Abstract
The literature is inconsistent regarding associations between parental smoking and childhood leukemia, possibly because previous studies used self-reported smoking habits as surrogates for children's true exposures to cigarette smoke. Here, the authors investigated the use of nicotine concentrations in house dust as measures of children's exposure to cigarette smoke in 469 households from the Northern California Childhood Leukemia Study (1999–2007). House dust was collected by using high-volume surface samplers and household vacuum cleaners and was analyzed for nicotine via gas chromatography–mass spectrometry. Using multivariable linear regression, the authors evaluated the effects of self-reported parental smoking, parental demographics, house characteristics, and other covariates on house-dust nicotine concentrations. They observed that nicotine concentrations in house dust were associated with self-reported smoking for periods of months and years before dust collection. Furthermore, the authors found that the relation between nicotine dust levels and self-reported smoking varied by parental age and socioeconomic status. These findings suggest that house-dust nicotine concentrations reflect long-term exposures to cigarette smoke in the home and that they may be less biased surrogates for children's exposures to cigarette smoke than self-reported smoking habits.
Keywords: child, dust, environmental exposure, infant, leukemia, linear models, nicotine, smoking
Although parental smoking is a potential contributor to childhood leukemia risk, the evidence supporting such an association is inconsistent. Studies have suggested variously that maternal smoking during pregnancy increases the risk of acute lymphocytic leukemia (1–3) and acute myeloid leukemia (4, 5); that paternal smoking increases the risk of infant leukemia (6), acute lymphocytic leukemia (7–10), and acute myeloid leukemia (8, 10); and that neither maternal nor paternal smoking is associated with childhood leukemia (11–15).
All previous research on potential associations between childhood leukemia and parental smoking has relied on self-reported smoking histories, which can result in misclassification of children's true exposures to cigarette smoke and subsequent bias in the exposure-response relation (16). Studies using nicotine-specific cotinine biomarkers as “gold” standards have shown that only about 5% of professed nonsmokers are actually smokers (17–19); however, deception rates as high as 25% have been observed when parents report their smoking habits in studies involving their children's health (20, 21). Interestingly, researchers observed that for 11,083 self-reported nonsmokers, the likelihood of an elevated urinary cotinine level (consistent with smoking) decreased with increasing education (22). This finding indicates that less educated subjects may be more likely to underreport their smoking exposure.
To reduce misclassification of exposure to cigarette smoke, it is beneficial to use an objective measure of exposure such as nicotine in indoor air (23), cotinine in urine (24), or nicotine in hair (25). Alternatively, researchers have suggested using nicotine levels in house dust as surrogates for in-home exposures to cigarette smoke (26–29). Indeed, previous research has shown that nicotine concentrations in house dust are highly correlated with children's levels of urinary cotinine (rS = 0.77, n = 15) in households with smokers (28). However, previous investigations of nicotine levels in house dust (26–29) involved small numbers of households (n = 72, n = 49, n = 23, and n = 37, respectively) and were unable to thoroughly examine the determinants of house-dust nicotine concentrations.
In this paper, we report results from 469 households in which nicotine was measured in house dust and from which extensive questionnaire data, including smoking habits, were also obtained from residents. These data were collected as part of the Northern California Childhood Leukemia Study (NCCLS), a large case-control study (8). The objectives of the current study were to compare house-dust nicotine levels with self-reported smoking at various times before and during a child's life and to identify determinants of house-dust nicotine levels. Although this information is directly relevant to researchers considering the effect of parental smoking on childhood leukemia risk, it should also be pertinent for any epidemiologic study that seeks to quantify exposure to cigarette smoke.
MATERIALS AND METHODS
Study population
The NCCLS is an ongoing study conducted in the San Francisco Bay Area and California Central Valley in which cases aged 0–14 years are ascertained from 9 pediatric clinical centers. Controls, matched to cases on date of birth, gender, race, Hispanic ethnicity, and maternal residence, are selected from the California birth registry (8). The homes of cases and controls aged 0–7 years who lived at the home they occupied at the time of diagnosis (and a similar reference date for controls) from December 1999 through November 2007 were eligible for household dust collection. Among 324 cases and 407 controls determined to be eligible, 296 cases (91%) and 333 controls (82%) participated. We obtained informed consent from the children's parent or legal guardian in accordance with the institutional review boards’ requirements at the University of California, Berkeley; the National Cancer Institute; and other participating institutions.
