Abstract
Di-(2-ethylhexyl) phthalate (DEHP) is a plasticizer used in consumer and medical products that can cross the placenta, disrupt steroid hormone synthesis, and activate peroxisome proliferator-activated receptor γ. The authors examined DEHP exposure in relation to the timing of labor in a pregnancy cohort study of 283 women recruited in 4 US states (California, Iowa, Minnesota, and Missouri) between 2000 and 2004. The authors estimated associations between concentrations of DEHP metabolites and gestational age at delivery using linear regression models and associations between DEHP metabolites and clinical outcomes using logistic regression models. After covariate adjustment, women at the 75th percentile of DEHP metabolite concentrations had a 2-day-longer mean length of gestation than women at the 25th percentile (95% confidence interval: 1.4, 3.3). Log-unit increases in mono-2-ethylhexyl phthalate and mono-2-ethyl-5-oxohexyl phthalate concentrations were associated with increased odds of cesarean section delivery (30% and 50% increased odds, respectively), increased odds of delivering at 41 weeks or later (100% and 120% increased odds), and reduced odds of preterm delivery (50% and 60% decreased odds). These data suggest that DEHP may interfere with signaling related to the timing of parturition.
Keywords: creatinine, diethylhexyl phthalate, endocrine disruptors, gestational age, parturition, placenta, PPAR γ, pregnancy
Di-(2-ethylhexyl) phthalate (DEHP) is a plasticizer that is widely used in consumer products, such as polyvinyl chloride flooring, carpeting, roofing, vinyl, upholstery, clothing, and packaging (1). DEHP exposure occurs through ingestion of food and water (2, 3) and inhalation of household dust (4), as well as parenterally from medical devices (1). DEHP metabolites have been detected in 95% of the US population aged ≥6 years (5).
The hydrolytic metabolite of DEHP, mono-(2-ethylhexyl) phthalate (MEHP), can cross the placenta and enter fetal circulation (6, 7). Metabolites of DEHP have been measured in umbilical cord blood (8), amniotic fluid (9), maternal urine (10), placental tissue (7, 11), and neonatal urine and meconium (12, 13). MEHP is further metabolized into oxidative metabolites, including mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP) and mono-2-ethyl-5-oxohexyl phthalate (MEOHP). MEHP and its oxidative metabolites can be glucuronidated and excreted in urine and feces. These 3 metabolites account for approximately 50% of the received dose of DEHP (14).
Among rodents, DEHP suppresses fetal testosterone synthesis in males (15, 16) and inhibits ovarian aromatase transcription in adult females (17, 18). Aromatase, which is responsible for the conversion of androgens to estradiol, is expressed in several tissues, including the human placenta (19). Inhibition of steroid hormone synthesis in the ovary of the adult rodent, fetal testis, and fetal brain has been linked to binding and activation of the transcription factor peroxisome proliferator-activated receptor γ (PPARγ) (20–22). MEHP can activate PPARγ in primate fibroblasts, in rodent granulosa and trophoblast cells, and in human cervical cancer cells (21–24).
PPARγ activation increases early in pregnancy to promote zygote implantation and placental development (25–27). During pregnancy, high levels of placental PPARγ contribute to uterine quiescence by down-regulating cyclooxygenase 2 and inhibiting prostaglandin synthesis. By contrast, at parturition, PPARγ activation decreases in order to allow for increased prostaglandin production, which is essential in the stimulation of uterine contractions (26, 28). It has been hypothesized that exposure to PPARγ ligands during pregnancy reduces the risk of preterm labor by suppressing the inflammatory response in fetal tissues (27).
We tested the hypothesis that exposure to DEHP alters the timing of labor by measuring the associations between maternal urinary concentrations of DEHP metabolites during pregnancy and gestational age at delivery, as well as between these metabolites and risk of cesarean delivery, preterm birth, and delivering at greater than 41 weeks’ gestation.
MATERIALS AND METHODS
Women included in this analysis were recruited into the Study for Future Families (SFF) between March 2000 and August 2004 at 4 US sites. Details on the SFF are available elsewhere (29). Briefly, women were recruited at prenatal clinics associated with university hospitals in Los Angeles, California (Harbor-UCLA and Cedars-Sinai); Minneapolis, Minnesota (University of Minnesota Health Center); Columbia, Missouri (University of Missouri School of Medicine); and Iowa City, Iowa (University of Iowa School of Medicine). Women were eligible if they were at least 18 years of age, conception had been natural, and the pregnancy was not medically threatened.
Of the 783 pregnant women enrolled in the SFF between 2000 and 2004, only those who were fully enrolled in a follow-up study for postnatal evaluation of their babies were eligible for inclusion in the phthalate study (n = 441). For a woman to be eligible for follow-up, the pregnancy had to end in a livebirth, the baby had to be 2–36 months of age, the mother and baby had to live within 50 miles (80 km) of the clinic, and the mother had to attend at least 1 study visit. Reasons for exclusion were unlikely to be related to either phthalate exposure or gestational age at delivery (except possibly a nonviable birth outcome, which was extremely rare). The current analysis (n = 283) was restricted to mothers for whom we had urinary phthalate concentrations (n = 304) and complete medical record data (n = 298) after excluding twin births (12 babies), 2 babies with missing data, and 1 baby born at 30 weeks with eclampsia. Women provided 1 urine sample at the time of recruitment, which was on average 12.2 weeks (standard deviation, 7.6 weeks) before delivery or the beginning of the third trimester.
Human subject committees at each of the participating centers approved study procedures, and all participants gave signed informed consent. Data were stripped of identifying information before analysis.
Gestational age at delivery was calculated in 2 ways. First, the date of the last menstrual period as reported by the woman at study entry was subtracted from the date of delivery to obtain an estimate in days. Second, the clinical estimate recorded by the obstetrician was abstracted directly from the birth record. The clinical estimate was based on ultrasound data, examination of the newborn, and dates reported by the mother and was rounded up to the next-highest week. There was a high correlation between the 2 estimates (r = 0.92, n = 283) after we substituted the clinical estimate for cases for which there was a discrepancy of 14 or more days (30). We used the clinical estimate in the current analysis.
