Abstract
Validation of early childhood diet recalls by surrogate responders decades later has not been possible because of a lack of diet records from the distant past. Between 1948 and 1970, parents of children participating in the Fels Longitudinal Study (Kettering, Ohio) completed a 7-day diet record for their children every year from birth to age 18 years. In 2005–2006, all surviving women (n = 59) with a child aged 3–5 years when diet records had been collected were asked to complete a 42-item food frequency questionnaire (FFQ) pertaining to 1 of their children's diets at age 3–5 years. One or more diet records were available for 48 children. The authors calculated Spearman correlation coefficients for correlations between food, food-group, and nutrient intakes from the diet records and the FFQ and deattenuated them to account for the effects of within-person variation in the diet records on the association. For foods, the median deattenuated correlation coefficient was 0.19 (range, −0.31 to 0.85); moderate-to-high correlations were found for some specific foods. Correlations for food groups were slightly higher (median, 0.27; range, −0.14 to 0.85). Correlations for nutrient intakes were consistently low (median, 0.06; range, −0.35 to 0.27). Overall, the FFQ did not validly reflect overall preschool diet when completed by mothers 4 decades later.
Keywords: child, data collection, diet, epidemiologic methods, mental recall, mothers, questionnaires, validation studies as topic
Much evidence suggests that diet during early childhood could be an important determinant of chronic disease risk later in life (1–7). A comprehensive evaluation of this hypothesis requires either the establishment of large prospective cohort studies that follow young children into late adulthood, when chronic diseases are frequent enough to permit analysis, or the retrospective assessment of diet in case-control studies or large cohorts of adults. Both approaches have important drawbacks. The enormous investment of time and resources required to establish cohorts suitable for evaluation of the relation between childhood diet and risk of chronic diseases of adulthood diminishes enthusiasm for this approach. On the other hand, the usefulness of studies based on retrospective assessments of childhood diet depends on the validity of recall. Original records of diet during this period of life are seldom available; thus, in most cases, it is impossible to evaluate the validity of recall.
Although diet up to about 25 years in the past appears to be recalled accurately by adults (8, 9), it is less clear whether adults or surrogate responders can validly report childhood diet 3 or more decades before. To date, only 1 study has evaluated the validity of childhood diet recall by adults after more than 3 decades; that study suggested that adult recall of childhood diet may not be sufficiently valid for use in epidemiologic research (10). In an alternative approach, we evaluated whether mothers could validly recall, 3–5 decades later, the diets of their children around preschool age.
MATERIALS AND METHODS
Study participants
Study subjects were mothers of active member participants in the Fels Longitudinal Study. The Fels Longitudinal Study was established in Ohio in 1929 and is the oldest continuous study of growth, development, and aging in the world (11). Subject recruitment has continued to the present, and more than 5,000 members have been accrued, 1,300 of whom remain under active follow-up. Most member participants were recruited in utero or at birth. Each participant is followed from enrollment (usually birth) to death, regardless of changes in his or her health, but participants are not examined when transient conditions (e.g., infectious disease) that could affect data collection arise. At each visit, extensive age-appropriate growth, body composition, and other health-related data are collected. For the last 78 years, the data collection protocol has specified that participant visits occur at birth and at 3, 6, 9, and 12 months of age; then every 6 months at half-birthdays and birthdays until age 18 years; and every 2–5 years afterwards.
The current study was approved by the institutional review boards of the Fels Research Institute (Kettering, Ohio), Wright State University (Dayton, Ohio), and Brigham and Women's Hospital (Boston, Massachusetts).
Study design and dietary assessments
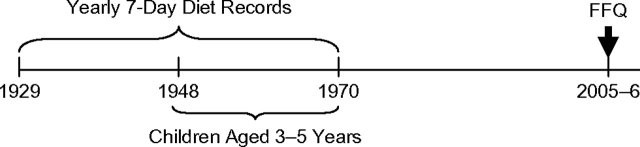
Figure 1 illustrates the study design. Between 1929 and 1970, parents of study members completed a 7-day diet record every year from their child's birth until age 18 years. The diet records were distributed to the members’ parents at their regularly scheduled visits. The parents were instructed to keep a record of all foods eaten on 7 consecutive days by their study member child, including details of portion size, and to return the records after completion. Parents did not receive any specific training in completing the diet records. The records were almost always completed by the mother. Diet records were not checked for completeness upon return. In a subsequent review of the records, it was found that 97% of the diet records were completed for all 7 days and returned to the Fels investigators (12).
Figure 1.
Overview of the FFQ validation study design in the Fels Longitudinal Study, 1948–2006. FFQ, food frequency questionnaire.
Between September 2005 and January 2006, all surviving mothers who had had a child between 3 and 5 years of age when the diet records were collected (n = 59; diet records collected between 1948 and 1970) were contacted by mail and asked to complete a 42-item food frequency questionnaire (FFQ) pertaining to their child's diet when the child had been 3–5 years of age. This FFQ is based on a similar one previously used in a case-control study of preschool diet and breast cancer (2), and it was revised after a preliminary validation study. The questionnaire was designed to be answered by mothers of middle-aged persons. Thus, we decided to limit recall to 5 years of age, to include only the period of childhood when diet could be reasonably assumed to be mostly controlled by the parents: the years before entrance into the school system.
The FFQ focuses on foods commonly consumed in the United States during the target historical period (1948–1970) that could also potentially allow estimation of some nutrients of interest. It includes 40 food or nutrient supplement items and 2 questions about the types of fat used in food preparation (to improve the estimation of specific fatty acid intakes). Participants were asked to report their child's intake of “an average serving” of each food item between 3 and 5 years of age. Serving sizes were further specified in the response categories of food items with a “natural” serving size—for example, glasses (milk, juice), slices (cheese), pats (margarine, butter), and whole units (apples, bananas, oranges, eggs).
All women contacted returned a completed FFQ. One or more diet records from the relevant time period were located for 48 of the participants’ children. For these 48 children, 1 diet record was available for 14 children, 2 diet records were available for 12 children, and 3 diet records were available for 22 children.