House-dust nicotine measurement
Dust was collected by using a high-volume surface sampler (HVS3) in the room where the child spent the most time while awake (commonly the family room) as well as from household vacuum cleaners, as previously described (30); data derived from both methods were used in our analyses. In HVS3-sampled homes (82%), we recorded the area of the carpet sampled, but this information was not relevant in homes where vacuum cleaner dust was used.
For the nicotine analyses, each 0.5-g dust aliquot was spiked with 250 ng of d4-nicotine, extracted by ultrasonication in dichloromethane, concentrated, and analyzed by using a gas chromatograph–mass spectrometer in the multiple ion detection mode. The gas chromatograph analysis utilized a DB-1701 column (30 m, 0.25-mm internal diameter, 0.15-μm film) that was programmed from 130°C to 220°C at 2°C/minute and then from 220°C to 280°C at 10°C/minute. Dibromobiphenyl was used as an internal standard; a 9-point calibration curve (range: 2–750 ng/mL) and a zero-level standard were analyzed with each sample set (12 field samples, a duplicate, a duplicate spike (250 ng), and a solvent method blank). We used d4-nicotine as a surrogate recovery standard to correct for variable nicotine recovery on a sample-by-sample basis. Recoveries of nicotine and d4-nicotine in spiked samples were 57% (standard deviation, 45) and 59% (standard deviation, 45), respectively. The median relative percentage difference for duplicates was 17% after surrogate recovery standard correction.
Self-reported smoking
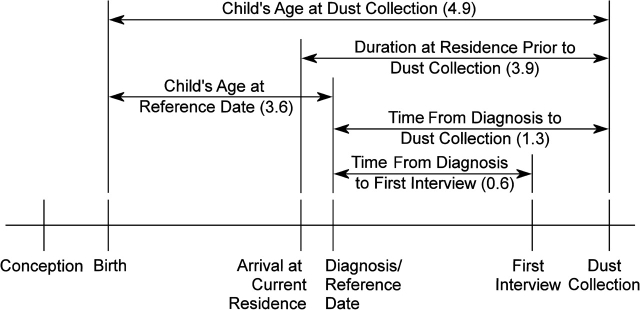
Parents, primarily the mother (97%), responded to 2 sets of questionnaires, each with inquiries about smoking habits, as outlined in Figure 1. The initial interview ascertained the smoking status of the mother, father, and others in the household at several time points of interest (Table 1). Additionally, the first interview asked the respondent for the number of cigarettes smoked per day for some but not all of the time periods. A subsequent interview at the time of dust collection ascertained the total number of cigarettes smoked per day inside the house during the previous month. This additional question dealt specifically with smoking inside the home and was therefore expected to correspond to the concurrent house-dust nicotine measurements. However, we also considered responses from the first interview as potential determinants of house-dust nicotine concentrations because nicotine is known to persist indoors (31), where it is protected from degradation by moisture, sunlight, and microbial action.
Figure 1.
Conceptual timeline of the Northern California Childhood Leukemia Study, 1999–2007, showing time variables included in the statistical analysis as potential modifiers of house-dust nicotine concentrations. For each variable, the median value for the study population (measured in years) is shown in parentheses.
Table 1.
Prevalence of Smoking at Various Time Periods Indicated by Variables Derived From Interviews, Northern California Childhood Leukemia Study, 1999–2007
| Self-reported Cigarette Smoking Variable | Response |
Median Nicotine Concentration (ng/g) |
||||
| Yes | No | % Yes | Yes | No | P Valuea | |
| Mother ever smoked | 137 | 326 | 30 | 465 | 203 | <0.0001 |
| Mother smoked at first interview | 41 | 422 | 9 | 1,256 | 240 | <0.0001 |
| Mother smoked during 3 months before conceptionb | 53 | 410 | 11 | 1,071 | 237 | <0.0001 |
| Mother smoked during pregnancyb | 29 | 434 | 6 | 1,634 | 245 | <0.0001 |
| Mother smoked after birthb | 53 | 409 | 11 | 847 | 235 | <0.0001 |
| Father ever smoked | 182 | 277 | 40 | 415 | 184 | <0.0001 |
| Father smoked at first interview | 76 | 380 | 17 | 774 | 214 | <0.0001 |
| Father smoked during 3 months before conceptionb | 95 | 363 | 21 | 613 | 215 | <0.0001 |
| Anyone else smoked during 1 year before birth | 25 | 438 | 5 | 1,109 | 252 | <0.0001 |
| Anyone else smoked during 1 year after birth | 17 | 443 | 4 | 1,109 | 255 | <0.0001 |
| Anyone smoked in the house during the month before dust collectionb | 21 | 446 | 4 | 1,256 | 256 | <0.0001 |
Kruskal-Wallis one-way analysis of variance.
