | 0.01 |
| Dietary supplement use | 54.0 | 63.0 | 67.0 | 71.6 | 0.001 |
| C-reactive protein, mg/L | 2.4 | 2.0 | 2.0 | 1.9 | 0.09 |
| Total cholesterol, mg/dL | 199.0 | 204.9 | 209.9 | 220.8 | <0.001 |
| High density lipoprotein cholesterol, mg/dL | 52.5 | 54.9 | 54.6 | 56.3 | 0.01 |
| Cholesterol-lowering-medication use | 16.7 | 15.3 | 19.7 | 20.4 | 0.04 |
| Systolic blood pressure, mm Hg | 125.3 | 126.2 | 129.2 | 128.8 | 0.02 |
| Blood-pressure-lowering-medication use | 27.8 | 25.6 | 29.7 | 31.2 | 0.18 |
| Diabetes | 10.6 | 8.8 | 10.0 | 13.9 | 0.25 |
| Glomerular filtration rate <60 mL/minute per 1.73 m2 | 5.6 | 6.0 | 6.3 | 7.3 | 0.24 |
| Peripheral arterial disease | 5.0 | 3.3 | 2.5 | 2.5 | 0.02 |
Values are expressed as percentages for categorical variables or means for continuous variables adjusted for age (years), sex, and race-ethnicity; for serum cotinine and C-reactive protein, adjusted geometric means are reported.
P value for linear trend in percentages or means across quartiles of serum selenium adjusted for age (years), sex, and race-ethnicity.
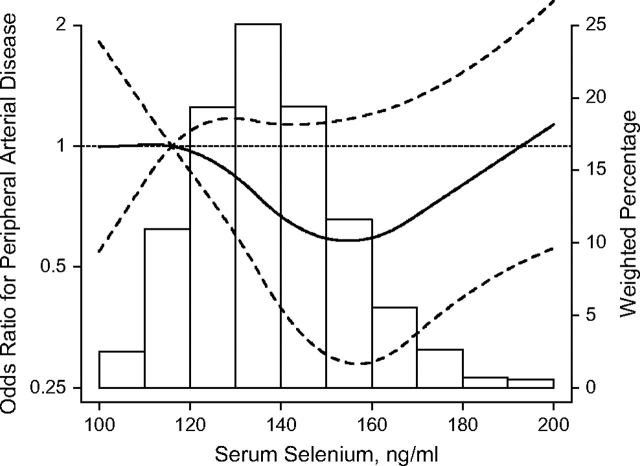
The age-, sex-, and race-adjusted prevalence of peripheral arterial disease decreased with increasing serum selenium levels (P for linear trend = 0.02) (Table 3). However, there was an indication of an upturn in risk trend in the highest quartile of serum selenium, particularly after adjusting for cardiovascular risk factors. The fully adjusted odds ratios for peripheral arterial disease comparing selenium quartiles 2, 3, and 4 with the lowest quartile were 0.75 (95% confidence interval: 0.37, 1.52), 0.58 (95% confidence interval: 0.28, 1.19), and 0.67 (95% confidence interval: 0.34, 1.31), respectively. In spline regression models, peripheral arterial disease prevalence decreased with increasing serum selenium levels up to 150–160 ng/mL (80th–91st percentiles of the serum selenium distribution in the study population), followed by a gradual increase at higher selenium levels (Figure 1). Consistently, there was a marginally significant U-shaped dose-response relation between serum selenium and peripheral arterial disease prevalence (P for quadratic spline terms = 0.06 and 0.12 after adjustment for age, sex, and race and after full adjustment, respectively).
Table 3.
Odds Ratios and 95% Confidence Intervals for Peripheral Arterial Disease by Quartile of Serum Selenium Level, National Health and Nutrition Examination Survey, 2003–2004
| Quartile of Serum Selenium (ng/mL) |
P Value for Linear Trenda | ||||||||
| Quartile 1 (<125) |
Quartile 2 (125–134) |
Quartile 3 (135–147) |
Quartile 4 (≥148) |
||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Median serum selenium, ng/mL | 118 | 131 | 142 | 157 | |||||
| Cases/noncases | 50/440 | 43/453 | 38/515 | 38/485 | |||||
| Model 1b | 1.00 | Reference | 0.65 | 0.40, 1.07 | 0.48 | 0.26, 0.90 | 0.49 | 0.28, 0.85 | 0.02 |
| Model 2c | 1.00 | Reference | 0.73 | 0.41, 1.30 | 0.57 | 0.29, 1.11 | 0.64 | 0.33, 1.23 | 0.16 |
| Model 3d | 1.00 | Reference | 0.75 | 0.37, 1.52 | 0.58 | 0.28, 1.19 | 0.67 | 0.34, 1.31 | 0.18 |
Abbreviations: CI, confidence interval; OR, odds ratio.
