Abstract
Among men of European ancestry, diabetics have a lower risk of prostate cancer than do nondiabetics. The biologic basis of this association is unknown. The authors have examined whether the association is robust across populations in a population-based prospective study. The analysis included 5,941 prostate cancer cases identified over a 12-year period (1993–2005) among 86,303 European-American, African-American, Latino, Japanese-American, and Native Hawaiian men from the Multiethnic Cohort. The association between diabetes and prostate-specific antigen (PSA) levels (n = 2,874) and PSA screening frequencies (n = 46,970) was also examined. Diabetics had significantly lower risk of prostate cancer than did nondiabetics (relative risk = 0.81, 95% confidence interval (CI): 0.74, 0.87; P < 0.001), with relative risks ranging from 0.65 (95% CI: 0.50, 0.84; P = 0.001) among European Americans to 0.89 (95% CI: 0.77, 1.03; P = 0.13) among African Americans. Mean PSA levels were significantly lower in diabetics than in nondiabetics (mean PSA levels, 1.07 and 1.28, respectively; P = 0.003) as were PSA screening frequencies (44.7% vs. 48.6%; P < 0.001); however, this difference could explain only a small portion (∼20%) of the inverse association between these diseases. Diabetes is a protective factor for prostate cancer across populations, suggesting shared risk factors that influence a common mechanism.
Keywords: cohort studies; diabetes mellitus, type 2; ethnology; prostate-specific antigen; prostatic neoplasms
Prostate cancer and type 2 diabetes are 2 of the most common chronic diseases that afflict the aging male population. Epidemiologic studies conducted primarily in populations of European ancestry have provided evidence of an inverse relation between these diseases, with diabetics having ∼20% lower risk of developing prostate cancer than do nondiabetics (1–8). However, considerable effect heterogeneity has been noted among studies, highlighting the need for additional prospective analyses of these endpoints in large representative population-based studies. The biologic basis of this suspected relation is currently unknown and, aside from age and perhaps obesity (4, 9, 10), these 2 diseases share no known nongenetic risk factors.
The inverse relation between these endpoints may be due to direct effects on prostate cancer growth and development, as men with type 2 diabetes have been found to have lower prostate-specific antigen (PSA) levels, on average, than do men without type 2 diabetes (11, 12). The reported protective effects of type 2 diabetes may also be attributed to differences in prostate cancer screening in diabetic and nondiabetic patients. Differences in health maintenance, access to medical care, and the presence of serious medical conditions may result in more (or less) medical attention and preventive measures (13). Thus, examining the association between type 2 diabetes status and prostate cancer screening frequencies is important to quantify the degree to which detection bias may explain the apparent relation.
The incidence rates of type 2 diabetes and prostate cancer vary widely across populations. However, the ethnic disparities for these common diseases are not correlated; that is, not all populations with high rates of diabetes are at low risk of prostate cancer (14–20). The extent to which these diseases are linked in non-European populations is not clear. To confirm the previously reported association in a large, representative population-based prospective study, as well as to examine the consistency of the association across racial/ethnic populations with differing rates of prostate cancer, we evaluated prostate cancer incidence by type 2 diabetes status in a multiethnic sample of 86,303 men from the Multiethnic Cohort (21). We also assessed the presumed effect of diabetes status in the etiology of prostate cancer by examining PSA levels in a multiethnic sample of men with and without type 2 diabetes. We also evaluated PSA screening frequencies in diabetics and nondiabetics to define the role of screening bias in explaining the observed association between these common diseases.
MATERIALS AND METHODS
Study population
The Multiethnic Cohort is a prospective cohort study that includes 215,251 men and women, the majority from 5 racial/ethnic groups in Hawaii and Los Angeles, California (African Americans, European Americans, Native Hawaiians, Japanese Americans, and Latinos) (21). Between 1993 and 1996, participants entered the cohort by completing a 26-page, self-administered questionnaire that asked about diet and demographic factors, personal behaviors (e.g., physical activity), history of prior medical conditions (e.g., diabetes), and family history of common cancers. Potential cohort members were identified primarily through Department of Motor Vehicles drivers’ license files and, additionally for African Americans, Health Care Financing Administration data files. Participants were between the ages of 45 and 75 years at the time of recruitment.
In the cohort, incident prostate cancer cases are identified annually through cohort linkage to the population-based Surveillance, Epidemiology, and End Results (SEER) cancer registries in Hawaii and Los Angeles County, as well as the California Cancer Registry. Information on stage and grade of disease is also obtained through these registries. Linkage with these registries is complete through December 31, 2004, in Hawaii and December 31, 2005, in California. Over this period, 5,941 incident cases of invasive prostate cancer were identified. Deaths within the cohort are determined from linkages to the death certificate files in Hawaii and California, supplemented with linkages to the National Death Index. In the Multiethnic Cohort, diabetes status is defined on the basis of self-report on the baseline questionnaire. This question did not differentiate between type 1 diabetes and type 2 diabetes, and thus we expect a small fraction (<10%) of the respondents to have type 1 diabetes and to be potentially misclassified (22).
In addition to self-reported race/ethnicity, the following risk factors were included in the analysis: body mass index (weight (kg)/height (m)2), educational level (≤12 years, some college or vocational, and college graduate), first-degree family history of prostate cancer, and amount of vigorous physical activity (0, >0–1.5, >1.5–5, and >5 hours/week). Vigorous activity includes both vigorous sports and vigorous work.