Indicates that cigarette consumption (cigarettes smoked per day) was obtained for this category.
Statistical analysis
Because the distribution of nicotine in house dust was highly skewed, the nonparametric Kruskal-Wallis one-way analysis of variance (KW ANOVA) was used to compare the distribution of nicotine in house dust between various groups throughout the analysis. The data had an approximate log-normal distribution, so the natural log of the concentration was used in all analyses involving the continuous variable. House-dust nicotine measurements below the limit of detection, that is, 20 ng/g (n = 53, 11% of households), were assigned a value of one-half the limit of detection. Pairwise correlation coefficients between the natural log-transformed house-dust nicotine concentrations and self-reported cigarette smoking (as well as other variables of interest) were estimated. Although Pearson correlation coefficients are reported here, results were similar when we used Spearman rank coefficients.
Seven groups of variables were considered for inclusion in the house-dust nicotine regression models: self-reported smoking, parental demographics, house characteristics, child-specific variables, sampling conditions, time effects, and ethnicity (refer to Appendix Table 1 for the full list of variables considered). Groups of highly correlated variables were examined by principal components analysis to produce simpler, but meaningful, summary measures of the variables within these groups for inclusion in the final house-dust nicotine regression models (32). The remaining groups of candidate variables were modeled individually by using backward elimination (P < 0.10) to identify other variables used in the final models. In addition to main effects, we included significant interactions (P < 0.10) between self-reported smoking variables and parental demographic variables and between self-reported smoking variables and case-control status.
Using the variables identified in group screening, we performed 3 subsequent regression analyses with case and control households combined. The first analysis used all possible households regardless of the sampling method (both HVS3 and vacuum cleaner dust samples were used). The second analysis used only HVS3-sampled households; this analysis included size of sampling area, a variable relevant to only those homes where HVS3 sampling was conducted. The third analysis evaluated the effect of data censoring (due to values below the limit of detection) on the regression coefficients from the first model. We used Tobit regression to model the logged house-dust nicotine concentrations, estimating the parameters of the uncensored data by using maximum likelihood and assuming a normally distributed error term.
RESULTS
Nicotine in house dust
Our analysis included 233 cases and 236 controls for whom house-dust nicotine measurements were available. Nicotine was detected in 89% (416 of 469) of the households. The nicotine concentrations ranged from not detected (less than 20 ng/g) to a maximum of 35,000 ng/g, with a median value of 265 ng/g and an interquartile range of 96–612 ng/g. Table 1 shows the prevalence of smoking during various time periods, the median concentration of nicotine in house dust for smokers and nonsmokers in each category, and the P value from the KW ANOVA comparing the distributions of house-dust nicotine concentrations for smokers versus nonsmokers. Significant differences in house-dust nicotine concentrations were observed for all self-reported smoking categories.
Univariate analysis
Pearson correlation coefficients for covariates of interest (those that were continuous and significantly correlated with the log-transformed house-dust nicotine concentrations) are shown in Table 2. The group of smoking variables was highly correlated, as was the group of parental demographic variables, whereas the 2 groups of variables were negatively correlated with each other. Other variables correlated with house-dust nicotine were age of residence, breastfeeding duration, size of sampling area (HVS3 dust samples only), and vacuum use frequency.
Table 2.