Urinary phthalate metabolite concentrations were measured using analytical methods described in detail elsewhere (31). Briefly, the phthalate metabolites were first enzymatically deconjugated and then extracted from the urine using automated on-line solid phase extraction, separated by high-performance liquid chromatography, and detected by isotope-dilution tandem mass spectrometry. Each analytical run included calibration standards, reagent blanks, and quality control materials of high and low concentration to monitor for accuracy and precision. The limits of detection were 0.98 ng/mL (MEHP), 0.95 ng/mL (MEHHP), and 1.07 ng/mL (MEOHP).
Because of the high correlation and pharmacokinetic similarities between MEOHP and MEHHP (r = 0.99), only estimates for MEOHP are described below.
Statistical analysis
Geometric mean values for urinary phthalates and their 95% confidence intervals, unadjusted and adjusted for creatinine concentration, were calculated and compared with US population estimates for women of reproductive age (i.e., 18–40 years) derived from 1999–2000 and 2001–2002 National Health and Nutrition Examination Survey (NHANES) data (32, 33). We also calculated geometric mean values and 95% confidence intervals for US pregnant women using the NHANES data (n = 209 for MEHP and n = 104 for MEOHP, MEHHP, and %MEHP (defined below)). Because the NHANES sample was nonrandom, we used the recommended weights to correctly estimate variances (34). Phthalate metabolite concentrations, which were all right-skewed, were transformed using the natural logarithm. For concentrations below the limit of detection, we assigned a value equal to the limit of detection divided by the square root of 2 (35). A potential phenotypic marker of DEHP metabolism, %MEHP, was calculated as the ratio of MEHP concentration to the sum of the 3 DEHP metabolite concentrations (in nanomoles) and transformed using the natural logarithm (36).
We adjusted for urinary dilution in 2 ways. For comparisons with NHANES data, we used metabolite concentrations divided by creatinine concentrations (μg/g creatinine). However, because creatinine is associated with demographic and physiologic parameters that may be on the causal pathway from exposure to outcome (37, 38), we included a square root transformation of creatinine (mg/dL) as a covariate in all regression models rather than dividing the concentration by creatinine.
Spearman correlation coefficients were used to estimate pairwise associations among phthalate concentrations. Multivariate linear regression models were used to evaluate associations between urinary phthalate concentrations and gestational age at delivery. Although the distribution of gestational ages was slightly skewed, a sensitivity analysis using generalized estimating equations, which is valid even under departure from normality, produced similar results. Logistic regression was used to calculate associations of phthalate concentrations with binary outcomes, such as cesarean section delivery.
Covariates, including demographic characteristics (race, geographic site, mother's education, mother's age, mother's employment status during pregnancy), sample characteristics (timing of urine sample, creatinine), previous pregnancy history (parity, history of miscarriage), sex of the baby, maternal and paternal smoking, job-related stress, and mother's prepregnancy health (nongestational diabetes, thyroid disorders, fibroids, high blood pressure, and respiratory conditions (asthma and chronic obstructive pulmonary disease)) were considered as potential confounders (Table 1) and were retained in the model if they were significant at P ≤ 0.15. Potential effect modifiers evaluated included sex of the baby, %MEHP status (low/high), geographic location, timing of the urine sample, and parity. We had no information on mother's weight prior to pregnancy, body mass index, or weight gain during pregnancy.
Table 1.
Characteristics of Participants (n = 283) in the Study for Future Families, a US Multicenter Pregnancy Cohort Study, 2000–2004
| Characteristic | Overall |
Study Center |
||||||||
| California |
Iowa |
Minnesota |
Missouri |
|||||||
| No. or Mean | % | No. or Mean | % | No. or Mean | % | No. or Mean | % | No. or Mean | % | |
| No. of subjects | 283 | 100 | 48 | 17 | 80 | 28 | 87 | 31 | 68 | 24 |
| Time between urine collection and delivery, weeksa | ||||||||||
| Mean | 12 (8)b | 14 (8) | 11 (8) | 12 (8) | 12 (6) | |||||
| First trimester | 5 | 2 | 1 | 2 | 0 | 0 | 4 | 5 | 0 | 0 |
| Second trimester | 119 | 42 | 24 | 51 | 34 | 43 | 34 | 39 | 27 | 40 |
| Third trimester | 157 | 56 | 22 | 47 | 45 | 57 | 49 | 56 | 41 | 60 |
| Sex of baby | ||||||||||
| Male | 143 | 51 | 24 | 50 | 38 | 47 | 45 | 52 | 36 | 53 |
| Female | 140 | 49 | 24 | 50 | 42 | 53 | 42 | 48 | 32 | 47 |
| Parity (no. of prior livebirths)*** | ||||||||||
| 0 | 145 | 51 | 30 | 63 | 32 | 40 | 52 | 60 | 31 | 46 |
| 1 | 87 | 31 | 11 | 23 | 25 | 31 | 27 | 31 | 24 | 35 |
| ≥2 | 51 | 18 | 7 | 15 | 23 | 29 | 8 | 9 | 13 | 19 |
| Racec,*** | ||||||||||
| White | 238 | 84 | 22 | 46 | 72 | 91 | 84 | 97 | 60 | 88 |
| Hispanic | 26 | 9 | 20 | 42 | 2 | 3 | 0 | 0 | 4 | 6 |
| Other | 18 | 6 | 6 | 13 | 5 | 6 | 3 | 3 | 4 | 6 |
| Education* | ||||||||||