To allow estimation of nutrient intakes from the FFQ, a nutrient database pertaining to the relevant time period was developed by a team of research dietitians (L. S., P. T., and C. W.) based on data from the US Department of Agriculture (13–19), complemented with other information as necessary (20–28). Nutrient intakes were calculated by summing the nutrient contribution of each food in the FFQ. Because we were unable to find modern diet record analysis software that supported a nutrient database from the target historical period, we analyzed diet records using the 1986 version of the CBORD Diet Analyzer System (The CBORD Group, Inc., Ithaca, New York). This software package uses the 1986 version of the ESHA nutrient database (ESHA Research, Salem, Oregon), which is based on data published by the US Department of Agriculture between 1975 and 1981 (18, 29).
Statistical analyses
Intake of 1 item included on the FFQ (cod liver oil) was not reported in any diet record, and thus this item was excluded from the analysis. Since food groups may be recalled better than individual food items in this setting (10), we also aggregated individual food items into 10 food groups. We adjusted nutrient intakes for total energy intake using the nutrient residual method (30) to account for extraneous variation in intakes. When more than 1 diet record was available for a participant, the average across all of the available diet records was considered the gold standard intake measure for the individual. We calculated mean values and standard deviations for food and nutrient intakes as reported in the diet records and the FFQ and for the difference between diet-record and FFQ intakes. To evaluate the validity of recall, we calculated Spearman correlation coefficients and 95% confidence intervals (31) for correlations between food, food-group, and nutrient intakes reported in the diet records and the FFQ (validation coefficients).
To correct for the attenuation in the correlation coefficients introduced by random within-person variation (32) while simultaneously allowing the number of repeated diet records to vary across individuals in the study, we corrected the observed Spearman correlation coefficients and calculated their 95% confidence intervals using an extension of the weighted sib-mean estimator previously described for Pearson correlations (33). Pooled intraclass correlation coefficients (ICCs) (33) were estimated as a measure of within-person variation relative to between-person variation in intakes between 3 and 5 years of age. Lastly, we evaluated whether the validity of food or nutrient recall was related to the frequency or variability of intake by calculating Spearman correlation coefficients for correlations between the validation coefficients and summary measures of food, food-group, and nutrient intake frequency (mean intakes in diet record and FFQ) and within-person variation (ICC and λ, the ratio of within- to between-person variance).
RESULTS
FFQs were completed by the mothers of member participants, on average, 43 years (range, 35–58) after they had completed the diet records. At the time of completion, the mean maternal age was 73 years (range, 60–92). Mean food intakes as reported in the diet records and the FFQ were generally close, although large differences were apparent for milk and bakery products (Table 1). The mean difference between diet-record and FFQ food intakes across all foods was 0.15 (95% confidence interval (CI): −0.11, 0.42) servings per day. Intakes of most nutrients were systematically lower in the FFQ, with the exception of vitamin A intake, whose FFQ intakes were, on average, more than 3 times the diet-record-estimated intakes (Table 2). Estimated total energy intake was approximately one-third lower in the FFQ than in the diet records.
Table 1.
Mean Food Intakes as Assessed Through Diet Records and a Food Frequency Questionnaire (n = 48), Fels Longitudinal Study, 1948–2006
| Food | Mean Intake, servings/day |
Mean Difference | 95% Confidence Interval | |
| Diet Records | Food Frequency Questionnaire | |||
| Milk | 6.80 (6.03)a | 2.28 (0.81) | 4.52 | 2.75, 6.29 |
| Ice cream | 0.24 (0.21) | 0.16 (0.13) | 0.08 | 0.00, 0.17 |
| Cheese | 0.34 (0.38) | 0.34 (0.31) | 0.00 | −0.13, 0.14 |
| Margarine | 0.25 (0.48) | 1.13 (1.13) | −0.88 | −1.25, 0.51 |
| Butter | 0.43 (0.49) | 0.48 (0.86) | −0.05 | −0.27, 0.17 |
| Peanut butter | 0.23 (0.25) | 0.36 (0.19) | −0.13 | −0.20, −0.06 |
| Mayonnaise | 0.06 (0.15) | 0.15 (0.18) | −0.09 | −0.15, −0.03 |
| Apples (raw or sauce) | 0.31 (0.33) | 0.33 (0.19) | −0.03 | −0.13, 0.08 |
| Bananas | 0.13 (0.16) | 0.28 (0.21) | −0.16 | −0.23, −0.08 |
| Raisins | 0.05 (0.18) | 0.13 (0.12) | −0.08 | −0.14, −0.01 |
| Oranges | 0.04 (0.09) | 0.15 (0.16) | −0.11 | −0.16, −0.06 |
| Orange juice | 1.11 (1.69) | 0.55 (0.38) | 0.55 | 0.10, 1.01 |
| Apple juice | 0.14 (0.43) | 0.09 (0.20) | 0.04 | −0.10, 0.18 |
| Broccoli | 0.03 (0.06) | 0.04 (0.04) | −0.02 | −0.04, 0.01 |
| Carrots | 0.07 (0.11) | 0.20 (0.21) | −0.13 | −0.19, −0.07 |
| String beans | 0.11 (0.17) | 0.23 (0.20) | −0.12 | −0.19, −0.04 |
| Peas | 0.16 (0.22) | 0.19 (0.16) | −0.03 | −0.10, 0.05 |
| Corn | 0.11 (0.16) | 0.21 (0.18) | −0.10 | −0.15, −0.04 |
| Spinach | 0.03 (0.13) | 0.06 (0.09) | −0.03 | −0.07, 0.02 |
| Eggs | 0.33 (0.29) | 0.30 (0.19) | 0.03 | −0.05, 0.12 |
| Hot dogs | 0.12 (0.14) | 0.14 (0.11) | −0.02 | −0.08, 0.03 |
| Other processed meats | 0.55 (0.44) | 0.23 (0.18) | 0.32 | 0.18, 0.45 |
| Ground beef | 0.31 (0.40) | 0.30 (0.18) | 0.01 | −0.12, 0.15 |
| Beef, pork, or lamb | 0.60 (0.66) | 0.29 (0.20) | 0.31 | 0.11, 0.51 |
| Chicken or turkey | 0.22 (0.28) | 0.25 (0.17) | −0.03 | −0.13, 0.07 |
| Fish/seafood | 0.13 (0.28) | 0.11 (0.12) | 0.03 | −0.05, 0.10 |
| Liver | 0.04 (0.10) | 0.02 (0.04) | 0.01 | −0.02, 0.04 |
| Tomato or spaghetti sauce | 0.06 (0.15) | 0.14 (0.11) | −0.08 | −0.13, −0.02 |
| Pizza | 0.02 (0.08) | 0.05 (0.05) | −0.03 | −0.05, −0.01 |
| Pasta | 0.18 (0.24) | 0.16 (0.16) | 0.02 | −0.06, 0.11 |
| Bread | 1.18 (0.65) | 1.36 (0.82) | −0.18 | −0.48, 0.12 |
| Bakery products | 2.80 (1.76) | 0.45 (0.23) | 2.35 | 1.83, 2.88 |
| Rice | 0.03 (0.06) | 0.08 (0.09) | −0.04 | −0.07, −0.02 |
| Breakfast cereal | 0.63 (0.86) | 0.54 (0.30) | 0.08 | −0.17, 0.33 |
| Sweet potatoes or yams | 0.03 (0.17) | 0.06 (0.10) | −0.02 | −0.08, 0.03 |
| Potatoes, not fried | 0.31 (0.31) | 0.36 (0.17) | −0.05 | −0.15, 0.05 |
| French fries | 0.54 (0.70) | 0.07 (0.09) | 0.47 | 0.26, 0.68 |
| Other fried potatoes | 0.09 (0.25) | 0.10 (0.11) | −0.01 | −0.09, 0.07 |
| Multivitamins | 0.09 (0.23) | 0.49 (0.32) | −0.41 | −0.51, −0.30 |
| Total | 0.15 | −0.11, 0.42 | ||
Numbers in parentheses, standard deviation.