Pearson Correlation Coefficient Matrix of Continuous Variables Significantly Correlated (P < 0.05) With (Logged) House-Dust Nicotine Concentration, Northern California Childhood Leukemia Study, 1999–2007
| Lognormal (House-Dust Nicotine Concentration) | Cigarette Consumptiona of Father 3 Months Before Conception | Cigarette Consumption of Mother 3 Months Before Conception | Cigarette Consumption of Mother While Pregnant | Cigarette Consumption of Mother After Birth | Household Cigarette Consumption at Dust Collection | Mother's Age | Father's Age | Mother's Education | Father's Education | Household Annual Income | Age of Residence | Breastfeeding Duration | Size of Sampling Area | Vacuum Use Frequency | |
| 469 | 454 | 463 | 462 | 462 | 467 | 463 | 459 | 469 | 458 | 469 | 413 | 462 | 385 | 457 | |
| Lognormal (house-dust nicotine concentration) | 1 | 0.37** | 0.24** | 0.17* | 0.31** | 0.29** | −0.26** | −0.16* | −0.27** | −0.28** | −0.24** | 0.13* | −0.18** | −0.16* | 0.15* |
| Cigarette consumption of father 3 months before conception | 1 | 0.41** | 0.20** | 0.46** | 0.29** | −0.14* | −0.05 | −0.18* | −0.21** | −0.16* | 0.00 | −0.08 | −0.05 | 0.12* | |
| Cigarette consumption of mother 3 months before conception | 1 | 0.75** | 0.87** | 0.30** | −0.13* | −0.04 | −0.11* | −0.09* | −0.01 | −0.04 | −0.07 | −0.02 | 0.04 | ||
| Cigarette consumption of mother while pregnant | 1 | 0.63** | 0.26** | −0.09* | −0.08 | −0.06 | −0.03 | 0.00 | −0.01 | −0.06 | 0.03 | −0.01 | |||
| Cigarette consumption of mother after birth | 1 | 0.38** | −0.15* | −0.07 | −0.14* | −0.12* | −0.04 | −0.04 | −0.10* | −0.08 | 0.08 | ||||
| Household cigarette consumption at dust collection | 1 | −0.12* | −0.03 | −0.16* | −0.15* | −0.14* | 0.04 | −0.07 | −0.03 | 0.09 | |||||
| Mother's age | 1 | 0.75** | 0.42** | 0.39** | 0.37** | 0.11* | 0.24** | 0.11* | −0.26** | ||||||
| Father's age | 1 | 0.36** | 0.34** | 0.32** | 0.09 | 0.20** | 0.05 | −0.23** | |||||||
| Mother's education | 1 | 0.69** | 0.60** | 0.06 | 0.17* | 0.06 | −0.35** | ||||||||
| Father's education | 1 | 0.59** | 0.03 | 0.23** | 0.09 | −0.37** | |||||||||
| Household annual income | 1 | −0.05 | 0.11* | 0.13* | −0.33** | ||||||||||
| Age of residence | 1 | 0.10* | −0.14* | −0.04 | |||||||||||
| Breastfeeding duration | 1 | 0.00 | −0.21** | ||||||||||||
| Size of sampling area | 1 | 0.09 | |||||||||||||
| Vacuum use frequency | 1 |
* P < 0.05; **P < 0.0001.
Cigarettes smoked per day.
Principal components analysis
Tables 3 and 4 show the results of the principal components analysis for the 2 groups of highly correlated variables: self-reported smoking and parental demographics, respectively. Three meaningful factors were chosen to represent the 15 self-reported smoking variables, and 2 factors were chosen to represent the 5 parental demographic variables. A variable was said to load on a given component if the factor loading was 0.40 or greater (32). When this criterion was used, 12 variables describing parental smoking were found to load on the first smoking component, which was subsequently labeled the parental smoking component. Similarly, the 4 father's smoking variables loaded on the second smoking component (father smoking component), and 3 variables, describing other household smoking, loaded on the third component (other household smoking component). Combined, the smoking-related principal components accounted for 65% of the total variance of all smoking variables. The demographic variable group, shown in Table 4, was described by a parental socioeconomic status (SES) component, which was loaded by parental education and income, and a parental age component, which was loaded by mother's age and father's age. Combined, the summary demographic principal components accounted for 80% of the total variance explained by all demographic variables.
Table 3.
Principal Components Analysis Factor Loadings for Self-reported Smoking Variables, Northern California Childhood Leukemia Study, 1999–2007
| Variable | Component Loadings |
||
| Parental Smoking | Father Smoking | Other Household Smoking | |
| Lifetime smoking status of mother | 0.59a | −0.13 | −0.22 |
| Smoking status of mother at initial interview | 0.74a | −0.23 | −0.18 |
| Smoking status of mother 3 months before conception | 0.83a | −0.29 | −0.11 |
| Smoking status of mother while pregnant | 0.74a | −0.35 | 0.07 |
| Smoking status of mother after birth | 0.80a | −0.19 | −0.19 |
| Cigarette consumptionb of mother 3 months before conception | 0.85a | −0.28 | 0.09 |
| Cigarette consumption of mother while pregnant | 0.65a | −0.39 | 0.04 |
| Cigarette consumption of mother after birth | 0.84a | −0.19 | 0.09 |
| Lifetime smoking status of father | 0.43a | 0.60a | −0.25 |
| Smoking status of father at initial interview | 0.49a | 0.71a | −0.14 |
| Smoking status of father 3 months before conception | 0.60a | 0.67a | −0.16 |
| Cigarette consumption of father 3 months before conception | 0.65a | 0.56a | 0.02 |
| Smoking status of others in the house before birth | 0.39 | 0.18 | 0.65a |
| Smoking status of others in the house after birth | 0.20 | 0.14 | 0.58a |
| Household cigarette consumption during the month before dust collection | 0.34 | 0.19 | 0.50a |
| Proportion (%) of group variance explained | 41 | 15 | 8 |
A variable was said to load on a given component if the factor loading was ≥0.40.