P value for linear risk trend across quartiles of serum selenium.
Adjusted for age (years), sex (men, women), and race-ethnicity (white, black, Mexican American, other).
Further adjusted for education (<high school, ≥high school), family income (<$20,000, ≥$20,000), postmenopausal status for women (yes, no), cigarette smoking (never, former, current), serum cotinine (log-transformed), alcohol consumption (yes, no), body mass index (kg/m2), and dietary supplement use (yes, no).
Further adjusted for C-reactive protein (log-transformed), total cholesterol (mg/dL), high density lipoprotein cholesterol (mg/dL), cholesterol-lowering-medication use (yes, no), systolic blood pressure (mm Hg), blood-pressure-lowering-medication use (yes, no), diabetes (yes, no), and glomerular filtration rate (<60, 60–<90, ≥90 mL/minute per 1.73 m2).
Figure 1.
Odds ratios for peripheral arterial disease by serum selenium levels, National Health and Nutrition Examination Survey, 2003–2004. Curves represent adjusted odds ratios (solid line) and their 95% confidence intervals (dashed lines) based on restricted quadratic splines for serum selenium levels with knots at the 5th, 50th, and 95th percentiles. The reference value (odds ratio = 1) was set at the 10th percentile of serum selenium distribution (116 ng/mL). Odd ratios were adjusted for age, sex, race-ethnicity, education, family income, postmenopausal status, smoking, serum cotinine, alcohol consumption, body mass index, dietary supplement use, C-reactive protein, total cholesterol, high density lipoprotein cholesterol, cholesterol-lowering medication use, systolic blood pressure, blood-pressure-lowering medication use, diabetes, and glomerular filtration rate. Bars represent the weighted histogram of serum selenium distribution.
DISCUSSION
In this cross-sectional study, conducted in a representative sample of the US population, the association between serum selenium levels and the prevalence of peripheral arterial disease was not statistically significant, although a U-shaped relation was suggested: the prevalence of peripheral arterial disease decreased with increasing serum selenium levels up to 150 ng/mL but increased with increasing selenium levels above 160 ng/mL. Selenium intake varies around the world primarily because of geographic variation in the amount of selenium in the soil (1, 23). In the United States, estimated selenium intake ranges from 60 μg/day to 220 μg/day (23), higher than the recommended dietary allowance for healthy adults (55 μg/day) (4). As a consequence, serum selenium levels in the United States are high: in NHANES 2003–2004, the median selenium level was 134 ng/mL, and 99% of study participants had serum selenium levels above 95 ng/mL. These concentrations are considerably higher than in other countries. In Europe, for instance, average serum selenium levels range from 50 ng/mL to 90 ng/mL (23, 24).
Very limited data are available on the association of selenium with peripheral arterial disease. Two small studies found similar selenium levels in patients with peripheral arterial disease compared with controls, but the dose-response relation was not evaluated (12, 13). For other cardiovascular outcomes, most prospective studies have been conducted in populations with suboptimal selenium levels in Europe or China (3, 25–36). These studies tended to report inverse associations between serum selenium levels and coronary heart disease incidence, but their sample sizes were too small for detailed dose-response analyses.
Findings from the only 2 prospective studies of serum selenium levels and coronary heart disease conducted in the United States, however, are consistent with a U-shaped relation (6, 8). In the Physicians’ Health Study, the relative risks for incident myocardial infarction comparing quintiles 2–5 of plasma selenium with the lowest quintile were 0.87, 0.82, 0.60, and 1.53, respectively (6). The cutoff levels for quintiles 1 and 5 of serum selenium in this study were 92 ng/mL and 134 ng/mL, respectively. In the NHANES III Mortality Study, the relative risks for cardiovascular disease mortality comparing tertiles 2 and 3 of serum selenium with the lowest tertile were 0.90 and 0.98, respectively; for stroke mortality, the corresponding relative risks were 0.73 and 1.23 (8). The cutoff levels for serum selenium tertiles in NHANES III were 117.3 ng/mL and 130.4 ng/mL, respectively. In this study, a dose-response analysis showed that cardiovascular and coronary heart disease mortality decreased with increasing serum selenium levels up to 120 ng/mL followed by an increase at higher levels, although the U-shaped relation was not statistically significant (8). Finally, in the Health Professionals Follow-up Study, the odds ratios for incident coronary heart disease comparing quintiles 2–5 of toenail selenium levels with the first quintile were 1.03, 0.99, 1.32, and 0.86, respectively, with no clear dose-response relation (7). Both serum and toenail selenium levels reflect selenium status, although toenails reflect longer-term exposure. It is unclear, however, whether both biomarkers are comparable in their ability to capture the different types of selenium compounds.