Table 2.
Mean Nutrient Intakes as Assessed Through Diet Records and a Food Frequency Questionnaire (n = 48), Fels Longitudinal Study, 1948–2006
| Nutrient | Mean Intake |
Mean Difference | 95% Confidence Interval | |
| Diet Records | Food Frequency Questionnaire | |||
| Calories, kcal/day | 1,552 (403)a | 1,027 (242) | 524.1 | 377.9, 670.2 |
| Protein, g/day | 57.0 (17.0) | 44.5 (12.3) | 12.5 | 6.2, 18.8 |
| Total fat, g/day | 62.9 (16.6) | 46.7 (13.0) | 16.1 | 9.9, 22.4 |
| Carbohydrates, g/day | 189.5 (57.7) | 110.7 (26.5) | 78.8 | 85.9, 98.6 |
| Fiber, g/day | 9.2 (3.1) | 6.5 (1.8) | 2.7 | 1.6, 3.8 |
| Saturated fat, g/day | 26.7 (7.8) | 21.4 (6.5) | 5.3 | 2.4, 8.2 |
| Monounsaturated fat, g/day | 22.6 (5.8) | 15.6 (4.4) | 7.0 | 4.8, 9.2 |
| Polyunsaturated fat, g/day | 8.9 (3.0) | 4.8 (1.6) | 4.1 | 3.0, 5.1 |
| Cholesterol, mg/day | 298 (136) | 218 (74) | 79.4 | 36.5, 122.3 |
| Vitamin A, IU/day | 1,205 (559) | 5,622 (2,467) | −4,416 | −5,135, −3,798 |
| Vitamin B1, mg/day | 1.6 (0.6) | 1.3 (0.5) | 0.2 | 0.0, 0.5 |
| Vitamin B2, mg/day | 2.1 (0.7) | 1.7 (0.5) | 0.4 | 0.2, 0.7 |
| Niacin, mg/day | 15.8 (7.2) | 12.1 (4.1) | 3.7 | 1.4, 6.0 |
| Vitamin B6, mg/day | 1.6 (0.7) | 1.2 (0.4) | 0.4 | 0.1, 0.6 |
| Folate, μg/day | 237 (140) | 125 (38) | 112.1 | 70.5, 153.7 |
| Vitamin B12, μg/day | 5.6 (2.8) | 5.1 (2.0) | 0.5 | −0.4, 1.5 |
| Vitamin C, mg/day | 85.1 (40.5) | 81.2 (33.4) | 3.9 | −10.9, 18.7 |
| Vitamin E, mg/day | 7.4 (4.2) | 3.4 (1.3) | 4.0 | 2.7, 5.4 |
| Calcium, mg/day | 908 (318) | 678 (203) | 229.6 | 126.7, 332.6 |
| Iron, mg/day | 9.3 (2.6) | 4.8 (1.2) | 4.5 | 3.7, 5.4 |
Numbers in parentheses, standard deviation.
The correlations for food intakes reported in diet records and FFQs were generally low, although moderate-to-high correlations were found for some foods, including eggs, orange juice, butter, French fries, corn, and peanut butter (Table 3). The median observed correlation was 0.14, and correlations ranged from −0.25 for ice cream to 0.42 for butter. Correcting for the effects of random within-person variation slightly improved the correlations. As expected, when observed correlations were negative (e.g., those for hot dogs and broccoli), deattenuation made them more negative. The median deattenuated correlation was 0.19, and deattenuated correlations ranged from −0.31 for ice cream to 0.85 for multivitamins. Because the degree of correction depends on how large within-person variation in intake is relative to between-person variation, as expected, the degrees of correction for foods with very high within-person variation (reflected in a low ICC) were very large, and the width of their confidence intervals suggested that the deattenuated estimates for these foods were not very informative. For example, the correlation for raisins (ICC = 0.09) went from 0.09 (95% CI: −0.21, 0.37) to 0.53 (95% CI: −1.00, 1.00). On the other hand, foods with little within-person variation relative to between-person variation, such as pizza (ICC = 0.87), had a very small correction. The observed and deattenuated correlations for pizza were 0.35 (95% CI: 0.07, 0.59) and 0.36 (95% CI: 0.09, 0.58), respectively. When foods with an ICC below 0.20 were excluded, the median deattenuated correlation was 0.18, with the same lower bound and a maximum of 0.47 for orange juice and eggs.
Table 3.