We limited our analysis to 91,018 men in the Multiethnic Cohort from the 5 major racial/ethnic groups. We excluded men with a prevalent report of prostate cancer (n = 3,004) based on self-report or from the Surveillance, Epidemiology, and End Results registries. We excluded men with missing information for body mass index (n = 838), educational level (n = 872), and diabetes status (n = 1). The prospective analysis of the association between diabetes status and prostate cancer incidence in this study includes 86,303 men.
PSA levels were previously measured on 4,623 men in the Multiethnic Cohort (23). These men were randomly selected from the cohort to evaluate the distribution of PSA levels across ethnic groups. We excluded 194 men with prevalent prostate cancer at baseline. We also excluded 1,527 men with incident prostate cancer during the follow-up period, to ensure that elevated PSA levels among undiagnosed cases did not influence the results. Another 28 men with missing body mass index data were excluded from the analysis, leaving 2,874 men who are included in the final analysis of the effect of type 2 diabetes on PSA levels.
In 2001, we sent a short follow-up questionnaire to cohort members. On this questionnaire, we also asked about PSA screening prior to 1999. Of the 86,303 men included in the primary analysis of type 2 diabetes and prostate cancer, 23,768 (27.5%) did not complete the follow-up questionnaire. We also excluded 4,649 men with incident prostate cancer. Finally, we excluded men under the age of 50 years (n = 10,916) because annual PSA screening is recommended to begin at age 50 (24). This leaves 46,970 men included in the analysis of the association between type 2 diabetes and PSA screening.
The informed consent and study protocol were approved by the institutional review boards at the University of Southern California and the University of Hawaii.
Statistical analysis
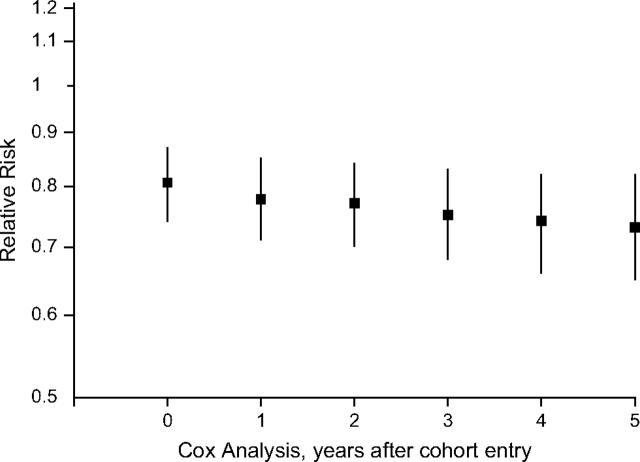
Cox regression was used to estimate hazard ratios (reported as relative risks) for the effect of type 2 diabetes on prostate cancer incidence (STATA, version 8, software; StataCorp LP, College Station, Texas). We adjusted for age, body mass index, educational level, and race/ethnicity (in pooled analyses). Neither body mass index nor educational level was associated with prostate cancer risk, but both remained in the model as the former was found to be associated with PSA levels and the latter was found to be a highly significant predictor of PSA screening. Physical activity and family history of prostate cancer were left out of the final model because neither had an effect on the association between type 2 diabetes and prostate cancer. Stratified analyses were performed in older age groups to assess whether type 2 diabetes duration and long-term exposure to declining insulin levels may be important in prostate cancer development. Because men may be at increased risk of prostate cancer within the first few years following a diabetes diagnosis as a result of higher insulin levels, and since the date of type 2 diabetes diagnosis is unknown for cohort members, we also performed a sensitivity analysis to examine whether the association might be attenuated in recently diagnosed diabetics. In this analysis, we censored follow-up of incident prostate cancer cases incrementally by year from 1 to 5 years after cohort entry. We also examined the association in analyses stratified by body mass index (≥25 kg/m2 and <25 kg/m2). Analyses stratified by Gleason score to determine the effect of type 2 diabetes status on prostate cancer severity were also conducted. This latter analysis excludes 370 prostate cancer cases with missing information on the Gleason score.
In the analysis of PSA levels, generalized linear models were used to estimate least-squared mean PSA levels by type 2 diabetes status (SAS, version 9.1, software; SAS Institute, Inc., Cary, North Carolina). Models were adjusted for the putative confounders of age, body mass index, and race/ethnicity. We calculated PSA screening frequencies adjusted for both age and educational level by type 2 diabetes status, and we tested for a difference using logistic regression; body mass index was not found to influence the effect of type 2 diabetes on PSA screening. The fraction of the association between type 2 diabetes and prostate cancer that may be attributable to PSA screening was estimated. Assuming that prostate cancer incidence roughly doubled since the initiation of PSA screening (25), with about 50% of men being screened, we estimate that incidence rates have increased by 0.02 per 1% of the population screened. We then used this slope to estimate the relative impact of screening on prostate cancer incidence in diabetic and nondiabetic men as follows: relative risk (RR)PSA = (1 + 0.02 × screening frequency in nondiabetics)/(1 + 0.02 × screening frequency in diabetics), with (1 − RRPSA/1 − RRT2D) being an estimate of the fraction of the association between type 2 diabetes (T2D) and prostate cancer incidence attributable to PSA screening.