| High school or less | 25 | 9 | 9 | 19 | 6 | 8 | 3 | 3 | 7 | 10 |
| Some college | 63 | 22 | 19 | 40 | 14 | 18 | 20 | 23 | 10 | 15 |
| College graduation | 106 | 38 | 10 | 21 | 34 | 43 | 33 | 38 | 29 | 43 |
| Graduate school | 89 | 31 | 10 | 21 | 26 | 33 | 31 | 36 | 22 | 32 |
| History of miscarriage (yes/no) | 60 | 21 | 9 | 19 | 20 | 25 | 14 | 16 | 17 | 25 |
| Employed during pregnancy (yes/no) | 231 | 82 | 33 | 69 | 63 | 79 | 79 | 91 | 56 | 82 |
| Job-related stress | ||||||||||
| No work-related stress | 52 | 18 | 15 | 31 | 17 | 21 | 8 | 9 | 12 | 18 |
| Not at all stressful | 17 | 6 | 2 | 4 | 4 | 5 | 6 | 7 | 5 | 7 |
| Not too stressful | 80 | 28 | 14 | 29 | 21 | 26 | 29 | 33 | 16 | 24 |
| Somewhat stressful | 105 | 37 | 12 | 25 | 32 | 40 | 35 | 40 | 26 | 38 |
| Very stressful | 29 | 10 | 5 | 10 | 6 | 8 | 9 | 10 | 9 | 13 |
| Maternal health | ||||||||||
| Diabetes | 14 | 5 | 4 | 8 | 6 | 8 | 1 | 1 | 3 | 4 |
| Thyroid disorders | 17 | 6 | 5 | 10 | 5 | 6 | 2 | 2 | 5 | 7 |
| Fibroids* | 16 | 6 | 1 | 2 | 8 | 10 | 2 | 2 | 5 | 7 |
| High blood pressure | 19 | 7 | 4 | 8 | 5 | 6 | 5 | 6 | 5 | 7 |
| Respiratory conditions | 26 | 9 | 4 | 8 | 10 | 13 | 5 | 6 | 7 | 10 |
| Mean creatinine level, mg/dL | 92 (61) | 99 (63) | 93 (64) | 82 (51) | 97 (66) | |||||
| Mean maternal age, years* | 30.2 (6.0) | 28.3 (6.0) | 30.6 (4.6) | 30.8 (5.3) | 30.3 (4.6) | |||||
| Gestational age at delivery (clinical estimate), weeksd | ||||||||||
| Mean** | 39.2 (1.5) | 38.9 (1.3) | 38.9 (1.3) | 39.7 (1.3) | 39.1 (1.8) | |||||
| <37 | 14 | 5 | 3 | 6 | 4 | 5 | 2 | 2 | 5 | 7 |
| 37–41 | 262 | 93 | 45 | 94 | 76 | 95 | 81 | 93 | 60 | 88 |
| >41 | 7 | 2 | 0 | 0 | 0 | 0 | 4 | 5 | 3 | 4 |
* P < 0.05; **P < 0.01; ***P < 0.001 (P for difference between at least 2 of the 4 study centers).
Information on timing of urine sample collection was missing for 2 participants.
Numbers in parentheses, standard deviation.
Information on race was missing for 1 participant.
Clinical estimate of gestational age abstracted from the birth medical record; values were reported rounded to the next-highest week. Information was missing for 1 participant.
Statistical significance was defined by a (2-sided) P value of 0.05 or lower and was expressed as a 95% confidence interval. SAS 9.1 software (SAS Institute Inc., Cary, North Carolina) was used to conduct all analyses.
RESULTS
Demographic and other sample characteristics varied by geographic site (Table 1), with significant differences in parity, race, education, history of fibroids, and gestational age. After creatinine adjustment, DEHP metabolite concentrations varied across geographic locations, with significantly higher concentrations of MEHP among women from Missouri and Minnesota than among women from California and Iowa. White non-Hispanic mothers, who made up 84% of the study population, had marginally higher concentrations of MEHP and MEOHP than Hispanics. Women with some college education had somewhat lower concentrations of MEOHP than women with a high school education or less. Higher levels of job-related stress were slightly associated with higher MEHP concentrations. Women with a previous miscarriage had significantly lower urinary concentrations of MEHP. Metabolite and creatinine concentrations were not related to the timing of the urine sample.
The unadjusted mean concentrations of all 3 DEHP metabolites in the SFF women were somewhat lower than the NHANES US population estimates for pregnant women and women of reproductive age (Table 2), but only the mean MEHHP concentration in SFF women was significantly lower than that for nonpregnant women (11.9 ng/mL vs. 19.8 ng/mL). Neither the unadjusted nor the creatinine-adjusted mean values for pregnant women differed significantly between the SFF and the NHANES.
Table 2.
Urinary Concentrations (ng/mL) of Di-(2-Ethylhexyl) Phthalate Metabolites Among Participants (n = 283) in the Study for Future Families (2000–2004) as Compared With Participants in the National Health and Nutrition Examination Survey (1999–2002)
| Di-(2-Ethylhexyl) Phthalate Metabolite | % of Samples With Values Greater Than Limit of Detection | Percentil e |
Study for Future Families |
NHANES Pregnant Womena |
NHANES Reproductive-Age Womenb |
|||||||
| 5th | 25th | 50th | 75th | 95th | GM | 95% CI | GM | 95% CI | GM | 95% CI | ||
| MEHP | 76 | 0.6 | 1.1 | 3.5 | 8.2 | 40.2 | 3.6 | 3.1, 4.3 | 4.8 | 3.8, 6.0 | 4.5 | 4.0, 5.0 |
| MEHHP | 97 | 1.1 | 5.6 | 11.2 | 25.5 | 99.4 | 11.9 | 10.1, 13.9 | 19.0 | 13.5, 26.7 | 19.8 | 14.9, 26.2 |
| MEOHP | 96 | 1.2 | 5.1 | 9.9 | 24.6 | 68.4 | 10.9 | 9.3, 12.6 | 15.4 | 11.3, 21.1 | 13.8 | 10.4, 18.3 |
| %MEHPc | NA | 4 | 8 | 14 | 23 | 48 | 14 | 12, 15 | 17 | 13, 20 | 13 | 11, 14 |
Abbreviations: CI, confidence interval; GM, geometric mean; MEHHP, mono-2-ethyl-5-hydrohexyl phthalate; MEHP, mono-2-ethylhexyl phthalate; MEOHP, mono-2-ethyl-5-oxohexyl phthalate; NA, not applicable; NHANES, National Health and Nutrition Examination Survey.
NHANES data on pregnant women, 1999–2000 (MEHP) and 2001–2002 (MEHP, MEOHP, and MEHHP) (n = 209 for MEHP and n = 104 for MEHHP, MEOHP, and %MEHP).
NHANES data on women aged 18–40 years, 1999–2000 (MEHP) and 2001–2002 (MEHP, MEOHP, and MEHHP) (n = 853 for MEHP and n = 437 for MEHHP, MEOHP, and %MEHP).
MEHP/Σ(MEHP, MEOHP, MEHHP) × 100.