Observed and Deattenuated Spearman Correlation Coefficients (r) for Correlations Between Food Intakes Assessed Through Diet Records and a Food Frequency Questionnaire (n = 48), Fels Longitudinal Study, 1948–2006
| Food | Observed |
Deattenuated |
Intraclass Correlation Coefficient | ||
| r | 95% CI | r | 95% CI | ||
| Milk | 0.01 | −0.28, 0.30 | 0.02 | −0.27, 0.31 | 0.59 |
| Ice cream | −0.25 | −0.50, 0.05 | −0.31 | −0.59, 0.02 | 0.46 |
| Cheese | 0.16 | −0.14, 0.43 | 0.19 | −0.13, 0.47 | 0.48 |
| Margarine | 0.10 | −0.19, 0.38 | 0.19 | −0.21, 0.53 | 0.39 |
| Butter | 0.42 | 0.14, 0.63 | 0.46 | 0.17, 0.67 | 0.57 |
| Peanut butter | 0.33 | 0.05, 0.57 | 0.43 | 0.06, 0.70 | 0.48 |
| Mayonnaise | 0.21 | −0.08, 0.48 | 0.24 | −0.08, 0.52 | 0.41 |
| Apples (raw or sauce) | −0.06 | −0.35, 0.23 | −0.05 | −0.37, 0.27 | 0.38 |
| Bananas | 0.14 | −0.15, 0.42 | 0.18 | −0.15, 0.47 | 0.48 |
| Raisins | 0.09 | −0.21, 0.37 | 0.53 | −1.00, 1.00 | 0.09 |
| Oranges | 0.13 | −0.17, 0.41 | 0.21 | −0.26, 0.60 | 0.21 |
| Orange juice | 0.41 | 0.13, 0.62 | 0.47 | 0.16, 0.70 | 0.49 |
| Apple juice | 0.09 | −0.21, 0.37 | 0.16 | −0.22, 0.50 | 0.26 |
| Broccoli | −0.12 | −0.40, 0.18 | −0.26 | −0.67, 0.26 | 0.14 |
| Carrots | 0.22 | −0.07, 0.49 | 0.64 | −0.82, 0.99 | 0.11 |
| String beans | 0.16 | −0.14, 0.43 | 0.18 | −0.13, 0.45 | 0.58 |
| Peas | 0.13 | −0.17, 0.41 | 0.14 | −0.19, 0.45 | 0.36 |
| Corn | 0.40 | 0.13, 0.62 | 0.45 | 0.13, 0.69 | 0.36 |
| Spinach | 0.10 | −0.19, 0.38 | 0.14 | −0.19, 0.45 | 0.41 |
| Eggs | 0.39 | 0.11, 0.61 | 0.47 | 0.14, 0.71 | 0.45 |
| Hot dogs | −0.15 | −0.42, 0.15 | −0.20 | −0.55, 0.21 | 0.21 |
| Other processed meats | 0.09 | −0.20, 0.37 | 0.12 | −0.23, 0.44 | 0.31 |
| Ground beef | −0.07 | −0.36, 0.22 | −0.10 | −0.40, 0.22 | 0.49 |
| Beef, pork, or lamb | 0.11 | −0.19, 0.39 | 0.09 | −0.37, 0.52 | 0.12 |
| Chicken or turkey | −0.10 | −0.38, 0.20 | −0.10 | −0.41, 0.23 | 0.38 |
| Fish/seafood | 0.24 | −0.05, 0.50 | 0.35 | −0.05, 0.66 | 0.28 |
| Liver | 0.06 | −0.23, 0.35 | 0.12 | −0.27, 0.47 | 0.23 |
| Tomato sauce | 0.14 | −0.15, 0.37 | 0.17 | −0.18, 0.48 | 0.32 |
| Pizza | 0.35 | 0.07, 0.59 | 0.36 | 0.09, 0.58 | 0.87 |
| Pasta | 0.11 | −0.19, 0.39 | 0.15 | −0.20, 0.48 | 0.31 |
| Bread | 0.07 | −0.23, 0.35 | 0.08 | −0.25, 0.39 | 0.37 |
| Bakery products | −0.13 | −0.40, 0.17 | −0.15 | −0.45, 0.18 | 0.47 |
| Rice | 0.24 | −0.05, 0.50 | 0.65 | −1.00, 1.00 | 0.07 |
| Breakfast cereal | 0.26 | −0.03, 0.51 | 0.29 | −0.02, 0.54 | 0.55 |
| Sweet potatoes or yams | 0.15 | −0.15, 0.42 | 0.22 | −0.15, 0.53 | 0.37 |
| Potatoes, not fried | 0.19 | −0.11, 0.46 | 0.22 | −0.12, 0.52 | 0.37 |
| French fries | 0.34 | 0.05, 0.57 | 0.45 | 0.04, 0.73 | 0.27 |
| Other fried potatoes | 0.31 | 0.02, 0.55 | 0.40 | 0.05, 0.67 | 0.40 |
| Multivitamins | 0.29 | 0.00, 0.54 | 0.85 | −1.00, 1.00 | 0.11 |
| Median | 0.14 | 0.19 | 0.37 | ||
Abbreviation: CI, confidence interval.
Food groups tended to be recalled better than individual food items (Table 4). Moderate correlations were found for high-carbohydrate foods, fruits and fruit juices, vegetables, and condiments. The median observed correlation was 0.20, and observed correlations ranged from −0.12 for red meats to 0.39 for eggs. Deattenuation improved the correlations between diet records and FFQs: The median deattenuated correlation was 0.27 (range, −0.14 to 0.85). When the 2 food groups with an ICC below 0.20 (multivitamins and high-carbohydrate foods) were excluded, the median deattenuated correlation was 0.20 (range, −0.14 to 0.47). When the 3 individual food items that were considered separate food groups (milk, eggs, and multivitamins) were excluded, the median deattenuated correlation was 0.26 (range, −0.14 to 0.40).
Table 4.