Cigarettes smoked per day.
Table 4.
Principal Components Analysis Factor Loadings for Parental Demographic Variables, Northern California Childhood Leukemia Study, 1999–2007
| Variable | Component Loadings |
|
| Parental Socioeconomic Status | Parental Age | |
| Father's education | 0.86a | 0.19 |
| Mother's education | 0.85a | 0.23 |
| Household annual income | 0.81a | 0.20 |
| Father's age | 0.27 | 0.89a |
| Mother's age | 0.18 | 0.92a |
| Proportion (%) of group variance explained | 59 | 21 |
A variable was said to load on a given component if the factor loading was ≥0.40.
Multivariable regression models
We identified 13 variables for the model with all homes (Table 5) based on a priori screening of groups of variables for significant associations with logged house-dust nicotine concentrations. The variables are ordered in Table 5 by their significance in the final model. We included 2 significant interactions between the parental SES component and the father smoking component and between the parental age component and the father smoking component. After adjustment for the model degrees of freedom, the overall model fit was R2adjusted = 0.31. Table 6 shows predicted house-dust nicotine concentrations for various combinations of smoking scenarios and parental demographics based on the model with all homes.
Table 5.
Final Multivariate Regression Models of Determinants of House-Dust Nicotine Concentrations, Northern California Childhood Leukemia Study, 1999–2007
| Variable | All Homes (n = 381) |
HVS3 Homes (n = 312) |
||||
| Regression Coefficient | t | P Value | Regression Coefficient | t | P Value | |
| Intercept | 5.09 | 5.51 | ||||
| Parental smoking componenta | 0.55 | 7.5 | <0.0001 | 0.50 | 6.3 | <0.0001 |
| Father smoking componenta | 0.34 | 4.5 | <0.0001 | 0.32 | 4.0 | <0.0001 |
| Age of residenceb | 0.11 | 3.8 | 0.0002 | 0.09 | 2.7 | 0.008 |
| Parental socioeconomic status componenta | −0.26 | −3.0 | 0.003 | −0.26 | −2.7 | 0.007 |
| Parental age componenta | −0.20 | −2.6 | 0.011 | −0.18 | −2.1 | 0.035 |
| Residence is apartmentc | 0.84 | 2.3 | 0.022 | 0.93 | 2.4 | 0.015 |
| Residence is townhousec | 0.61 | 1.9 | 0.056 | 0.56 | 1.5 | 0.13 |
| Season is fallc | −0.25 | −1.5 | 0.13 | −0.33 | −1.6 | 0.11 |
| Breastfeeding durationd | −0.02 | −1.5 | 0.13 | −0.01 | −1.2 | 0.24 |
| Residence is mobile homec | 0.65 | 1.2 | 0.23 | 0.58 | 1.1 | 0.28 |
| Other household smoking componenta | 0.09 | 1.1 | 0.26 | 0.06 | 0.7 | 0.47 |
| Child is neither Hispanic nor whitec | −0.10 | −0.6 | 0.56 | −0.13 | −0.7 | 0.51 |
| Vacuum use frequencye | 0.02 | 0.3 | 0.80 | 0.05 | 0.5 | 0.59 |
| Father smoking × parental socioeconomic status | 0.34 | 3.8 | 0.0002 | 0.30 | 3.1 | 0.002 |
| Father smoking × parental age | 0.18 | 2.4 | 0.019 | 0.17 | 2.0 | 0.042 |
| Size of sampling areaf | −0.16 | −2.4 | 0.018 | |||
Abbreviation: HVS3, high-volume surface sampler.
Each principal component is a unitless variable with a mean of 0 and standard deviation of 1; values were calculated based on component loadings shown in Tables 3 and 4.
Categorical variable (age in 5-year increments).
Dichotomous variable.
Continuous variable (duration in months).
Categorical variable (vacuum frequency less than once a month, 1–3 times a month, once a week, or more than once a week).
Continuous variable (area in square meters).
Table 6.