Few randomized trials have evaluated the effect of selenium supplementation on cardiovascular outcomes or atherosclerosis progression, and most of these studies combined selenium with other vitamins and minerals (3, 37). Only 2 of these trials were conducted in the United States, both reporting null results (38, 39). In the Nutritional Prevention of Cancer trial, the relative risk for cardiovascular disease incidence comparing 200 μg/day of selenium supplementation with placebo was 1.03 (95% confidence interval: 0.78, 1.37) (38). In the HDL-Atherosclerosis Treatment Study, the progression of atherosclerosis measured by coronary angiography in patients with coronary artery disease was similar among participants randomized to an antioxidant supplement containing 100 μg/day of selenium, 800 IU/day of vitamin E, 1 g/day of vitamin C, and 25 mg/day of β-carotene and participants randomized to placebo (39). Overall, limited evidence from randomized trials has not shown a protective effect of selenium supplementation in US studies. With respect to observational studies, those in the United States have not been able to detect a significant linear association between serum selenium and cardiovascular outcomes, but the dose-response associations in these studies were U-shaped.
The biologic mechanisms underlying a potential effect of selenium on cardiovascular disease are likely complex, but they may be related to the dual role of selenium as an essential and toxic element. Selenium is an essential micronutrient that is incorporated into glutathione peroxidases and other selenoproteins (4). Increasing serum selenium levels increase the concentration and activity of glutathione peroxidases, but this dose-response relation reaches a plateau at serum selenium levels of 70–90 ng/mL (4). As a consequence, higher selenium levels could potentially prevent atherosclerosis development and progression in populations whose selenium exposure is below the levels needed to maximize glutathione peroxidases (1–3). In selenium-replete populations such as in the United States, in which virtually all participants have serum selenium levels above 70–90 ng/mL, the mechanisms underlying a potential beneficial effect of increased selenium levels are unclear. Since selenium supplementation is actively promoted in the United States, and large randomized controlled trials testing the efficacy of selenium supplementation in prostate cancer prevention are under way (40, 41), mechanistic studies are urgently needed to establish the biologic basis for a protective effect of selenium in populations whose selenium status is already high.
Selenium, however, has a narrow therapeutic range (4), and it may even be harmful at intake levels below the current tolerable Upper Intake Level of 400 μg/day (2). In fact, some selenium compounds have been documented to generate reactive oxygen species (42–44), and the upturn in peripheral arterial disease prevalence that we observed at selenium levels above 160 ng/mL could be associated with selenium-induced increased oxidative stress. This upturn in risk is also consistent with recent reports showing increased risk of diabetes (45, 46) and elevated lipid levels (47) with high selenium levels in US populations. For instance, the Nutritional Prevention of Cancer trial showed an increased risk of diabetes for participants receiving 200 μg/day of selenium compared with placebo (hazard ratio = 1.50, 95% confidence interval: 1.03, 2.33) (46). Interestingly, the excess risk was limited to participants in the upper tertile of the serum selenium distribution (>121.6 ng/mL), who had a hazard ratio for diabetes of 2.70 (95% confidence interval: 1.30, 5.61). Further research is needed to establish the mechanisms underlying the association of high-normal selenium levels with peripheral arterial disease and with metabolic abnormalities, and to determine whether the change point in risk associated with elevated selenium levels depends on genetic polymorphisms in candidate genes for selenium metabolism (48).