Observed and Deattenuated Spearman Correlation Coefficients (r) for Correlations Between Food Group Intakes Assessed Through Diet Records and a Food Frequency Questionnaire (n = 48), Fels Longitudinal Study, 1948–2006
| Food Group | Observed |
Deattenuated |
Intraclass Correlation Coefficient | ||
| r | 95% CI | r | 95% CI | ||
| Milk | 0.01 | −0.28, 0.30 | 0.02 | −0.27, 0.31 | 0.59 |
| Other dairy foodsa | 0.12 | −0.18, 0.40 | 0.13 | −0.18, 0.41 | 0.52 |
| Condimentsb | 0.18 | −0.12, 0.45 | 0.26 | −0.12, 0.57 | 0.46 |
| Fruits and fruit juicesc | 0.24 | −0.05, 0.50 | 0.30 | −0.02, 0.57 | 0.53 |
| Vegetablesd | 0.21 | −0.09, 0.47 | 0.27 | −0.07, 0.55 | 0.39 |
| Eggs | 0.39 | 0.11, 0.61 | 0.47 | 0.14, 0.71 | 0.45 |
| Red meatse | −0.12 | −0.40, 0.18 | −0.14 | −0.43, 0.17 | 0.49 |
| Fish and poultryf | 0.03 | −0.25, 0.31 | 0.05 | −0.26, 0.35 | 0.54 |
| High-carbohydrate foodsg | 0.25 | −0.03, 0.50 | 0.40 | −0.18, 0.78 | 0.18 |
| Multivitamins | 0.29 | 0.00, 0.54 | 0.85 | −1.00, 1.00 | 0.11 |
| Median | 0.20 | 0.27 | 0.48 | ||
Abbreviation: CI, confidence interval.
Ice cream, cheese, and butter.
Margarine, peanut butter, and mayonnaise.
Apples, apple juice, bananas, raisins, oranges, and orange juice.
Broccoli, carrots, corn, peas, spinach, string beans, and tomato sauce.
Beef, pork, or lamb, ground beef, hot dogs, other processed meats, and liver.
Chicken or turkey and fish/seafood.
Breakfast cereal, bakery products, bread, pasta, pizza, rice, French fries, other fried potatoes, sweet potatoes or yams, and potatoes, not fried.
The correlations for nutrient intakes derived from diet records and the FFQ were low without exception (Table 5). The median observed correlation coefficient was 0.01, with a range between −0.24 for carbohydrates and 0.18 for calcium. Calorie adjustment and deattenuation of the calorie-adjusted correlations did not substantially improve the results. The median calorie-adjusted correlation was 0.02 (range, −0.23 to 0.20), and the median deattenuated correlation was 0.06 (range, −0.35 to 0.27). Excluding vitamin A from the analyses (ICC = 0.17) did not change the overall picture. The median deattenuated correlation became 0.05, with the same range of values as before the exclusion.
Table 5.
Observed, Calorie-adjusted, and Deattenuated Spearman Correlation Coefficients (r) for Correlations Between Nutrient Intakes Assessed Through Diet Records and a Food Frequency Questionnaire (n = 48), Fels Longitudinal Study, 1948–2006
| Nutrient | Observed |
Calorie-adjusted |
Intraclass Correlation Coefficient | ||||
| Observed |
Deattenuated |
||||||
| r | 95% CI | r | 95% CI | r | 95% CI | ||
| Calories | −0.16 | −0.42, 0.11 | |||||
| Protein | −0.04 | −0.31, 0.24 | −0.12 | −0.40, 0.18 | −0.15 | −0.46, 0.19 | 0.47 |
| Total fat | −0.05 | −0.32, 0.23 | −0.04 | −0.33, 0.25 | −0.03 | −0.34, 0.29 | 0.48 |
| Carbohydrates | −0.24 | −0.48, 0.04 | −0.08 | −0.36, 0.22 | −0.08 | −0.37, 0.22 | 0.61 |
| Fiber | −0.15 | −0.41, 0.12 | 0.20 | −0.09, 0.47 | 0.27 | −0.08, 0.57 | 0.37 |
| Saturated fat | 0.03 | −0.24, 0.30 | 0.15 | −0.14, 0.43 | 0.20 | −0.13, 0.50 | 0.46 |
| Monounsaturated fat | −0.04 | −0.31, 0.24 | 0.01 | −0.28, 0.30 | 0.06 | −0.30, 0.40 | 0.37 |
| Polyunsaturated fat | −0.18 | −0.44, 0.09 | −0.15 | −0.42, 0.15 | −0.16 | −0.46, 0.17 | 0.37 |
| Cholesterol | 0.05 | −0.23, 0.32 | 0.19 | −0.11, 0.46 | 0.22 | −0.09, 0.49 | 0.60 |
| Vitamin A | 0.10 | −0.18, 0.36 | 0.18 | −0.12, 0.44 | 0.27 | −0.18, 0.63 | 0.17 |
| Vitamin B1 | −0.05 | −0.32, 0.23 | −0.23 | −0.48, 0.07 | −0.35 | −0.68, 0.09 | 0.22 |
| Vitamin B2 | 0.08 | −0.20, 0.35 | −0.12 | −0.40, 0.17 | −0.17 | −0.47, 0.17 | 0.37 |
| Niacin | 0.02 | −0.25, 0.29 | −0.14 | −0.41, 0.16 | −0.17 | −0.48, 0.18 | 0.33 |
| Vitamin B6 | 0.08 | −0.19, 0.35 | −0.03 | −0.32, 0.26 | −0.09 | −0.44, 0.29 | 0.26 |
| Folate | 0.11 | −0.16, 0.37 | −0.01 | −0.30, 0.28 | 0.00 | −0.33, 0.33 | 0.40 |
| Vitamin B12 | 0.00 | 0.27, 0.27 | 0.03 | −0.26, 0.32 | 0.05 | −0.33, 0.41 | 0.22 |
| Vitamin C | 0.13 | −0.14, 0.39 | 0.12 | −0.17, 0.40 | 0.14 | −0.17, 0.43 | 0.47 |
| Vitamin E | −0.14 | −0.40, 0.14 | 0.06 | −0.24, 0.34 | 0.06 | −0.28, 0.38 | 0.35 |
| Calcium | 0.18 | −0.10, 0.43 | 0.12 | −0.18, 0.40 | 0.14 | −0.16, 0.42 | 0.58 |
| Iron | −0.08 | −0.34, 0.20 | 0.08 | −0.22, 0.36 | 0.09 | −0.23, 0.40 | 0.47 |
| Median | 0.01 | 0.02 | 0.06 | 0.38 | |||
Abbreviation: CI, confidence interval.