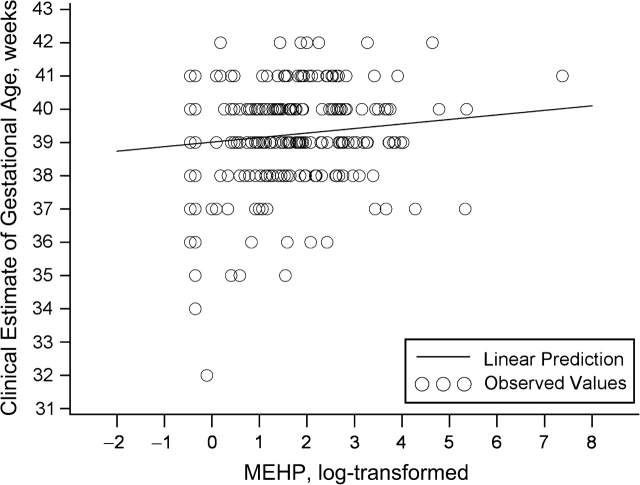
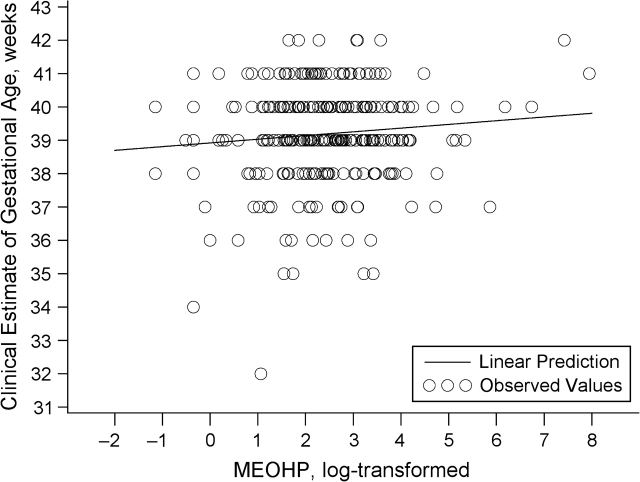
Associations of the DEHP metabolites with gestational age, unadjusted and adjusted for covariates, are presented in Table 3 and Figures 1 and 2. Overall, a log-unit increase in the urinary concentrations of metabolites was associated with a 1.3- to 1.5-day increase in gestational age. The effect size was slightly greater for MEOHP than for MEHP, and we saw no association between %MEHP and gestational age. Effect estimates were somewhat attenuated after adjustment for potential confounders. Each tertile increase in MEHP concentration was associated with a 1.8-day (95% confidence interval (CI): 0.2, 3.4) increase in gestational age, and each tertile increase in MEOHP concentration was associated with a 1.9-day increase (95% CI: 0.1, 3.8). We compared estimates after removing 1 outlier for urinary MEHP concentration (1,600 ng/mL) and 1 outlier for gestational age (32 weeks) (Figure 1). The effect estimates did not change appreciably. Since we did not see any reasons, medical or otherwise, for excluding these subjects from the analysis, they were included.
Table 3.
Relations of Urinary Concentrations of Di-(2-Ethylhexyl) Phthalate Metabolites in Pregnant Women to Gestational Age at Delivery in Linear Regression Models (n = 283), Study for Future Families, 2000–2004a*
| Model | MEHP |
MEOHP |
MEHHP |
|||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Adjusted for creatinine | 0.18 | 0.04, 0.32 | 0.21 | 0.05, 0.38 | 0.19 | 0.05, 0.34 |
| Adjusted for creatinine + demographic factorsb | 0.17 | 0.03, 0.32 | 0.20 | 0.03, 0.36 | 0.18 | 0.02, 0.33 |
| Adjusted for above factors + maternal healthc | 0.17 | 0.03, 0.31 | 0.19 | 0.03, 0.35 | 0.17 | 0.02, 0.32 |
| Adjusted for above factors + parity | 0.16 | 0.02, 0.30 | 0.19 | 0.03, 0.35 | 0.16 | 0.01, 0.31 |
Abbreviations: CI, confidence interval; MEHHP, mono-2-ethyl-5-hydrohexyl phthalate; MEHP, mono-2-ethylhexyl phthalate; MEOHP, mono-2-ethyl-5-oxohexyl phthalate.
P < 0.05 for all results shown in table.
Change in gestational age at delivery (weeks) per log-unit increase in urinary di-(2-ethylhexyl) phthalate metabolite concentration.
Geographic center, mother's educational level, and job-related stress.
Nongestational diabetes, thyroid disorders, and fibroids.
Figure 1.
Clinically estimated gestational age as a function of log urinary mono-2-ethylhexyl phthalate (MEHP) concentration (ng/mL) (n = 283) after adjustment for creatinine, Study for Future Families, 2000–2004.
Figure 2.
Clinically estimated gestational age as a function of log urinary mono-2-ethyl-5-oxohexyl phthalate (MEOHP) concentration (ng/mL) (n = 283) after adjustment for creatinine, Study for Future Families, 2000–2004.
The odds of giving birth at greater than 41 weeks increased by 100% and 120% with each log-unit increase in MEHP and MEOHP concentrations, respectively, after adjustment for covariates (Table 4). Conversely, we observed 50% and 60% reductions in the odds of preterm delivery with a log-unit increase in MEHP and MEOHP concentrations, respectively. The frequency of premature delivery (6%) was slightly lower than the NHANES US population estimate of 11.5% for whites, because we excluded twins and an eclampsia case, which would bring the overall prevalence to 9.5%. The higher socioeconomic status of this cohort also contributed to the slightly lower prevalence. The odds of a cesarean section delivery increased by 50% with each log-unit increase in MEOHP concentration and by 30% with each log-unit increase in MEHP concentration. We explored potential explanations for the association of cesarean section with DEHP metabolite concentrations. Women who had “failure of labor to progress” listed as a reason for cesarean section delivery had a mean MEOHP concentration that was 0.33 log units higher (95% CI: 0.08, 0.57) than that of all other women, after controlling for other reasons for cesarean section. We found no associations of DEHP metabolite concentrations with breech presentation (n = 8), repeat cesarean section (n = 15), or medically induced (n = 85) or augmented (n = 55) labor or with duration of labor measured in minutes.