Predicted House-Dust Nicotine Concentrations (ng/g) for Various Combinations of Self-reported Smoking and Parental Demographics,a Northern California Childhood Leukemia Study, 1999–2007
| Description of Self-reported Smoking | Parental Demographics |
||
| Younger and Lower SESb | Median Age and Median SESc | Older and Higher SESd | |
| No smoking by anyone at any time | 210 | 130 | 90 |
| Only the mother smoked—stopped at least 3 months before conceptione | 230 | 130 | 90 |
| Only the father smoked—stopped at least 3 months before conceptione | 210 | 160 | 130 |
| Both the mother and father smoked—stopped at least 3 months before conceptione | 230 | 170 | 130 |
| No parental smoking at any time; only others smoked in the home before and after birthe | 400 | 330 | 280 |
| Only the mother smoked at all time periodsf | 1,000 | 230 | 70 |
| Only the father smoked at all time periodsf | 230 | 370 | 530 |
| Both the mother and father smoked at all time periodsf | 1,100 | 650 | 420 |
| Both the mother and father smoked at all time periodsf and in-home smoking reported at dust collectionf | 1,300 | 800 | 540 |
| Both the mother and father smoked at all time periodsf and others smoked before and after birthe | 2,200 | 1,700 | 1,300 |
| Both the mother and father smoked at all time periodsf and others smoked before and after birthe and in-home smoking reported at dust collectionf | 2,500 | 2,000 | 1,700 |
Abbreviation: SES, socioeconomic status.
Predicted values based on the “All Homes” model, assuming a 10-year-old single-family house, measurement season is not fall, no breastfeeding, child is white or Hispanic, and usual vacuum frequency of less than once a month.
25th percentile values: mother's age (years) = 26, father's age (years) = 28, household annual income = $30,000–$44,000, parents have high school degrees.
Median values: mother's age (years) = 30, father's age = 33, household annual income = $60,000–$74,000, parents have some post–high school education.
75th percentile values: mother's age (years) = 34, father's age (years) = 37, household annual income = ≥$75,000, parents have bachelor's degrees.
Dichotomous (yes/no) smoking variable.
Continuous smoking variable, showing model predictions for smoking 5 cigarettes per day.
Restricting the analysis to only HVS3-sampled homes (and including the variable size of sampling area) yielded a model with similar regression coefficients and P values (Table 5, HVS3 homes). The variable size of sampling area was significant in the model with only HVS3-sampled homes. When the effect of data censoring was considered, parameter estimates from the Tobit regression model were very similar to those in Table 5 (data not shown), a finding consistent with the relatively small proportion of measurements below the limit of detection (11%).
DISCUSSION
The house-dust nicotine concentrations measured in the NCCLS were lower than those previously reported (26, 28, 29). These lower levels might be explained by the low prevalence of in-home smoking in our population (4% vs. 19%–65% in previous studies) or by the low prevalence of smoking in California in general. However, these differences persisted when we considered only the self-reported nonsmokers from each study; our measured concentrations in these homes were substantially lower (median of 0.3 μg/g vs.12–20 μg/g in previous studies). Alternatively, these differences may partly reflect differences in analytical methodology. Despite the lower nicotine concentrations, we confirmed that nicotine concentrations in house-dust samples were correlated with concurrently self-reported household cigarette smoking (rP = 0.29, in log scale).
We identified several determinants of house-dust nicotine concentrations (Table 5). Notably, 2 principal components summarizing self-reported smoking variables (parental smoking component and father smoking component) were highly significant predictors of house-dust nicotine in the final models (P < 0.0001). These principal components represented self-reported smoking for time periods of months and years before dust collection. As shown in Table 6, a lifetime history of parental smoking did not substantially increase predicted house-dust nicotine concentrations above nonsmoking levels, unless more recent smoking had also occurred (3 months before conception or later). However, consistent parental smoking caused the predicted house-dust nicotine concentrations to increase, even in the absence of in-home smoking reported at dust collection. Indeed, the additional effect of in-home smoking reported at dust collection on predicted house-dust nicotine concentrations was small. This observation shows that household cigarette smoking during the month before dust collection was a weak predictor of house-dust nicotine levels in the final models. Our finding suggests that nicotine concentrations in house dust reflect cumulative smoking habits of residents over periods of up to several years rather than simply the current smoking pattern in the home.