Lastly, we evaluated whether the validity of recall was dependent on the frequency of food consumption or the within-person variation in food, food-group, or nutrient intake. Neither the observed nor the deattenuated validation coefficients for foods or food groups were related to the mean intakes as reported in the diet records or the FFQ. Similarly, none of the validation coefficients for food or nutrient intakes were significantly related to the ICC (Table 6). However, the validity of recall for food-group intakes improved as the within-person variation in intake increased relative to the between-person variation, as reflected in inverse associations between the observed and deattenuated validation coefficients for food groups with the ICC and positive correlations with λ.
Table 6.
Spearman Correlation Coefficients (r) for Correlations Between Validation Coefficients for Food, Food-Group, and Nutrient Intakes and Measures of Food, Food-Group, and Nutrient-Intake Frequency and Variability, Fels Longitudinal Study, 1948–2006
| r | No.a | Diet Records |
Food Frequency Questionnaire |
Within-Person Variation |
|||||
| Mean Intake | 95% CI | Mean Intake | 95% CI | ICC | 95% CI | λb | 95% CI | ||
| Foods | |||||||||
| Observed | 39 | –0.06 | –0.37, 0.25 | 0.08 | –0.23, 0.39 | 0.17 | –0.14, 0.47 | –0.17 | –0.47, 0.14 |
| Deattenuated | 39 | –0.21 | –0.49, 0.11 | 0.04 | –0.28, 0.35 | –0.17 | –0.47, 0.14 | 0.17 | –0.14, 0.47 |
| Food groups | |||||||||
| Observed | 10 | –0.49 | –0.86, 0.17 | –0.24 | –0.76, 0.45 | –0.67 | –0.92, –0.11 | 0.67 | 0.11, 0.92 |
| Deattenuated | 10 | –0.52 | –0.87, 0.13 | –0.19 | –0.74, 0.49 | –0.74 | –0.94, –0.24 | 0.74 | 0.24, 0.94 |
| Nutrients | |||||||||
| Observed | 19 | –0.02 | –0.47, 0.43 | 0.02 | –0.43, 0.47 | ||||
| Calorie-adjusted | |||||||||
| Observed | 19 | 0.16 | –0.31, 0.58 | –0.16 | –0.58, 0.31 | ||||
| Deattenuated | 19 | 0.14 | –0.33, 0.56 | –0.14 | –0.56, 0.33 | ||||
Abbreviations: CI, confidence interval; ICC, intraclass correlation coefficient.
Number of foods, food groups, or nutrients (see Materials and Methods).
λ = σ2within-person/σ2between-person.
DISCUSSION
We evaluated whether mothers of middle-aged persons could validly recall their children's preschool diets, on average, 43 years later. The validity of food intake recall was inadequate, although recall of consumption of some specific foods (eggs, orange juice, butter, French fries, other fried potatoes, corn, peanut butter, pizza, fish/seafood, and breakfast cereals) and food groups (high-carbohydrate foods, fruits and fruit juices, vegetables, and condiments) was acceptable. On the other hand, validity for nutrient intakes was consistently poor, without any notable exceptions. Further, while the validity of food and nutrient intakes was unrelated to intake frequency or variability, the validity of food-group recall was related to the variability of intake.
Many studies have examined whether past diet can be validly recalled by adults. Diet appears to be recalled with sufficient validity for use in epidemiologic studies when the recall period ranges from a few years to 25 years in the past (8, 9, 34–39). However, longer recall times or recall of specific periods in childhood may also be of interest in epidemiologic research. To date, only 1 study has evaluated whether diet during childhood can be validly recalled more than 3 decades later. Dwyer et al. (10) asked middle-aged persons to recall their diets between 5 and 7 years of age 44 years later and compared the recalled food intakes with those recorded in prospectively collected diet records. The median correlation between diet-record and FFQ reported intakes was 0.12, and correlation appeared to be slightly better for food groups (median r, 0.14) than for individual foods. Although the findings of Dwyer et al. are not directly comparable to our results because the recalls refer to different ages (5–7 years in Dwyer et al.’s study and 3–5 years in ours) and the person recalling was different (the self in Dwyer et al.’s study and the mother in ours), the median correlations for food intakes are very similar and suggest that overall childhood diet cannot be validly recalled after 4 decades, either by the individual or by a surrogate responder. As in Dwyer et al.’s report (10), the validity of food-group intake recall was better than the validity of recall of individual foods, although in our study this difference was larger than in Dwyer et al.’s report and some food groups appeared to be recalled with reasonable validity. Although more studies should be conducted, these data suggest that maternal recall of specific food items or food groupings found to be remembered reasonably well could be a useful tool in epidemiologic studies with a hypothesis focused on these dietary exposures.
The validity of nutrient intakes derived from the FFQ was poor across the board. This was surprising given that food and nutrient intakes were estimated from the same instruments and, in other studies, the validity of nutrient intakes follows closely that of food intakes (40–43). A possible explanation for this discrepancy is the fact that the nutrient databases used to analyze the FFQ and the diet records did not reflect the same historical periods and had only minimal overlap. A specific nutrient database was designed for the FFQ, which used the best nutrient composition sources available for the historical period in which the diet records had been collected (1948–1970). In addition, when assigning specific nutrient contents to individuals, we used their year of birth for guidance as to which nutrient content to assign to each food, so that results would closely reflect changes in the nutrient composition of the food over time. On the other hand, the diet record nutrient database may have been suboptimal in at least 2 regards. First, it may not have reflected the correct nutrient composition of some foods during the target historical period. Despite multiple efforts, we were unable to locate an electronic nutrient database for the historical period in which the diet records had been collected. Because building a nutrient database of the correct historical period for the analysis of diet records was an undertaking well above us, considering the resources available, we opted to use instead the oldest available nutrient database, whose sources reflected the nutrient composition of foods starting in 1975 (18, 29).
Second, this nutrient database did not allow for changes in the nutrient composition of foods over time. These shortcomings of the diet record nutrient database may have contributed to error in nutrient intake estimation, leading to attenuation of the correlation coefficients. For example, consider 2 hypothetical subjects born 10 years apart with identical food intakes in their FFQ and diet records. In our study, these subjects would correctly have different estimated FFQ nutrient intakes (reflecting changes in food nutrient composition over those 10 years) but incorrectly identical diet record estimated nutrient intakes. In this hypothetical scenario, the correlations for food intakes would be unaffected, while the correlations for nutrient intakes would be artificially attenuated.