RESULTS
The mean age of the men (n = 86,303) in this study was 59.9 (standard deviation, 8.8) years and ranged from 56.6 for Native Hawaiians to 61.3 for African Americans (Table 1). The age-standardized prostate cancer incidence rate of 830.2 (per 100,000) was nearly 2 times greater in African Americans than in the other populations (Table 1). The age-adjusted prevalence of type 2 diabetes also varied widely across populations, from 6.9% in European Americans to 18.0% in Native Hawaiians. The mean age of the diabetic men in our study at baseline was slightly higher than that of the nondiabetic men for each racial/ethnic group, ranging from 59.2 years (vs. 56.1 in nondiabetics) in Native Hawaiians to 63.6 years (vs. 60.6 in nondiabetics) in Japanese Americans. The age-standardized prostate cancer incidence rates were lower in diabetic men than in nondiabetic men for each racial/ethnic group, ranging from 242.8 (per 100,000) in European-American diabetics (vs. 413.9 in nondiabetics) to 686.2 in African-American diabetics (vs. 845.2 in nondiabetics). First-degree family history of prostate cancer was also less common in diabetic men than in nondiabetic men for each racial/ethnic group, ranging from 5.1% in Latino diabetic men (vs. 5.8% in nondiabetics) to 7.9% in African-American diabetic men (vs. 8.8% in nondiabetics). As expected, in each population, diabetic men were more likely to be overweight and less physically active than men without type 2 diabetes (Table 1).
Table 1.
Descriptive Characteristics by Race/Ethnicity and Diabetes Status (Yes/No) in the Multiethnic Cohort (n = 86,303), Los Angeles, California, and Hawaii, 1993–2005
| European Americans |
African Americans |
Native Hawaiians |
Japanese Americans |
Latinos |
Total | ||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | ||
| No. of men | 1,440 | 20,606 | 1,791 | 9,475 | 988 | 5,088 | 3,160 | 22,927 | 3,446 | 17,382 | 86,303 |
| Mean age, years (SD) | 62.8 (8.1) | 58.5 (9.0) | 63.4 (8.0) | 60.9 (8.9) | 59.2 (8.0) | 56.1 (8.6) | 63.6 (8.3) | 60.6 (9.2) | 61.7 (7.0) | 59.7 (7.8) | |
| No. of prostate cancer cases | 60 | 1,193 | 215 | 1,295 | 35 | 213 | 160 | 1,302 | 198 | 1,270 | 5,941 |
| Prostate cancer incidence ratesa | 242.8 | 413.9 | 686.2 | 845.2 | 257.9 | 373.8 | 270.1 | 354.9 | 304.2 | 433.7 | |
| Family history of prostate cancer, %b | 7.0 | 7.9 | 7.9 | 8.8 | 5.6 | 5.7 | 6.1 | 6.2 | 5.1 | 5.8 | |
| Body mass index (kg/m2), %b | |||||||||||
| <23 | 13.1 | 21.3 | 11.1 | 17.5 | 7.6 | 13.8 | 21.0 | 26.3 | 12.3 | 16.7 | |
| 23–24.99 | 8.1 | 17.9 | 9.6 | 14.6 | 4.7 | 11.1 | 17.3 | 25.1 | 8.1 | 10.8 | |
| 25–29.99 | 42.7 | 45.7 | 44.5 | 47.8 | 40.0 | 45.6 | 45.1 | 41.4 | 49.1 | 53.8 | |
| 30–34.99 | 23.7 | 11.9 | 24.4 | 15.9 | 28.1 | 20.3 | 12.9 | 6.2 | 22.8 | 15.1 | |
| ≥35 | 12.3 | 3.1 | 10.0 | 4.3 | 19.6 | 9.1 | 3.7 | 1.0 | 7.7 | 3.5 | |
| Educational level, %b | |||||||||||
| ≤12 years | 34.6 | 23.2 | 41.8 | 40.0 | 63.0 | 53.8 | 36.6 | 34.8 | 67.4 | 64.1 | |
| Some college or vocational | 29.0 | 29.1 | 36.9 | 37.0 | 25.8 | 28.5 | 34.3 | 30.5 | 22.5 | 23.5 | |
| College graduate | 36.4 | 47.7 | 20.8 | 22.9 | 11.2 | 17.7 | 29.1 | 34.6 | 10.2 | 12.3 | |
| Physical activity (hours/week), %bc | |||||||||||
| 0 | 39.5 | 27.9 | 43.7 | 34.1 | 29.7 | 22.1 | 39.3 | 33.0 | 37.7 | 28.9 | |
| >0–1.5 | 16.0 | 14.4 | 17.5 | 15.6 | 13.9 | 13.4 | 19.6 | 18.2 | 14.3 | 14.3 | |
| >1.5–5 | 20.8 | 23.5 | 15.7 | 22.0 | 23.4 | 25.7 | 20.9 | 23.4 | 18.0 | 20.9 | |
| >5 | 20.1 | 31.6 | 17.2 | 23.9 | 29.3 | 35.7 | 17.3 | 23.0 | 24.4 | 31.1 | |
Abbreviation: SD, standard deviation.
Adjusted to the 1970 US standard population.
Age standardized (5-year age groups) to the total population included in the study.
Percentages do not add up to 100% because of missing values.
In multivariate analyses, men with type 2 diabetes had significantly lower risk of prostate cancer than did men without type 2 diabetes (RR = 0.81, 95% confidence interval (CI): 0.74, 0.87; P < 0.001) (Table 2). The inverse association was observed consistently in all 5 populations and ranged from 0.65 (95% CI: 0.50, 0.84) in European Americans to 0.89 (95% CI: 0.77, 1.03) in African Americans (Pheterogeneity = 0.32). We also examined the effect of type 2 diabetes status on prostate cancer incidence by age at entry into the cohort as a surrogate for duration of type 2 diabetes, as the progressive decline of insulin levels with age among type 2 diabetics has been suggested to be protective for prostate cancer (1, 3, 6, 8). We found no evidence that the inverse association was strengthened among older men (Table 2). However, we did observe a slight, yet consistent, decrease in the relative risk when censoring the follow-up of incident cases, by year, within the first 5 years of follow-up (Appendix Figure 1). We observed no significant difference in the association when stratified by body mass index (≥25 kg/m2: RR = 0.78, 95% CI: 0.71, 0.86; <25 kg/m2: RR = 0.86, 95% CI: 0.74, 1.00; Pinteraction = 0.24). We also observed consistent effects by disease severity (Gleason score ≤7, n = 3,853: RR = 0.81, 95% CI: 0.73, 0.90; Gleason score >7, n = 1,703: RR = 0.76, 95% CI: 0.65, 0.89).