Table 4.
Relations of Urinary Concentrations of Di-(2-Ethylhexyl) Phthalate Metabolites in Pregnant Women to Clinical Outcomes Related to Parturition and Labor in Logistic Regression Models (n = 283), Study for Future Families, 2000–2004a
| Outcome | No. of Subjects | % | MEHP |
MEOHP |
MEHHP |
|||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |||
| Gestational age >41 weeks | 7 | 2 | ||||||
| Adjusted for creatinine | 1.8* | 1.1, 3.0 | 2.1** | 1.2, 3.4 | 1.9* | 1.1, 3.2 | ||
| Adjusted for creatinine + covariatesb | 2.0* | 1.1, 3.5 | 2.2** | 1.3, 4.0 | 2.1* | 1.3, 3.7 | ||
| Preterm delivery (<37 weeks) | 14 | 5 | ||||||
| Adjusted for creatinine | 0.5* | 0.3, 0.9 | 0.5* | 0.2, 0.9 | 0.5* | 0.3, 0.9 | ||
| Adjusted for creatinine + covariatesc | 0.5* | 0.3, 0.9 | 0.4* | 0.2, 0.9 | 0.5* | 0.3, 0.9 | ||
| Cesarean section delivery | 62 | 22 | ||||||
| Adjusted for creatinine | 1.3 | 1.0, 1.6 | 1.4* | 1.1, 1.9 | 1.4* | 1.1, 1.8 | ||
| Adjusted for creatinine + covariatesd | 1.3* | 1.0, 1.6 | 1.5** | 1.1, 1.9 | 1.4* | 1.1, 1.8 | ||
| Failure to progress as reason for cesarean section delivery | 18 | 6 | ||||||
| Adjusted for creatinine | 1.3 | 0.9, 1.8 | 1.6* | 1.1, 2.4 | 1.5* | 1.1, 2.2 | ||
| Adjusted for creatinine + covariatese | 1.2 | 0.9, 1.7 | 1.6* | 1.1, 2.3 | 1.5* | 1.0, 2.1 | ||
Abbreviations: CI, confidence interval; MEHHP, mono-2-ethylhexyl phthalate; MEHP, mono-2-ethylhexyl phthalate; MEOHP, mono-2-ethyl-5-oxohexyl phthalate; OR, odds ratio.
* P < 0.05; **P < 0.01.
Change in log odds per log-unit increase in urinary di-(2-ethylhexyl) phthalate metabolite concentration.
Adjusted for geographic site (Minnesota vs. California, Iowa, and Missouri) and respiratory conditions.
Adjusted for high blood pressure and nongestational diabetes.
Adjusted for mother's age (≥35 years vs. <35 years), geographic site (Minnesota vs. California, Iowa, and Missouri), nongestational diabetes, and fibroids.
Adjusted for parity, thyroid conditions, and high blood pressure. Information on the reason for cesarean section was missing for 2 subjects.
We conducted stratified analyses by geographic site and %MEHP status (low/high). In Missouri, Iowa, and California, gestational age at delivery tended to increase with higher MEHP and MEOHP concentrations, while in Minnesota gestational age tended to decrease (though not significantly). After adjustment for creatinine, mother's education, parity, and job-related stress, the difference in slopes for the 2 groups (California, Iowa, and Missouri vs. Minnesota) was significant for MEOHP (P = 0.02) and marginally significant for MEHP (P = 0.06).
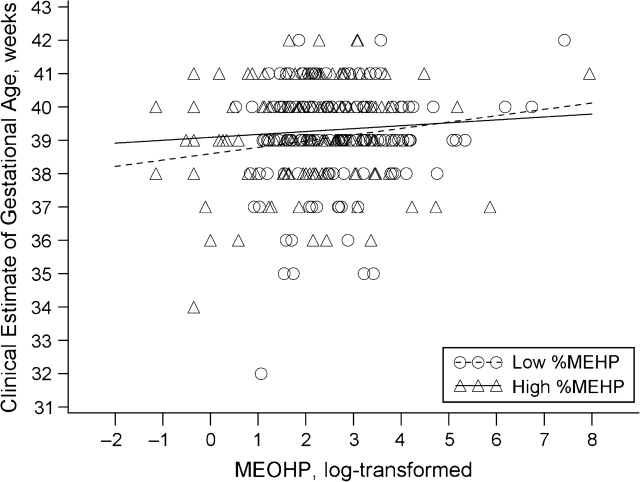
The association of MEOHP concentrations with gestational age among women in the low-%MEHP category was approximately 3 times stronger in magnitude than in the high-%MEHP group after adjustment for creatinine, geographic site, mother's education, and job-related stress. When modeled as a multiplicative interaction term, the P value was 0.10 for differences in slope between the 2 groups (Figure 3). There was no difference in MEHP slope between the low- and high-%MEHP categories.
Figure 3.
Clinically estimated gestational age as a function of log urinary mono-2-ethyl-5-oxohexyl phthalate (MEOHP) concentration (ng/mL), by percent mono-2-ethylhexyl phthalate (%MEHP) status (low/high) (n = 283), after adjustment for creatinine and covariates, Study for Future Families, 2000–2004. %MEHP was calculated as the ratio of MEHP concentration to the sum of the concentrations of 3 di-(2-ethylhexyl) phthalate metabolites (MEHP, MEOHP, and mono-2-ethylhexyl phthalate) (in nanomoles) and transformed using the natural logarithm.
DISCUSSION
In approximately 300 pregnant women from 4 US locations, we found that gestation was 1.1 days and 1.3 days longer with each log-unit increase in urinary concentrations of the DEHP metabolites MEHP and MEOHP, respectively. Women at the 75th percentile of urinary MEHP concentration had a duration of gestation that was 2.3 days (95% CI: 1.4, 3.3) longer than that of women at the 25th percentile of exposure, after we controlled for urinary creatinine concentration, demographic factors, maternal health, stress, and parity. DEHP exposure in this cohort was similar to the NHANES US population estimate for pregnant females.