Furthermore, while the mean food intakes derived from the diet record and the FFQ were generally close, nutrient intakes were systematically lower in the FFQ than in the diet records, with the exception of vitamin A intake. This suggests that the food list may have been too short for a comprehensive estimation of nutrient intakes from this questionnaire (44). Consequently, the low validity correlations for nutrient intakes may not reflect the true validity of their estimated intakes; however, we are not aware of any other studies that might provide more accurate data. These results also highlight the importance of adequately choosing nutrient databases in nutrient-based validation studies, especially those of remote diet recall when the nutrient composition of foods may have changed over the period of diet record collection.
We found that the validity of food-group recall improved as the within-person variation in intake increased relative to the between-person variation. An association between the deattenuated correlation coefficients and the ICC or λ could be expected, given that the degree of correction is a function of these parameters; yet there were also associations of the crude correlation coefficient with the ICC and λ. Although these findings are interesting and may have implications for the design of FFQs, they should be interpreted with caution. Despite being highly statistically significant, this association was based on only 10 observations (each of the food groups). In addition, we did not observe any association between the individual food items that we summed to create the food groups and any measure of frequency or variability of food intake, suggesting that this may be a chance finding. Further research on this issue is needed.
The strengths and limitations of our study should be considered. A potential limitation is the fact that only 59 mothers had survived until 2005, when the FFQ was administered, and 13% of them were aged 80 years or older. Nevertheless, if this questionnaire were to be used in studies of chronic diseases of adulthood, mothers of participants in those studies would probably share this and other characteristics with the mothers in our study, and therefore the recalls provided by participants in our study are probably a good reflection of what could be expected in other settings. The strengths of our study include the availability of prospectively collected records of diet during the period of interest; lack of this resource has been the most important limitation in attempts to evaluate the true validity of FFQs retrospectively assessing childhood diet many years later. A related strength is the availability of multiple records of diet during the period of interest, which allowed the estimation of within-person variability in food and nutrient intakes over time and the deattenuation of the correlation coefficients.
In summary, maternal recall of overall preschool diet many decades later does not seem to be valid enough for use in epidemiologic studies. Recall of some specific foods and food groups is acceptable, however. Although more validation studies of mothers’ recall of their offsprings’ childhood diets are desirable, this questionnaire could be a valid tool in studies with hypotheses focused on the foods and food groups with apparently good validity of recall and in the development of similar questionnaires in other populations.
Acknowledgments
Author affiliations: Channing Laboratory, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Jorge E. Chavarro, Karin B. Michels, Bernard A. Rosner, Walter C. Willett); Obstetrics and Gynecology Epidemiology Center, Department of Obstetrics, Gynecology and Reproductive Biology, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Karin B. Michels); Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Jorge E. Chavarro, Laura Sampson, Carol Willey, Paula Tocco, Walter C. Willett); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Walter C. Willett, Karin B. Michels); Department of Biostatistics, Harvard School of Public Health, Boston, Massachusetts (Bernard A. Rosner); and Lifespan Health Research Center, Department of Community Health, Boonshoft School of Medicine, Wright State University, Kettering, Ohio (Sheherazadh Isaq, William Cameron Chumlea).
This work was supported by National Cancer Institute grant R03 CA115235 (to K. B. M.), National Institute of Diabetes and Digestive and Kidney Diseases grant T32 DK007703 (to W. C. W.), and National Institute of Child Health and Human Development grant R01 HD12252 (to W. C. C.), as well as by the Yerby Postdoctoral Fellowship Program, Harvard School of Public Health (J. E. C.).
Preliminary findings of this study were presented in part at the 41st Annual Meeting of the Society for Epidemiologic Research, Chicago, Illinois, June 24–27, 2008.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- FFQ
food frequency questionnaire
- ICC
intraclass correlation coefficient
References
- 1.van der Pols JC, Bain C, Gunnell D, et al. Childhood dairy intake and adult cancer risk: 65-year follow-up of the Boyd Orr cohort. Am J Clin Nutr. 2007;86(6):1722–1729. doi: 10.1093/ajcn/86.5.1722. [DOI] [PubMed] [Google Scholar]
- 2.Michels KB, Rosner BA, Chumlea WC, et al. Preschool diet and adult risk of breast cancer. Int J Cancer. 2006;118(3):749–754. doi: 10.1002/ijc.21407. [DOI] [PubMed] [Google Scholar]
- 3.Ahlgren M, Melbye M, Wohlfahrt J, et al. Growth patterns and risk of breast cancer in women. N Engl J Med. 2004;351(16):1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 4.Maynard M, Gunnell D, Emmett P, et al. Fruit, vegetables and antioxidants in childhood and risk of adult cancer: the Boyd Orr cohort. J Epidemiol Community Health. 2003;57(3):218–225. doi: 10.1136/jech.57.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frankel S, Gunnell D, Peters TJ, et al. Childhood energy intake and adult mortality from cancer: the Boyd Orr cohort study. BMJ. 1998;316(7130):499–504. doi: 10.1136/bmj.316.7130.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ness AR, Maynard M, Frankel S, et al. Diet in childhood and adult cardiovascular and all cause mortality: the Boyd Orr cohort. Heart. 2005;91(7):894–898. doi: 10.1136/hrt.2004.043489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunnell DJ, Frankel SJ, Nanchahal K, et al. Childhood obesity and adult cardiovascular mortality: a 57-y follow-up study based on the Boyd Orr cohort. Am J Clin Nutr. 1998;67(6):1111–1118. doi: 10.1093/ajcn/67.6.1111. [DOI] [PubMed] [Google Scholar]