Table 2.
Relative Risk of Prostate Cancer Associated With Diabetes Status by Age and Gleason Score in the Multiethnic Cohort, Los Angeles, California, and Hawaii (n = 86,303), 1993–2005
| European Americans |
African Americans |
Native Hawaiians |
Japanese Americans |
Latinos |
All |
|||||||||||||
| No. of Cases | Relative Riska | 95% Confidence Interval | No. of Cases | Relative Riska | 95% Confidence Interval | No. of Cases | Relative Riska | 95% Confidence Interval | No. of Cases | Relative Riska | 95% Confidence Interval | No. of Cases | Relative Riska | 95% Confidence Interval | No. of Cases | Relative Riska | 95% Confidence Interval | |
| All men | 1,253 | 0.65 | 0.50, 0.84 | 1,510 | 0.89 | 0.77, 1.03 | 248 | 0.73 | 0.51, 1.05 | 1,462 | 0.81 | 0.69, 0.96 | 1,468 | 0.78 | 0.67, 0.91 | 5,941 | 0.81 | 0.74, 0.87 |
| P value | 0.001 | 0.13 | 0.09 | 0.01 | 0.001 | <0.001 | ||||||||||||
| Pheterogeneity | 0.32 | |||||||||||||||||
| Age ≥50 years | 1,192 | 0.66 | 0.51, 0.86 | 1,436 | 0.87 | 0.75, 1.01 | 233 | 0.72 | 0.49, 1.04 | 1,422 | 0.80 | 0.68, 0.95 | 1,434 | 0.79 | 0.68, 0.91 | 5,717 | 0.80 | 0.74, 0.87 |
| P value | 0.002 | 0.06 | 0.08 | 0.01 | 0.002 | <0.001 | ||||||||||||
| Pheterogeneity | 0.47 | |||||||||||||||||
| Age ≥60 years | 932 | 0.66 | 0.49, 0.87 | 1,109 | 0.90 | 0.77, 1.06 | 170 | 0.76 | 0.50, 1.14 | 1,216 | 0.81 | 0.68, 0.97 | 1,069 | 0.77 | 0.65, 0.92 | 4,496 | 0.81 | 0.74, 0.89 |
| P value | 0.004 | 0.22 | 0.19 | 0.02 | 0.003 | <0.001 | ||||||||||||
| Pheterogeneity | 0.25 | |||||||||||||||||
| Age ≥70 years | 346 | 1.03 | 0.70, 1.49 | 371 | 0.92 | 0.70, 1.21 | 47 | 0.71 | 0.31, 1.60 | 521 | 0.80 | 0.62, 1.05 | 293 | 0.69 | 0.49, 0.97 | 1,578 | 0.84 | 0.72, 0.97 |
| P value | 0.90 | 0.55 | 0.40 | 0.11 | 0.03 | 0.02 | ||||||||||||
| Pheterogeneity | 0.57 | |||||||||||||||||
| Gleason score ≤7 | 753 | 0.66 | 0.47, 0.92 | 1,075 | 0.87 | 0.73, 1.03 | 139 | 0.86 | 0.54, 1.35 | 830 | 0.78 | 0.63, 0.98 | 1,056 | 0.80 | 0.67, 0.95 | 3,853 | 0.81 | 0.73, 0.90 |
| P value | 0.02 | 0.11 | 0.51 | 0.03 | 0.01 | <0.001 | ||||||||||||
| Pheterogeneity | 0.60 | |||||||||||||||||
| Gleason score >7 | 384 | 0.68 | 0.43, 1.07 | 340 | 0.95 | 0.71, 1.29 | 95 | 0.61 | 0.32, 1.14 | 558 | 0.76 | 0.58, 1.00 | 341 | 0.68 | 0.49, 0.94 | 1,718 | 0.76 | 0.65, 0.89 |
| P value | 0.09 | 0.76 | 0.12 | 0.05 | 0.02 | <0.001 | ||||||||||||
| Pheterogeneity | 0.54 | |||||||||||||||||
Adjusted for age, body mass index, and educational level. Adjusted for race in pooled analysis.
Association of PSA levels with type 2 diabetes status and body mass index
In the subset of 2,874 men with PSA measurements, diabetic men (n = 344) were found to have significantly lower least square geometric mean PSA levels than did nondiabetic men (n = 2,530; 1.04 vs. 1.29 ng/mL; P < 0.001). Adjusting for body mass index had little effect on this association (1.07 vs. 1.28 ng/mL; P = 0.003) (Table 3). This association was noted in all populations except Native Hawaiians and was statistically significant in European Americans (0.62 vs. 1.21 ng/mL; P = 0.003) and Latinos (0.99 vs. 1.27 ng/mL; P = 0.02). Consistent with previous reports (11, 12), this report also shows an inverse relation between body mass index and PSA levels (Table 3). In ethnicity-pooled analyses, compared with men with a body mass index of <25 kg/m2, men with a body mass index of ≥30 had 13.8% lower mean PSA levels (P = 0.009). In multivariate generalized linear models, adjusted for type 2 diabetes status and age at blood draw, we estimated a 1-unit increase in body mass index to be associated with a 1.6% decrease in mean PSA level (P < 0.001).