Several limitations of our study need to be considered. The use of a cross-sectional design and of prevalent cases of peripheral arterial disease limited our ability to determine the direction and the causality of the observed association. It is possible that the pathophysiologic changes of atherosclerosis could modify serum selenium levels or that participants with peripheral arterial disease change their health behaviors, including selenium intake through diet and dietary supplements. As a consequence, our findings must be confirmed in prospective studies with incident cases of peripheral arterial disease. Another limitation of our study is the use of a single measurement of serum selenium, which reflects short-term selenium intake and may be subject to high within-person variability (49). Furthermore, our study measured only total serum selenium, and we did not have information on selenoprotein levels or activity or about nonspecific incorporation of selenium as selenomethionine in other plasma proteins. More detailed analysis of different compartments of serum selenium will be needed to better understand the association of selenium with peripheral arterial disease. The strengths of our study come from the rigorous sampling design and the quality of the study measurements used in NHANES; the representativeness of the NHANES sample; and the use of ABI, a noninvasive measure of subclinical atherosclerosis.
In summary, the association between serum selenium levels and the prevalence of peripheral arterial disease in NHANES 2003–2004 was not statistically significant, although a U-shaped relation was suggested. Other sources of evidence (6, 8) also suggest a U-shaped relation between serum selenium levels and cardiovascular outcomes in the United States, a selenium-replete population. In many populations worldwide, selenium intake is lower than in the United States (1, 23). At these lower levels of selenium intake, the association of selenium with peripheral arterial disease remains unknown. Prospective studies of selenium status across populations with different levels of selenium intake and randomized trials stratified by baseline selenium status are needed to establish the optimal selenium levels to minimize the risk of cardiovascular and other chronic diseases.
Acknowledgments
Author affiliations: Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Joachim Bleys, Ana Navas-Acien, Martin Laclaustra, Andy Menke, Eliseo Guallar); Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland (Eliseo Guallar); Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins Bloomberg School of Public Health and Johns Hopkins School of Medicine, Baltimore, Maryland (Joachim Bleys, Ana Navas-Acien, Martin Laclaustra, Andy Menke, Eliseo Guallar); Department of Environmental Health Sciences, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Ana Navas-Acien); Department of Cardiovascular Epidemiology and Population Genetics, National Center for Cardiovascular Research (CNIC), Madrid, Spain (Martin Laclaustra, Eliseo Guallar); National Center for Epidemiology, Instituto de Salud Carlos III, and the CIBER in Epidemiology and Public Health (CIBERESP), Madrid, Spain (Roberto Pastor-Barriuso); Friedman School of Nutrition Science and Policy at Tufts University, Boston, Massachusetts (Jose M. Ordovas); and Clinical Sciences Research Institute at Warwick Medical School, Coventry, United Kingdom (Saverio Stranges).
Supported by grants 1 R01 ES012673 from the National Institute of Environmental Health Sciences and 0230232N from the American Heart Association.
Conflict of interest: none declared.
Glossary
Abbreviations
- ABI
ankle-brachial blood pressure index
- NHANES
National Health and Nutrition Examination Survey
References
- 1.Rayman MP. Food-chain selenium and human health: emphasis on intake. Br J Nutr. 2008;100(2):254–268. doi: 10.1017/S0007114508939830. [DOI] [PubMed] [Google Scholar]
- 2.Navas-Acien A, Bleys J, Guallar E. Selenium intake and cardiovascular risk: what is new? Curr Opin Lipidol. 2008;19(1):43–49. doi: 10.1097/MOL.0b013e3282f2b261. [DOI] [PubMed] [Google Scholar]
- 3.Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, et al. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr. 2006;84(4):762–773. doi: 10.1093/ajcn/84.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Food and Nutrition Board, Institute of Medicine. Washington, DC: The National Academies Press; 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. A report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. [Google Scholar]