- 8.Byers TE, Rosenthal RI, Marshall JR, et al. Dietary history from the distant past: a methodological study. Nutr Cancer. 1983;5(2):69–77. doi: 10.1080/01635588309513781. [DOI] [PubMed] [Google Scholar]
- 9.Lindsted KD, Kuzma JW. Long-term (24-year) recall reliability in cancer cases and controls using a 21-item food frequency questionnaire. Nutr Cancer. 1989;12(2):135–149. doi: 10.1080/01635588909514012. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer JT, Gardner J, Halvorsen K, et al. Memory of food intake in the distant past. Am J Epidemiol. 1989;130(5):1033–1046. doi: 10.1093/oxfordjournals.aje.a115404. [DOI] [PubMed] [Google Scholar]
- 11.Roche AF. Growth, Maturation and Body Composition: The Fels Longitudinal Study 1929–1991. Cambridge, United Kingdom: Cambridge University Press; 1992. [Google Scholar]
- 12.Chumlea WC, Guo SS. CERIN Symposium: Nutrition and Personnes Âgées au-delà des Apports Recommandés. Paris, France: Centre de Recherche et d'Information Nutritionnelles; 1997. Milk consumption in childhood and bone mineral density in adulthood: The Fels Longitudinal Study; pp. 125–134. ( http://www.cerin.org/upload/_editor/fichiers/colloque%20PA.pdf) [Google Scholar]
- 13.US Department of Agriculture. Composition of Foods. (Agriculture Handbook no. 8) Washington, DC: US GPO; 1950. [Google Scholar]
- 14.US Department of Agriculture. Composition of Foods. (Agriculture Handbook no. 8) Washington, DC: US GPO; 1963. [Google Scholar]
- 15.US Department of Agriculture. Nutritive Value of Foods. (Home and Garden Bulletin no. 72) Washington, DC: US GPO; 1964. [Google Scholar]
- 16.US Department of Agriculture. Nutritive Value of Foods. (Home and Garden Bulletin no. 72) Washington, DC: US GPO; 1970. [Google Scholar]
- 17.US Department of Agriculture. Nutritive Value of Foods. (Home and Garden Bulletin no. 72) Washington, DC: US GPO; 1971. [Google Scholar]
- 18.Adams CF. Nutritive Value of American Foods in Common Units. (Agriculture Handbook no. 456) Washington, DC: US Department of Agriculture; 1975. [Google Scholar]
- 19.US Department of Agriculture. Nutritive Value of Foods. (Home and Garden Bulletin no. 72) Washington, DC: US GPO; 1978. [Google Scholar]
- 20.Medical Economics, Inc. Physicians’ Desk Reference, 1950. Rutherford, NJ: Medical Economics, Inc; 1949. [Google Scholar]
- 21.Medical Economics, Inc. Physicians’ Desk Reference. 14th ed. Oradell, NJ: Medical Economics, Inc; 1959. [Google Scholar]
- 22.Rice EE, Weiss TJ, Mattil KF. Composition of modern margarines. J Am Diet Assoc. 1962;41(4):319–322. [PubMed] [Google Scholar]
- 23.Bowes A, Church CF, Church HN. Food Values of Portions Commonly Used. 9th ed. Philadelphia, PA: J B Lippincott & Company; 1963. [Google Scholar]
- 24.Bowes A, Church CF, Church HN. Food Values of Portions Commonly Used. 10th ed. Philadelphia, PA: J B Lippincott & Company; 1966. [Google Scholar]
- 25.Medical Economics, Inc. Physicians’ Desk Reference. 24th ed. Oradell, NJ: Medical Economics, Inc; 1969. [Google Scholar]
- 26.Bowes A, Church CF, Church HN. Food Values of Portions Commonly Used. 11th ed. Philadelphia, PA: J B Lippincott & Company; 1970. [Google Scholar]
- 27.Bowes A, Church CF, Church HN. Food Values of Portions Commonly Used. 12th ed. Philadelphia, PA: J B Lippincott & Company; 1975. [Google Scholar]
- 28.Bowes A, Church HN, Pennington JAT. Bowes’ and Church's Food Values of Portions Commonly Used. 13th ed. Philadelphia, PA: J B Lippincott & Company; 1980. [Google Scholar]
- 29.US Department of Agriculture. Nutritive Value of Foods. (Home and Garden Bulletin no. 72) Washington, DC: US GPO; 1981. [Google Scholar]
- 30.Willett WC, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 31.Rosner BA, Glynn RJ. Interval estimation for rank correlation coefficients based on the probit transformation with extension to measurement error correction of correlated ranked data. Stat Med. 2007;26(3):633–646. doi: 10.1002/sim.2547. [DOI] [PubMed] [Google Scholar]
- 32.Rosner B, Willett WC. Interval estimates for correlation coefficients corrected for within-person variation: implications for study design and hypothesis testing. Am J Epidemiol. 1988;127(2):377–386. doi: 10.1093/oxfordjournals.aje.a114811. [DOI] [PubMed] [Google Scholar]
- 33.Perisic I, Rosner B. Comparisons of measures of interclass correlations: the general case of unequal group size. Stat Med. 1999;18(12):1451–1466. doi: 10.1002/(sici)1097-0258(19990630)18:12<1451::aid-sim142>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 34.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 35.Lindsted KD, Kuzma JW. Reliability of eight-year diet recall in cancer cases and controls. Epidemiology. 1990;1(5):392–401. doi: 10.1097/00001648-199009000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Byers T, Marshall J, Anthony E, et al. The reliability of dietary history from the distant past. Am J Epidemiol. 1987;125(6):999–1011. doi: 10.1093/oxfordjournals.aje.a114638. [DOI] [PubMed] [Google Scholar]
- 37.Sobell J, Block G, Koslowe P, et al. Validation of a retrospective questionnaire assessing diet 10–15 years ago. Am J Epidemiol. 1989;130(1):173–187. doi: 10.1093/oxfordjournals.aje.a115310. [DOI] [PubMed] [Google Scholar]
- 38.Wu ML, Whittemore AS, Jung DL. Errors in reported dietary intakes. II. Long-term recall. Am J Epidemiol. 1988;128(5):1137–1145. doi: 10.1093/oxfordjournals.aje.a115056. [DOI] [PubMed] [Google Scholar]
- 39.Friedenreich CM, Slimani N, Riboli E. Measurement of past diet: review of previous and proposed methods. Epidemiol Rev. 1992;14(1):177–196. doi: 10.1093/oxfordjournals.epirev.a036086. [DOI] [PubMed] [Google Scholar]
- 40.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 41.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 42.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 43.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 44.Willett WC, Lenart E. Reproducibility and validity of food-frequency questionnaires. In: Willett WC, editor. Nutritional Epidemiology. 2nd ed. New York, NY: Oxford University Press; 1998. pp. 101–147. [Google Scholar]