Table 3.
Geometric Mean Prostate-specific Antigen Levels by Ethnicity, Diabetes Status, and Body Mass Index in the Multiethnic Cohort (n = 2,874), Los Angeles, California, and Hawaii, 1993–2005
| European Americans |
African Americans |
Native Hawaiians |
Japanese Americans |
Latinos |
Alla |
||||||||||||||
| Mean, ng/mL | No. | P Value | Mean, ng/mL | No. | P Value | Mean, ng/mL | No. | P Value | Mean, ng/mL | No. | P Value | Mean, ng/mL | No. | P Value | Mean, ng/mL | No. | P Value | Pheterogeneity | |
| All menb | 1.19 | 446 | 1.50 | 916 | 1.14 | 313 | 1.22 | 485 | 1.26 | 714 | 1.26 | 2,874 | <0.001 | ||||||
| Diabetes statusc | |||||||||||||||||||
| Yes | 0.62 | 25 | 1.46 | 126 | 1.00 | 44 | 1.15 | 56 | 0.99 | 93 | 1.07 | 344 | |||||||
| No | 1.21 | 421 | 0.003 | 1.64 | 790 | 0.29 | 0.97 | 269 | 0.86 | 1.26 | 429 | 0.53 | 1.27 | 621 | 0.02 | 1.28 | 2,530 | 0.003 | 0.11 |
| Body mass index (kg/m2)d | |||||||||||||||||||
| <25 | 1.09 | 174 | Referent | 1.66 | 266 | Referent | 1.12 | 59 | Referent | 1.37 | 240 | Referent | 1.27 | 196 | Referent | 1.30 | 935 | Referent | |
| 25–29.99 | 1.27 | 198 | 0.18 | 1.69 | 457 | 0.84 | 0.96 | 148 | 0.26 | 1.14 | 216 | 0.03 | 1.27 | 365 | 0.97 | 1.28 | 1,384 | 0.70 | |
| ≥30 | 1.12 | 74 | 0.84 | 1.38 | 193 | 0.07 | 0.92 | 106 | 0.18 | 1.07 | 29 | 0.18 | 1.09 | 153 | 0.16 | 1.12 | 555 | 0.009 | 0.49 |
| Body mass index, % changee | −0.6 | 0.65 | −1.8 | 0.03 | −1.9 | 0.06 | −2.4 | 0.06 | −1.6 | 0.06 | −1.6 | <0.001 | 0.93 | ||||||
Adjusted for body mass index, diabetes status, ethnicity, and age at blood draw.
Adjusted for diabetes status, body mass index, and age at blood draw. African Americans versus each ethnic group, P < 0.001.
Adjusted for body mass index and age at blood draw.
Adjusted for diabetes status and age at blood draw.
Percent change in geometric prostate-specific antigen levels with increase of 1 body mass index unit adjusted for diabetes status and age at blood draw.
Association of type 2 diabetes status and education with PSA screening frequencies
In the sample of 46,970 men over 50 years of age with information on PSA screening, 48.2% reported a PSA screening test prior to 1999. European-American men were more likely to report having been screened (55.8%), while Native Hawaiians (34.3%) were the least likely to have had PSA testing (Pheterogeneity < 0.001). We observed a modest, yet highly statistically significant difference in age and educational level regarding standardized PSA screening frequencies between diabetics (44.7%) and nondiabetics (48.6%; P < 0.001) (Table 4). The lower PSA screening frequencies among diabetics were noted in all populations except in African Americans and were statistically significant in Japanese Americans (P < 0.001) and Latinos (P = 0.02). Men with higher educational levels were much more likely to have had a PSA test than were men with ≤12 years of schooling (Table 4). We adjusted for educational level as a surrogate for PSA screening in the primary cohort analyses discussed above, yet it had little impact on the association.
Table 4.
Prostate-specific Antigen Screening Frequencies by Level of Education and Diabetes Status in the Multiethnic Cohort (n = 46,970), Los Angeles, California, and Hawaii, 1993–2005
| European Americans |
African Americans |
Native Hawaiians |
Japanese Americans |
Latinos |
All |
||||||||||||||
| No.a | %b | P Value | No. | % | P Value | No. | % | P Value | No. | % | P Value | No. | % | P Value | No. | % | P Value | Pheterogeneity | |
| All menc | 12,035 | 55.8 | 4,946 | 52.1 | 2,995 | 34.3 | 16,021 | 46.3 | 10,973 | 44.7 | 46,970 | 48.2 | <0.001 | ||||||
| Educational levelcd | |||||||||||||||||||
| ≤12 years | 2,747 | 44.5 | Referent | 1,954 | 46.3 | Referent | 1,549 | 30.1 | Referent | 6,247 | 37.1 | Referent | 6,856 | 41.1 | Referent | 19,353 | 40.0 | Referent | |
| College or vocational | 3,347 | 53.6 | <0.001 | 1,819 | 53.3 | <0.001 | 874 | 35.6 | 0.001 | 4,846 | 46.0 | <0.001 | 2,702 | 50.8 | <0.001 | 13,588 | 49.1 | <0.001 | |
| College graduate | 5,941 | 63.4 | <0.001 | 1,173 | 59.8 | <0.001 | 572 | 45.7 | <0.001 | 4,928 | 56.4 | <0.001 | 1,415 | 50.5 | <0.001 | 14,029 | 58.6 | <0.001 | <0.001 |
| Diabetes statusef | |||||||||||||||||||
| Yes | 796 | 51.4 | 742 | 52.5 | 487 | 35.1 | 1,947 | 42.1 | 1,793 | 44.3 | 5,765 | 44.7 | |||||||
| No | 11,239 | 53.0 | 0.46 | 4,204 | 52.1 | 0.84 | 2,508 | 36.4 | 0.37 | 14,074 | 46.0 | <0.001 | 9,180 | 47.4 | 0.02 | 41,205 | 48.6 | <0.001 | 0.45 |
No. of men with prostate-specific antigen screening data.