To verify our hypothesis that house-dust nicotine levels reflect past smoking habits, we examined the NCCLS households that reported changes in their smoking status between the initial interview and dust collection. Of the households that reported no smoking in the month before dust collection, 90 households (21%) had previously reported some smoking at the initial interview. Nicotine concentrations in house dust from these 90 households did indeed remain elevated (median: 681 ng/g vs. 201 ng/g for consistently smoke-free homes, KW ANOVA P < 0.0001). Additionally, of the households that reported some smoking at the time of dust collection, 5 (24%) reported no smoking at the initial interview. These 5 households had lower house-dust nicotine concentrations than households that consistently reported smoking (median 314 ng/g vs. 1,730 ng/g, KW ANOVA P = 0.22). This finding supports the conjecture that current house-dust nicotine concentrations may be particularly good measures of cumulative household smoking habits. Furthermore, it suggests that, in studies that aim to estimate prenatal or postnatal cigarette smoking exposures retrospectively, house-dust nicotine could be a more useful surrogate than short-term exposure markers such as concentrations of nicotine in air or of cotinine in urine.
After considering self-reported smoking, age of the residence was a significant predictor of house-dust nicotine concentrations. The increase in house-dust nicotine concentrations with age of the residence offers another indication that nicotine accumulates in household carpets and that the nicotine concentration in house dust tends to reflect cumulative smoking habits in the household.
Two measures of parental demographics, the parental SES component and the parental age component, remained significant predictors of house-dust nicotine concentration after accounting for self-reported smoking. Table 6 illustrates that, in general, after adjusting for self-reported smoking, house-dust nicotine concentrations decreased with increasing parental SES and age. However, the interaction effects of the model (father smoking component × parental SES component and father smoking component × parental age component) caused this trend to be reversed when only the father smoked.
Interestingly, when we considered the 211 households that reported no smoking at any time, the households with below-median income had significantly higher house-dust nicotine concentrations than the households with above-median income (median: 279 ng/g vs. 113 ng/g, KW ANOVA P < 0.0001). Thus, even when no smoking was reported, low-income households had elevated house-dust nicotine concentrations compared with high-income households. Several explanations are possible for the discrepancy in house-dust nicotine levels in self-reported nonsmoking households: 1) low-SES houses may be physically unlike high-SES houses because of unmeasured differences in ventilation, carpet types, light, moisture, or microbial action; 2) low-SES parents may be more likely to be exposed to passive cigarette smoke, and they may convey nicotine into their homes on their clothing; 3) low-SES households may be more likely to have residual nicotine in house dust from previous residents (although the variables carpet age and residence stability were not significant predictors of house-dust nicotine); 4) low-SES households may use more smokeless tobacco products; or 5) low-SES households may have underreported their smoking habits. If differential self-reporting by SES or age is present, then an objective measure of exposure to household smoking, such as house-dust nicotine concentration, would be advantageous.
Three other variables were significant predictors of nicotine concentrations in house dust after we adjusted for self-reported smoking and parental demographics: residence is apartment, residence is townhouse, and size of sampling area. Since apartments and townhouses generally have less square footage than single-family homes, the positive regression coefficient for the variables residence is apartment and residence is townhouse is consistent with the observation of Hein et al. (26), who found that house-dust nicotine concentrations increased with decreasing square footage of the residence. The negative regression coefficient for the variable size of sampling area in the final model with HVS3-sampled homes indicates that, as the size of carpet sampled increased, the concentration of nicotine measured in house dust decreased. This finding is a property of the HVS3 sampling method and suggests that this variable should be measured and adjusted for in models of house-dust nicotine concentrations using HVS3 sampling. Still, including size of sampling area in our regression model had little effect on the other parameters.
Given that the ultimate purpose of the NCCLS is to compare leukemia cases and controls, we examined the effect of case-control status on measured nicotine concentration. Interestingly, case-control status was not a significant predictor of nicotine concentrations, and there was no indication that parents of cases were reporting their smoking differently from parents of controls (data not shown). This finding suggests that there was little differential misclassification of exposures in households of cases and controls in the previous analysis of self-reported cigarette smoking in the NCCLS population (8).
Although house-dust nicotine is a very specific indicator of cigarette smoke contamination in the home, its use to access children's exposure to cigarette smoke has limitations. First, it must be assumed that children are in the home when smoking occurs. This expectation is reasonable given the young age of the children in the NCCLS (median: 3.6 years at reference date). Second, it must be assumed that house-dust nicotine originated from cigarette smoke in the home. However, a previous study found that nicotine levels in house dust were elevated in homes where parents reported smoking outdoors only compared with homes where parents reported no smoking (27). Thus, parents exposed to cigarette smoke (either active or passive) may convey nicotine into carpets, via their clothing, without exposing their children to cigarette smoke. Indeed, this phenomenon could help explain the relatively weak performance in the house-dust nicotine models of the variable household cigarette consumption during month before dust collection, which was specific to in-home smoking. In contrast, the highly significant parental smoking component and father smoking component were based on smoking both inside and outside the home. Future studies should consider using a long-term biomarker of exposure to cigarette smoke, such as hair nicotine, to investigate the relation between house-dust nicotine and the corresponding biologic dose of nicotine in children.