- 5.Rayman MP. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc. 2005;64(4):527–542. doi: 10.1079/pns2005467. [DOI] [PubMed] [Google Scholar]
- 6.Salvini S, Hennekens CH, Morris JS, et al. Plasma levels of the antioxidant selenium and risk of myocardial infarction among U.S. physicians. Am J Cardiol. 1995;76(17):1218–1221. doi: 10.1016/s0002-9149(99)80344-0. [DOI] [PubMed] [Google Scholar]
- 7.Yoshizawa K, Ascherio A, Morris JS, et al. Prospective study of selenium levels in toenails and risk of coronary heart disease in men. Am J Epidemiol. 2003;158(9):852–860. doi: 10.1093/aje/kwg052. [DOI] [PubMed] [Google Scholar]
- 8.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168(4):404–410. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- 9.McDermott MM, Liu K, Criqui MH, et al. Ankle-brachial index and subclinical cardiac and carotid disease: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2005;162(1):33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 10.Murabito JM, Evans JC, Larson MG, et al. The ankle-brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study. Arch Intern Med. 2003;163(16):1939–1942. doi: 10.1001/archinte.163.16.1939. [DOI] [PubMed] [Google Scholar]
- 11.Newman AB, Shemanski L, Manolio TA, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19(3):538–545. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 12.Ondrus P, Alberty R, Vassanyiova Z. Importance of lipid peroxidation, protective enzymes and trace elements in chronic leg ischaemia. Eur J Clin Chem Clin Biochem. 1996;34(6):471–475. doi: 10.1515/cclm.1996.34.6.471. [DOI] [PubMed] [Google Scholar]
- 13.Mansoor MA, Bergmark C, Haswell SJ, et al. Correlation between plasma total homocysteine and copper in patients with peripheral vascular disease. Clin Chem. 2000;46(3):385–391. [PubMed] [Google Scholar]
- 14.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 15.Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics, Centers for Disease Control and Prevention. 2007. National Health and Nutrition Examination Survey, 2003–2004. ( http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/nhanes03_04.htm). (Accessed August 24, 2008) [Google Scholar]
- 17.National Center for Health Statistics, Centers for Disease Control and Prevention. 2007. NHANES 2003–2004 Data Documentation. Laboratory Assessment: Lab 39—Erythrocyte Protoporphyrin and Selenium. ( http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l39epp_c.pdf). (Accessed August 24, 2008) [Google Scholar]
- 18.National Center for Health Statistics, Centers for Disease Control and Prevention. 2005. NHANES 2003–2004 Data Documentation. Exam Component: Ankle-Brachial Blood Pressure Index (ABPI) Section of the Lower Extremity Disease Examination (LEXAB_C) ( http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/lexab_c.pdf). (Accessed August 24, 2008) [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6(4):356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 21.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. (ISBN 3-900051-07-0). ( http://www.R-project.org) [Google Scholar]
- 22.Lumley T. The Survey Package. ( http://cran.fhcrc.org/web/packages/survey/survey.pdf). (Accessed August 24, 2008) [Google Scholar]
- 23.Combs GF., Jr Selenium in global food systems. Br J Nutr. 2001;85(5):517–547. doi: 10.1079/bjn2000280. [DOI] [PubMed] [Google Scholar]
- 24.Rayman MP. The importance of selenium to human health. Lancet. 2000;356(9225):233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 25.Salonen JT, Alfthan G, Huttunen JK, et al. Association between cardiovascular death and myocardial infarction and serum selenium in a matched-pair longitudinal study. Lancet. 1982;2(8291):175–179. doi: 10.1016/s0140-6736(82)91028-5. [DOI] [PubMed] [Google Scholar]
- 26.Miettinen TA, Alfthan G, Huttunen JK, et al. Serum selenium concentration related to myocardial infarction and fatty acid content of serum lipids. Br Med J (Clin Res Ed) 1983;287(6391):517–519. doi: 10.1136/bmj.287.6391.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salonen JT, Salonen R, Penttila I, et al. Serum fatty acids, apolipoproteins, selenium and vitamin antioxidants and the risk of death from coronary artery disease. Am J Cardiol. 1985;56(4):226–231. doi: 10.1016/0002-9149(85)90839-2. [DOI] [PubMed] [Google Scholar]
- 28.Virtamo J, Valkeila E, Alfthan G, et al. Serum selenium and the risk of coronary heart disease and stroke. Am J Epidemiol. 1985;122(2):276–282. doi: 10.1093/oxfordjournals.aje.a114099. [DOI] [PubMed] [Google Scholar]