Percent screened.
Percentages are age standardized (5-year age groups) to the total population included in the study.
P values are from a logistic regression model and are adjusted for age, educational level, and race/ethnicity (pooled analysis).
Percentages are age (5-year age groups) and education standardized to the total population in the study.
P values are from a logistic regression model and are adjusted for age and race/ethnicity (pooled analysis).
Next, we examined the potential impact of detection bias on the observed association between type 2 diabetes and prostate cancer. On the basis of the 3.9% difference in PSA screening frequencies observed between diabetics and nondiabetics, we estimated that detection bias is likely to account for only ∼20% of the inverse association between type 2 diabetes and prostate cancer risk.
DISCUSSION
In this prospective analysis of 5 racial/ethnic populations, we found a highly significant association between type 2 diabetes status and prostate cancer incidence, with diabetics having ∼20% lower risk of developing prostate cancer. This inverse association was observed in all populations, with the magnitude of the effect being consistent with that of the majority of other studies conducted in men of European ancestry (1–8).
In this study, type 2 diabetes status was based on self-report, which may have led to misclassification. Previous studies, however, have shown that self-reported responses for many common chronic diseases such as diabetes are reliable when compared with medical records (26–28). The analysis does not account for incident cases of type 2 diabetes over the 8-year follow-up period. However, incident cases of diabetes in the nondiabetes group would make the 2 groups more similar and create an underestimation of the inverse association. Another limitation of our study is that we cannot differentiate between cases of type 1 diabetes and type 2 diabetes. Although they have similar phenotypes, type 1 diabetes and type 2 diabetes have distinct mechanisms of pathogenesis and may have dissimilar associations with prostate cancer incidence. However, we expect this differential misclassification to be minimal as the prevalence of type 1 diabetes is comparatively low in these populations (22).
Diabetes and prostate cancer are both traditionally underdiagnosed diseases. In this study, undiagnosed type 2 diabetes would result in prostate cancer incidence rates being more similar between the diabetic and nondiabetic groups. Undiagnosed cases of prostate cancer would result in lower rates of prostate cancer among both diabetics and nondiabetics. As a result, we would expect these simultaneous events of disease misclassification to counter the inverse association that we noted in this study toward the null. It is also possible that men who do not receive frequent medical care would be underdiagnosed and misclassified for both diseases. As a result, the underdiagnosis of prostate cancer and the lower risk of prostate cancer among the misclassified diabetics would also result in a bias toward the null of the underlying association. Although we expect that the underdiagnosis of these diseases is unable to explain their inverse association, future studies demanding regular blood glucose and PSA screening will be needed to quantify the impact of this bias.
In this study, we also found that men with diabetes are less likely to report PSA screening than are men without diabetes. These findings are contrary to those of a previous study that reported that men with diabetes are more likely to undergo screening for prostate cancer (3). PSA screening frequencies were lower among diabetics in all populations except in African Americans. Education, which is a surrogate for socioeconomic status and access to health care, was significantly associated with both PSA screening frequencies and diabetes status. However, further adjustment for education in the main cohort analyses did not change the results. We estimated that the potential bias incurred by differential PSA screening (∼4%) in diabetics and nondiabetics explained only ∼20% of the protective effect of type 2 diabetes on prostate cancer risk. In addition, if the association between these conditions was influenced by detection bias, then one would expect the inverse association to diminish among severe cases of prostate cancer, because they are likely to have been diagnosed without the use of PSA screening. However, we observed only a minimal change in the association between diabetes status and prostate cancer incidence when stratified by disease severity. Thus, detection bias associated with lower PSA levels and/or lower PSA screening frequencies in diabetics is unlikely to explain the strong and highly significant inverse association between type 2 diabetes and prostate cancer in this study.
Studies have suggested that the protective effect of diabetes on prostate cancer incidence may be greater among men with longstanding type 2 diabetes (3, 6, 8). One theory is that hyperinsulinemia, which is observed at onset, is associated with increased levels of growth factors (e.g., insulin-like growth factor-I) that may induce prostate cancer during the first few years of type 2 diabetes. Subsequently, prostate cancer rates would decrease in the later stages of type 2 diabetes when insulin levels decrease and men become hypoinsulinemic. We do not have data on the date of diagnosis for type 2 diabetes, but we did analyze the association between type 2 diabetes status and prostate cancer incidence by age at entry to the cohort as a surrogate for longstanding type 2 diabetes. With both of these theories, one would expect the magnitude of the inverse association between diabetes and prostate cancer to be greater among older men. However, we found no difference in the association in older men. We did, however, notice a modest increase in the magnitude of the inverse association when removing incident cases within the first 5 years of follow-up, which supports the hypothesis that men with newly diagnosed diabetes may have an increased risk of prostate cancer.