The clinical or population significance of an exposure-related shift of 2–3 days in gestational length is difficult to evaluate. For this reason, we also estimated associations with clinical outcomes and saw increased odds of delivering after 41 weeks, decreased odds of preterm delivery, and increased odds of delivering by cesarean section. Delivery after 41 weeks is associated with an increase in perinatal mortality due to meconium aspiration, fetal distress, asphyxia, pneumonia, malformations, shoulder dystocia, and traumatic injuries (39, 40). Women who undergo cesarean section are at increased risk in subsequent pregnancies of malpresentation, abnormal placentation, antepartum hemorrhage, placenta accreta, prolonged labor, uterine rupture, preterm birth, low birth weight, and stillbirth (41). The observed decrease in the risk of preterm delivery may be protective or may be indicative of abnormal function of the placenta (26); in this study, we could not distinguish between these possibilities.
A prior study using MEHP concentration in umbilical cord blood from 84 Italian mother/newborn pairs found an association that pointed in the opposite direction than was observed here (8). Latini et al. (8) reported that gestational age was shorter by 1.2 weeks in the MEHP-positive pairs than in the MEHP-negative subjects. In that study, they measured MEHP and DEHP in umbilical cord blood, which may have been subject to contamination by DEHP in the sampling and analytic equipment (42). Measuring MEHP in blood is not recommended because of its short half-life (14). In that analysis, exposure status was dichotomized as exposed and nonexposed. Given that more than 95% of the general US population has detectable urinary metabolites of DEHP (5), this approach could have resulted in misclassification among the nonexposed subjects. The discrepant results between these 2 studies could be due to differences in study design, exposure assessment, and/or the underlying characteristics of the populations.
When we stratified SFF subjects by geographic location, gestational age tended to increase with increasing phthalate metabolite concentrations for all sites except Minnesota, where it tended to remain flat or decrease slightly. The women from Minnesota tended to have higher MEOHP concentrations and %MEHP, higher gestational age, higher maternal age, and more education than women from other study centers, most markedly relative to California, and they were predominantly white (97%). We can speculate on 2 possible explanations. It is possible that the dose-response curve was nonlinear and essentially reversed at the higher doses among the Minnesota subjects. It is also possible that the site-specific populations differed in ways that modified the relation between DEHP exposure and placental function. The significant differences in race, education, maternal age, and parity between study centers could be proxies for other unmeasured effect modifiers, such as nutritional factors, coexposures, and/or lifestyle factors.
Other known causes of prolonged gestation include fish oil consumption during pregnancy (43, 44), deficiency in placental sulfatase, which is another cause of decreased estrogen synthesis (45), and living in a highly polluted area (46). Fish oil contains n-3 long-chain polyunsaturated fatty acids, which are also ligands of PPARγ and may contribute to a suppressed inflammatory response late in pregnancy (47, 48). If this were the case, it is possible that a competitive interaction between DEHP and fatty acids in the diet exists. We could not test this hypothesis, since fish consumption among our subjects was generally low (88% of those who consumed fish had 2 or fewer servings per week). We did not have information on the type of fish consumed or on how it was prepared.
We hypothesized that the association between DEHP exposure and timing of labor could also differ depending on a woman's ability to metabolize and excrete DEHP. To test this, we dichotomized %MEHP values at the median and compared metabolite associations within the low and high strata. The association of MEOHP concentrations with longer gestation was 3-fold stronger in the low-%MEHP group than in the high-%MEHP group. The association of MEOHP concentration with the timing of labor might be due to disruption in parturition and signaling by DEHP metabolites, but it might also be due to differences in a woman's ability to metabolize and excrete DEHP. In a previous report, we found %MEHP to be approximately twice as reproducible within a woman over the last 6 weeks of pregnancy as DEHP metabolites (10), suggesting that %MEHP may reflect stable interindividual differences that could be relevant to the metabolism and excretion of these compounds in pregnancy.
Concern exists about the potential for systematic error in estimating gestational age using the last-menstrual-period approach (49, 50). We addressed this by also using the clinical estimate, which takes into account ultrasound data and examination of the newborn in cases where there are inconsistencies in last-menstrual-period dating and clinical presentation, but the clinical estimate may have been less precise, given that it was rounded up. We found results to be consistent using both measures when including all gestational ages and less consistent when modeling associations with preterm and postterm delivery. Misclassification of gestational age by last menstrual period is most problematic among preterm and postterm births, with the degree of misclassification being associated with maternal race, age, education, parity, month that prenatal care began (51), and regularity of the menstrual period (52). Given that some of these factors are also related to phthalate exposure and pregnancy outcomes, we relied on the clinical estimate to model associations with DEHP metabolite concentrations.
Some misclassification of DEHP exposure was present in our data, given that we had a single spot urine sample for characterizing exposure. In a previous analysis, we showed that DEHP metabolite concentrations are not highly reproducible in pregnant women over the last 6 weeks of pregnancy, probably because of physiologic changes occurring in the third trimester (10). Of the SFF subjects, 55% were sampled in the third trimester. In addition to misclassification, there may have been other exposures and risk factors associated with urinary DEHP metabolite concentrations and birth outcomes that we were not able to adequately control for, resulting in residual confounding.
In conclusion, we observed an association between increased concentrations of DEHP metabolites in maternal urine measured during pregnancy and gestational age in a US multicenter pregnancy cohort study. The direction and size of the effect appeared to differ depending in part on geographic location and a woman's ability to metabolize and eliminate DEHP. Our results support the hypothesis that DEHP exposure may alter the dialogue between the maternal and fetal compartments that is essential for normal labor. Consistent with this hypothesis, urinary DEHP metabolite concentrations were associated with an increased risk of cesarean section delivery and of delivering at more than 41 weeks. The binding affinity of the metabolite MEHP for PPARγ and the central role PPARγ plays in regulating placental function may provide an explanation for this association, but this was not directly explored in the present study.
These results need to be replicated in other populations. There is likewise a need for more in vitro and in vivo research to better understand molecular mechanisms by which DEHP may alter placental development and/or function.
Acknowledgments
Author affiliations: Department of Environmental Health, Harvard School of Public Health, Boston, Massachusetts (Jennifer J. Adibi, Russ Hauser, Heather Nelson, Robert Herrick); Department of Biostatistics, Harvard School of Public Health, Boston, Massachusetts (Paige L. Williams); Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, New York (Robin M. Whyatt); National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, Georgia (Antonia M. Calafat); and Department of Obstetrics and Gynecology, University of Rochester Medical Center, Rochester, New York (Shanna H. Swan).