- 29.Ringstad J, Thelle D. Risk of myocardial infarction in relation to serum concentrations of selenium. Acta Pharmacol Toxicol (Copenh) 1986;59(suppl 7):336–339. doi: 10.1111/j.1600-0773.1986.tb02774.x. [DOI] [PubMed] [Google Scholar]
- 30.Kok FJ, de Bruijn AM, Hofman A, et al. Selenium status and chronic disease mortality: Dutch epidemiological findings. Int J Epidemiol. 1987;16(2):329–332. doi: 10.1093/ije/16.2.329. [DOI] [PubMed] [Google Scholar]
- 31.Ringstad J, Jacobsen BK, Thomassen Y, et al. The Tromsø Heart Study: serum selenium and risk of myocardial infarction a nested case-control study. J Epidemiol Community Health. 1987;41(4):329–332. doi: 10.1136/jech.41.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suadicani P, Hein HO, Gyntelberg F. Serum selenium concentration and risk of ischaemic heart disease in a prospective cohort study of 3000 males. Atherosclerosis. 1992;96(1):33–42. doi: 10.1016/0021-9150(92)90035-f. [DOI] [PubMed] [Google Scholar]
- 33.Marniemi J, Jarvisalo J, Toikka T, et al. Blood vitamins, mineral elements and inflammation markers as risk factors of vascular and non-vascular disease mortality in an elderly population. Int J Epidemiol. 1998;27(5):799–807. doi: 10.1093/ije/27.5.799. [DOI] [PubMed] [Google Scholar]
- 34.Kilander L, Berglund L, Boberg M, et al. Education, lifestyle factors and mortality from cardiovascular disease and cancer. A 25-year follow-up of Swedish 50-year-old men. Int J Epidemiol. 2001;30(5):1119–1126. doi: 10.1093/ije/30.5.1119. [DOI] [PubMed] [Google Scholar]
- 35.Wei WQ, Abnet CC, Qiao YL, et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr. 2004;79(1):80–85. doi: 10.1093/ajcn/79.1.80. [DOI] [PubMed] [Google Scholar]
- 36.Akbaraly NT, Arnaud J, Hininger-Favier I, et al. Selenium and mortality in the elderly: results from the EVA study. Clin Chem. 2005;51(11):2117–2123. doi: 10.1373/clinchem.2005.055301. [DOI] [PubMed] [Google Scholar]
- 37.Bleys J, Miller ER, III, Pastor-Barriuso R, et al. Vitamin-mineral supplementation and the progression of atherosclerosis: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006;84(4):880–887. doi: 10.1093/ajcn/84.4.880. [DOI] [PubMed] [Google Scholar]
- 38.Stranges S, Marshall JR, Trevisan M, et al. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. Am J Epidemiol. 2006;163(8):694–699. doi: 10.1093/aje/kwj097. [DOI] [PubMed] [Google Scholar]
- 39.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 40.Lippman SM, Goodman PJ, Klein EA, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97(2):94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 41.Marshall JR, Sakr W, Wood D, et al. Design and progress of a trial of selenium to prevent prostate cancer among men with high-grade prostatic intraepithelial neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1479–1484. doi: 10.1158/1055-9965.EPI-05-0585. [DOI] [PubMed] [Google Scholar]
- 42.Spallholz JE. On the nature of selenium toxicity and carcinostatic activity. Free Radic Biol Med. 1994;17(1):45–64. doi: 10.1016/0891-5849(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 43.Spallholz JE, Palace VP, Reid TW. Methioninase and selenomethionine but not Se-methylselenocysteine generate methylselenol and superoxide in an in vitro chemiluminescent assay: implications for the nutritional carcinostatic activity of selenoamino acids. Biochem Pharmacol. 2004;67(3):547–554. doi: 10.1016/j.bcp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Drake EN. Cancer chemoprevention: selenium as a prooxidant, not an antioxidant. Med Hypotheses. 2006;67(2):318–322. doi: 10.1016/j.mehy.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 45.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in U.S. adults. Diabetes Care. 2007;30(4):829–834. doi: 10.2337/dc06-1726. [DOI] [PubMed] [Google Scholar]
- 46.Stranges S, Marshall JR, Natarajan R, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(4):217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 47.Bleys J, Navas-Acien A, Stranges S, et al. Serum selenium and serum lipids in US adults. Am J Clin Nutr. 2008;88(2):416–423. doi: 10.1093/ajcn/88.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Méplan C, Crosley LK, Nicol F, et al. Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender-specific manner (the SELGEN study) FASEB J. 2007;21(12):3063–3074. doi: 10.1096/fj.07-8166com. [DOI] [PubMed] [Google Scholar]
- 49.Longnecker MP, Stampfer MJ, Morris JS, et al. A 1-y trial of the effect of high-selenium bread on selenium concentrations in blood and toenails. Am J Clin Nutr. 1993;57(3):408–413. doi: 10.1093/ajcn/57.3.408. [DOI] [PubMed] [Google Scholar]