Most, but not all, studies have shown that, on average, men with diabetes have lower PSA levels than do those without diabetes (11, 12, 29). In our multiethnic sample, PSA levels were lower in diabetic men. However, what this indicates is not clear. Lower PSA levels in diabetics may signal a lower prevalence of prostate cancer and an indication of a biologic effect of type 2 diabetes status on prostate growth and development. At the same time, the effect of diabetes status on PSA levels could result in decreased follow-up for prostate cancer diagnosis among diabetics, which may partially account for the inverse relation between type 2 diabetes and prostate cancer risk. Additional work will be needed to understand whether type 2 diabetes status influences the accuracy of PSA screening or directly contributes to prostate cancer risk. Consistent with previous studies (11, 12, 30), our analysis also suggests an inverse relation between body mass index and PSA levels. Further studies of this association are necessary to determine if obese men should have lower PSA thresholds to indicate further work-up for prostate cancer.
Only a small number of studies have investigated the relation between type 2 diabetes and prostate cancer risk in non-European populations (2, 31–33). Most of these studies have observed nonsignificant inverse associations; however, small sample sizes have limited interpretation of the findings. Our results, from a population-based prospective study of over 5,900 prostate cancer cases from 5 racial/ethnic populations, provide strong support for the pan-ethnic nature of the association between these common diseases.
Recently, a common variant in the hepatocyte nuclear factor-1 β gene (HNF1β) was found to be associated with an increased risk of prostate cancer. This same variant was also found to be associated with a decreased risk of type 2 diabetes (34). Common genetic variation in another gene, JAZF1, has also been associated with risks of both prostate cancer and type 2 diabetes (35, 36). These findings, along with findings from other studies that have shown that diabetes is inversely associated with a family history of prostate cancer (5, 37), which we also noted, point to both shared genetic risk and common molecular and/or metabolic pathways that are important in the etiology of these diseases.
In summary, in this large, multiethnic prospective study, we observed consistent inverse associations between type 2 diabetes and prostate cancer risk across multiple racial/ethnic populations. These findings provide strong support for the hypothesis that type 2 diabetes is a protective factor for prostate cancer. We also confirmed results from previous studies, showing that PSA levels are decreased in diabetic men. Our findings that diabetic men are less likely to be screened for prostate cancer could not account for these results. Future studies aimed at determining the biologic link between diabetes and prostate cancer are warranted and should focus on common environmental and genetic factors that are shared across populations.
Acknowledgments
Author affiliations: Department of Preventive Medicine, Keck School of Medicine, University of Southern California/Norris Comprehensive Cancer Center, Los Angeles, California (Kevin M. Waters, Brian E. Henderson, Daniel O. Stram, Peggy Wan, Christopher A. Haiman); and Epidemiology Program, Cancer Research Center, University of Hawaii, Honolulu, Hawaii (Laurence N. Kolonel).
This work was supported by the National Cancer Institute (grant CA54281).
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- PSA
prostate-specific antigen
- RR
relative risk
Appendix Figure 1.
Ethnically pooled association between type 2 diabetes and prostate cancer risk by analysis start point, Los Angeles, California, and Hawaii, 1993–2005. There were 5,941, 5,441, 4,933, 4,456, 3,941, and 3,373 cases at 0, 1, 2, 3, 4, and 5 years after cohort entry, respectively. Bars, 95% confidence interval.
References
- 1.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 2.Calton BA, Chang SC, Wright ME, et al. History of diabetes mellitus and subsequent prostate cancer risk in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2007;18(5):493–503. doi: 10.1007/s10552-007-0126-y. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E, Rimm EB, Stampfer MJ, et al. Diabetes mellitus and risk of prostate cancer. Cancer Causes Control. 1998;9(1):3–9. doi: 10.1023/a:1008822917449. [DOI] [PubMed] [Google Scholar]
- 4.Gong Z, Neuhouser ML, Goodman PJ, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1977–1983. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 5.Velicer CM, Dublin S, White E. Diabetes and the risk of prostate cancer: the role of diabetes treatment and complications. Prostate Cancer Prostatic Dis. 2007;10(1):46–51. doi: 10.1038/sj.pcan.4500914. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez C, Patel AV, Mondul AM, et al. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am J Epidemiol. 2005;161(2):147–152. doi: 10.1093/aje/kwh334. [DOI] [PubMed] [Google Scholar]
- 7.Pierce BL, Plymate S, Ostrander EA, et al. Diabetes mellitus and prostate cancer risk. Prostate. 2008;68(10):1126–1132. doi: 10.1002/pros.20777. [DOI] [PubMed] [Google Scholar]
- 8.Tavani A, Gallus S, Bosetti C, et al. Diabetes and the risk of prostate cancer. Eur J Cancer Prev. 2002;11(2):125–128. doi: 10.1097/00008469-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E, Rimm EB, Stampfer MJ, et al. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557–563. [PubMed] [Google Scholar]