This research was funded by the Environmental Protection Agency (Star Grant R-82943601-0) and the National Institute of Environmental Health Sciences (grant R01 ES013543). Jennifer Adibi’s doctoral training was funded by the Harvard Education and Research Center for Occupational Safety and Health (grant T42 OH008416).
The authors thank Maureen Nealon and Fan Liu for the processing and transfer of data, Dr. Sonia Hernandez-Diaz for her mentoring with regard to epidemiologic methods, Dr. Shruthi Mahalingaiah for her help in interpreting clinical data, and Dr. Rick Stalhut for his assistance in accessing and analyzing data from the National Health and Nutrition Examination Survey. The authors also thank Dr. Manori Silva, Dr. Jack Reidy, Jim Preau, and Ella Samandar of the Centers for Disease Control and Prevention for the phthalate metabolite measurements.
The findings and conclusions presented in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- DEHP
di-(2-ethylhexyl) phthalate
- MEHHP
mono-2-ethylhexyl phthalate
- MEHP
mono-2-ethylhexyl phthalate
- MEOHP
mono-2-ethyl-5-oxohexyl phthalate
- NHANES
National Health and Nutrition Examination Survey
- PPARγ
peroxisome proliferator-activated receptor γ
- SFF
Study for Future Families
References
- 1.Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29(1):134–139. doi: 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 2.Kavlock R, Barr D, Boekelheide K, et al. NTP-CERHR expert panel update on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol. 2006;22(3):291–399. doi: 10.1016/j.reprotox.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention. Toxicological Profile for Di(2-ethylhexyl)phthalate (DEHP) Atlanta, GA: Centers for Disease Control and Prevention; 2002. ( http://www.atsdr.cdc.gov/toxprofiles/tp9.html#9). (Accessed December 15, 2007) [PubMed] [Google Scholar]
- 4.Rudel RA, Camann DE, Spengler JD, et al. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol. 2003;37(20):4543–4553. doi: 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- 5.Kato K, Silva MJ, Reidy JA, et al. Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ Health Perspect. 2004;112(3):327–330. doi: 10.1289/ehp.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh AR, Lawrence WH, Autian J. Maternal-fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats. J Pharm Sci. 1975;64(8):1347–1350. doi: 10.1002/jps.2600640819. [DOI] [PubMed] [Google Scholar]
- 7.Mose T, Mortensen GK, Hedegaard M, et al. Phthalate monoesters in perfusate from a dual placenta perfusion system, the placenta tissue and umbilical cord blood. Reprod Toxicol. 2007;23(1):83–91. doi: 10.1016/j.reprotox.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Latini G, De Felice C, Presta G, et al. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect. 2003;111(14):1783–1785. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva MJ, Reidy JA, Herbert AR, et al. Detection of phthalate metabolites in human amniotic fluid. Bull Environ Contam Toxicol. 2004;72(6):1226–1231. doi: 10.1007/s00128-004-0374-4. [DOI] [PubMed] [Google Scholar]
- 10.Adibi JJ, Whyatt RM, Williams PL, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116(4):467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poole CF, Wibberley DG. Determination of di-(2-ethylhexyl)phthalate in human placenta. J Chromatogr. 1977;132(3):511–518. doi: 10.1016/s0021-9673(00)82915-5. [DOI] [PubMed] [Google Scholar]
- 12.Kato K, Silva MJ, Needham LL, et al. Quantifying phthalate metabolites in human meconium and semen using automated off-line solid-phase extraction coupled with on-line SPE and isotope-dilution high-performance liquid chromatography–tandem mass spectrometry. Anal Chem. 2006;78(18):6651–6655. doi: 10.1021/ac0608220. [DOI] [PubMed] [Google Scholar]
- 13.Weuve J, Sanchez BN, Calafat AM, et al. Exposure to phthalates in neonatal intensive care unit infants: urinary concentrations of monoesters and oxidative metabolites. Environ Health Perspect. 2006;114(9):1424–1431. doi: 10.1289/ehp.8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch HM, Bolt HM, Preuss R, et al. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79(7):367–376. doi: 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- 15.Parks LG, Ostby JS, Lambright CR, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000;58(2):339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- 16.Akingbemi BT, Youker RT, Sottas CM, et al. Modulation of rat Leydig cell steroidogenic function by di(2-ethylhexyl)phthalate. Biol Reprod. 2001;65(4):1252–1259. doi: 10.1095/biolreprod65.4.1252. [DOI] [PubMed] [Google Scholar]
- 17.Davis BJ, Maronpot RR, Heindel JJ. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol. 1994;128(2):216–223. doi: 10.1006/taap.1994.1200. [DOI] [PubMed] [Google Scholar]
- 18.Lovekamp TN, Davis BJ. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol. 2001;172(3):217–224. doi: 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- 19.Fournet-Dulguerov N, MacLusky NJ, Leranth CZ, et al. Immunohistochemical localization of aromatase cytochrome P-450 and estradiol dehydrogenase in the syncytiotrophoblast of the human placenta. J Clin Endocrinol Metab. 1987;65(4):757–764. doi: 10.1210/jcem-65-4-757. [DOI] [PubMed] [Google Scholar]
- 20.Borch J, Metzdorff SB, Vinggaard AM, et al. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology. 2006;223(1-2):144–155. doi: 10.1016/j.tox.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Lovekamp-Swan T, Jetten AM, Davis BJ. Dual activation of PPARα and PPARγ by mono-(2-ethylhexyl) phthalate in rat ovarian granulosa cells. Mol Cell Endocrinol. 2003;201(1-2):133–141. doi: 10.1016/s0303-7207(02)00423-9. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Cook TJ, Knipp GT. Effects of di-(2-ethylhexyl)-phthalate (DEHP) and its metabolites on fatty acid homeostasis regulating proteins in rat placental HRP-1 trophoblast cells. Toxicol Sci. 2005;84(2):287–300. doi: 10.1093/toxsci/kfi083. [DOI] [PubMed] [Google Scholar]
- 23.Feige JN, Gelman L, Rossi D, et al. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor γ modulator that promotes adipogenesis. J Biol Chem. 2007;282(26):19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- 24.Hurst CH, Waxman DJ. Activation of PPARα and PPARγ by environmental phthalate monoesters. Toxicol Sci. 2003;74(2):297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 25.Fournier T, Tsatsaris V, Handschuh K, et al. PPARs and the placenta. Placenta. 2007;28(2-3):65–76. doi: 10.1016/j.placenta.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Froment P, Gizard F, Defever D, et al. Peroxisome proliferator-activated receptors in reproductive tissues: from gametogenesis to parturition. J Endocrinol. 2006;189(2):199–209. doi: 10.1677/joe.1.06667. [DOI] [PubMed] [Google Scholar]
- 27.Schaiff WT, Barak Y, Sadovsky Y. The pleiotropic function of PPARγ in the placenta. Mol Cell Endocrinol. 2006;249(1-2):10–15. doi: 10.1016/j.mce.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Dunn-Albanese LR, Ackerman WE IV, Xie Y, et al. Reciprocal expression of peroxisome proliferator-activated receptor-gamma and cyclooxygenase-2 in human term parturition. Am J Obstet Gynecol. 2004;190(3):809–816. doi: 10.1016/j.ajog.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 29.Swan SH, Main KM, Liu F, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113(8):1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin C, Dietz PM, England LJ, et al. Effects of different data-editing methods on trends in race-specific preterm delivery rates, United States, 1990–2002. Paediatr Perinat Epidemiol. 2007;21(suppl 2):41–49. doi: 10.1111/j.1365-3016.2007.00860.x. [DOI] [PubMed] [Google Scholar]
- 31.Silva MJ, Slakman AR, Reidy JA, et al. Analysis of human urine for fifteen phthalate metabolites using automated solid-phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;805(1):161–167. doi: 10.1016/j.jchromb.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 32.National Center for Health Statistics. National Health and Nutrition Examination Survey. NHANES 1999–2000. Hyattsville, MD: National Center for Health Statistics; 2006. ( http://www.cdc.gov/nchs/about/major/nhanes/nhanes99_00.htm). (Accessed July 11, 2006) [Google Scholar]
- 33.National Center for Health Statistics. National Health and Nutrition Examination Survey. NHANES 2001–2002. Hyattsville, MD: National Center for Health Statistics; 2006. ( http://www.cdc.gov/nchs/about/major/nhanes/nhanes01-02.htm). (Accessed July 11, 2006) [Google Scholar]
- 34.National Center for Health Statistics. Continuous NHANES Web Tutorial. Hyattsville, MD: National Center for Health Statistics; 2007. ( http://www.cdc.gov/nchs/tutorials/NHANES/index_current.htm). (Accessed June 18, 2007) [Google Scholar]
- 35.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- 36.Hauser R, Meeker JD, Singh NP, et al. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum Reprod. 2007;22(3):688–695. doi: 10.1093/humrep/del428. [DOI] [PubMed] [Google Scholar]
- 37.Barr DB, Wilder LC, Caudill SP, et al. Urinary creatinine concentrations in the US population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schisterman EF, Whitcomb BW, Louis GM, et al. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005;113(7):853–857. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olesen AW, Westergaard JG, Olsen J. Perinatal and maternal complications related to postterm delivery: a national register-based study, 1978–1993. Am J Obstet Gynecol. 2003;189(1):222–227. doi: 10.1067/mob.2003.446. [DOI] [PubMed] [Google Scholar]
- 40.Hilder L, Costeloe K, Thilaganathan B. Prolonged pregnancy: evaluating gestation-specific risks of fetal and infant mortality. Br J Obstet Gynaecol. 1998;105(2):169–173. doi: 10.1111/j.1471-0528.1998.tb10047.x. [DOI] [PubMed] [Google Scholar]
- 41.Kennare R, Tucker G, Heard A, et al. Risks of adverse outcomes in the next birth after a first cesarean delivery. Obstet Gynecol. 2007;109(2):270–276. doi: 10.1097/01.AOG.0000250469.23047.73. [DOI] [PubMed] [Google Scholar]
- 42.Calafat AM, Needham LL. Factors affecting the evaluation of biomonitoring data for human exposure assessment. Int J Androl. 2008;31(2):139–143. doi: 10.1111/j.1365-2605.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- 43.Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD003402.pub2. CD003402. [DOI] [PubMed] [Google Scholar]
- 44.Szajewska H, Horvath A, Koletzko B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006;83(6):1337–1344. doi: 10.1093/ajcn/83.6.1337. [DOI] [PubMed] [Google Scholar]
- 45.Cunningham FG, Williams JW. Williams Obstetrics. New York, NY: McGraw-Hill Professional; 2005. [Google Scholar]
- 46.Shea KM, Wilcox AJ, Little RE. Postterm delivery: a challenge for epidemiologic research. Epidemiology. 1998;9(2):199–204. doi: 10.1097/00001648-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 47.Olsen SF, Østerdal ML, Salvig JD, et al. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: a randomised clinical trial with fish oil. Eur J Clin Nutr. 2007;61(8):976–985. doi: 10.1038/sj.ejcn.1602609. [DOI] [PubMed] [Google Scholar]
- 48.Schaiff WT, Knapp FF, Jr, Barak Y, et al. Ligand-activated peroxisome proliferator activated receptor gamma alters placental morphology and placental fatty acid uptake in mice. Endocrinology. 2007;148(8):3625–3634. doi: 10.1210/en.2007-0211. [DOI] [PubMed] [Google Scholar]
- 49.Hediger M, Kiely J. Foreword. Paediatr Perinat Epidemiol. 2007;21(suppl 2):1–3. [Google Scholar]
- 50.Savitz DA, Terry JW, Jr, Dole N, et al. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002;187(6):1660–1666. doi: 10.1067/mob.2002.127601. [DOI] [PubMed] [Google Scholar]
- 51.Dietz PM, England LJ, Callaghan WM, et al. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California livebirth and prenatal screening records. Paediatr Perinat Epidemiol. 2007;21(suppl 2):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 52.Ananth CV. Menstrual versus clinical estimate of gestational age dating in the United States: temporal trends and variability in indices of perinatal outcomes. Paediatr Perinat Epidemiol. 2007;21(suppl 2):22–30. doi: 10.1111/j.1365-3016.2007.00858.x. [DOI] [PubMed] [Google Scholar]