- 10.Huxley R, James WP, Barzi F, et al. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes Rev. 2008;9(suppl 1):53–61. doi: 10.1111/j.1467-789X.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 11.Fukui M, Tanaka M, Kadano M, et al. Serum prostate-specific antigen level in men with type 2 diabetes. Diabetes Care. 2008;31(5):930–931. doi: 10.2337/dc07-1962. [DOI] [PubMed] [Google Scholar]
- 12.Werny DM, Saraiya M, Gregg EW. Prostate-specific antigen values in diabetic and nondiabetic US men, 2001–2002. Am J Epidemiol. 2006;164(10):978–983. doi: 10.1093/aje/kwj311. [DOI] [PubMed] [Google Scholar]
- 13.Ross LE, Berkowitz Z, Ekwueme DU. Use of the prostate-specific antigen test among U.S. men: findings from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17(3):636–644. doi: 10.1158/1055-9965.EPI-07-2709. [DOI] [PubMed] [Google Scholar]
- 14.Abate N, Chandalia M. The impact of ethnicity on type 2 diabetes. J Diabetes Complications. 2003;17(1):39–58. doi: 10.1016/s1056-8727(02)00190-3. [DOI] [PubMed] [Google Scholar]
- 15.Borrell LN, Crawford ND, Dailo FJ. Race/ethnicity and self-reported diabetes among adults in the National Health Interview Survey: 2000–2003. Public Health Rep. 2007;122(5):616–625. doi: 10.1177/003335490712200509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mau MK, Glanz K, Severino R, et al. Mediators of lifestyle behavior change in Native Hawaiians: initial findings from the Native Hawaiian Diabetes Intervention Program. Diabetes Care. 2001;24(10):1770–1775. doi: 10.2337/diacare.24.10.1770. [DOI] [PubMed] [Google Scholar]
- 17.Aluli N. Prevalence of obesity in a Native Hawaiian population. Am J Clin Nutr. 1991;53(6 suppl):1556S–1560S. doi: 10.1093/ajcn/53.6.1556S. [DOI] [PubMed] [Google Scholar]
- 18.Brawley OW, Jani AB, Master V. Prostate cancer and race. Curr Probl Cancer. 2007;31(3):211–225. doi: 10.1016/j.currproblcancer.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 20.Kolonel LN, Altshuler D, Henderson BE. The Multiethnic Cohort Study: exploring genes, lifestyle, and cancer risk. Nat Rev Cancer. 2004;4(7):519–527. doi: 10.1038/nrc1389. [DOI] [PubMed] [Google Scholar]
- 21.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(4):346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atlanta, GA: Centers for Disease Control and Prevention; 2005. National Center for Chronic Disease Prevention and Health Promotion. National diabetes fact sheet. ( http://www.cdc.gov/diabetes/pubs/general.htm#what) [Google Scholar]
- 23.Cheng I, Yu MC, Koh WP, et al. Comparison of prostate-specific antigen and hormone levels among men in Singapore and the United States. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1692–1695. doi: 10.1158/1055-9965.EPI-04-0864. [DOI] [PubMed] [Google Scholar]
- 24.Smith RA, Cokkinides V, Eyre HJ, et al. American Cancer Society guidelines for the early detection of cancer, 2003. CA Cancer J Clin. 2003;53(1):27–43. doi: 10.3322/canjclin.53.1.27. [DOI] [PubMed] [Google Scholar]
- 25.Collin SM, Martin RM, Metcalfe C, et al. Prostate-cancer mortality in the USA and UK in 1975–2004: an ecological study. Lancet Oncol. 2008;9(5):445–452. doi: 10.1016/S1470-2045(08)70104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Midthjell K, Holmen J, Biøndal A, et al. Is questionnaire information valid in the study of a chronic disease such as diabetes? The Nord-Trøndelag Diabetes Study. J Epidemiol Community Health. 1992;46(5):537–542. doi: 10.1136/jech.46.5.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okura Y, Urban LH, Mahoney DW, et al. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not heart failure. J Clin Epidemiol. 2004;57(10):1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Walitt BT, Constantinescu F, Katz JD, et al. Validation of self-report of rheumatoid arthritis and systemic lupus erythematosus: the Women's Health Initiative. J Rheumatol. 2008;35(5):811–818. [PMC free article] [PubMed] [Google Scholar]
- 29.Chan JM, Latini DM, Cowan J, et al. History of diabetes, clinical features of prostate cancer, and prostate cancer recurrence-data for CaPSURE™ (United States) Cancer Causes Control. 2005;16(7):789–797. doi: 10.1007/s10552-005-3301-z. [DOI] [PubMed] [Google Scholar]
- 30.Fowke JH, Signorello LB, Chang SS, et al. Effects of obesity and height on prostate-specific antigen (PSA) and percentage of free PSA levels among African-American and Caucasian men. Cancer. 2006;107(10):2361–2367. doi: 10.1002/cncr.22249. [DOI] [PubMed] [Google Scholar]
- 31.Coker AL, Sanderson M, Zheng W, et al. Diabetes mellitus and prostate cancer risk among older men: population based case-control study. Br J Cancer. 2004;90(11):2171–2175. doi: 10.1038/sj.bjc.6601857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishina T, Watanabe H, Araki H, et al. Epidemiological study of prostatic cancer by matched-pair analysis. Prostate. 1985;6(4):423–436. doi: 10.1002/pros.2990060411. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg DJ, Neugut AI, Ahsan H, et al. Diabetes mellitus and the risk of prostate cancer. Cancer Invest. 2002;20(2):157–165. doi: 10.1081/cnv-120001141. [DOI] [PubMed] [Google Scholar]
- 34.Gudmundsson J, Sulem P, Steinthorsdottir V, et al. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39(8):977–983. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 35.Thomas G, Jacobs KB, Yeager M, et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40(3):310–315. doi: 10.1038/ng.91. [DOI] [PubMed] [Google Scholar]
- 36.Zeggini E, Scott LJ, Saxena R, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40(5):638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer P, Zuern C, Hermanns N, et al. The association between paternal prostate cancer and type 2 diabetes [electronic article] J Carcinog. 2007;6:14. doi: 10.1186/1477-3163-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]