A second limitation of house-dust nicotine as a measure of children's exposure to cigarette smoke relates to the temporality of exposure. It is suspected that specific time windows of exposure are critical to the etiology of childhood leukemia (8). However, since house-dust nicotine appears to be a cumulative measure of exposure to cigarette smoke, exposures received at particular times could be obscured. Future work should investigate this issue by comparing measurements of nicotine in house dust with time series of air samples collected from the same households.
Finally, our study included only a single measurement of house-dust nicotine in each household. This sampling strategy prevented us from analyzing the temporal (i.e., day-to-day) and spatial (i.e., room-to-room) variability of house-dust nicotine concentrations in a given household, and our analytical method introduced additional variability into the analyses (17% median relative difference in duplicate samples). Future studies should collect repeated samples of house dust to evaluate within-room, within-home, and between-home sources of variability of house-dust nicotine concentrations.
In summary, this study confirmed previous findings that house-dust nicotine concentrations are significantly associated with self-reported household smoking. Results suggest that house-dust nicotine can be used as a long-term surrogate for exposure to cigarette smoke in the home. Moreover, indirect evidence indicates that self-reported smoking may vary by parental SES and age, highlighting the utility of an objective surrogate of exposure to cigarette smoke in the home. Finally, these findings suggest that house-dust nicotine concentrations, in conjunction with self-reported questionnaires on smoking, will be useful in evaluating the association between childhood leukemia risk and parental smoking in the NCCLS population.
Acknowledgments
Author affiliations: School of Public Health, University of California, Berkeley, California (Todd Whitehead, Catherine Metayer, Steve Selvin, Patricia Buffler, Stephen M. Rappaport); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland (Mary H. Ward, Joanne S. Colt); Battelle Memorial Institute, Columbus, Ohio (Marcia G. Nishioka); and Northern California Cancer Center, Berkeley, California (Robert Gunier, Peggy Reynolds).
This work was supported by the National Institute of Environmental Health Sciences (grants R01ES009137, P-42-ES-04705-18), the Intramural Research Program of the National Cancer Institute, National Institute of Health (subcontracts 7590-S-04, 7590-S-01); and the National Cancer Institute (contract N02-CP-11015).
The authors thank clinical investigators at the collaborating California hospitals for help in recruiting patients, including the University of California Davis Medical Center (Dr. Jonathan Ducore), University of California San Francisco (Dr. Mignon Loh and Dr. Katherine Matthay), Children's Hospital of Central California (Dr. Vonda Crouse), Lucile Packard Children's Hospital (Dr. Gary Dahl), Children's Hospital Oakland (Dr. James Feusner), Kaiser Permanente Sacramento (Dr. Vincent Kiley), Kaiser Permanente Santa Clara (Dr. Carolyn Russo and Dr. Alan Wong), Kaiser Permanente San Francisco (Dr. Kenneth Leung), and Kaiser Permanente Oakland (Dr. Stacy Month). They also acknowledge the entire Northern California Childhood Leukemia Study staff and the Survey Research Center for their efforts and dedication.
Conflict of interest: none declared.
Glossary
Abbreviations
- HVS3
high-volume surface sampler
- KW ANOVA
Kruskal-Wallis one-way analysis of variance
- NCCLS
Northern California Childhood Leukemia Study
- SES
socioeconomic status
Appendix Table 1.
List of Potential Variables for Linear Regression, Northern California Childhood Leukemia Study, 1999-2007
| Variable Group | Variable | Unit |
| Self-reported smoking | Lifetime smoking status of mother | Dichotomous |
| Smoking status of mother 3 months before conception | Dichotomous | |
| Smoking status of mother while pregnant | Dichotomous | |
| Smoking status of mother after birth | Dichotomous | |
| Smoking status of mother at initial interview | Dichotomous | |
| Cigarette consumption of mother 3 months before conception | Continuous (cigarettes/day) | |
| Cigarette consumption of mother while pregnant | Continuous (cigarettes/day) | |
| Cigarette consumption of mother after birth | Continuous (cigarettes/day) | |
| Lifetime smoking status of father | Dichotomous | |
| Smoking status of father 3 months before conception | Dichotomous | |
| Smoking status of father at initial interview | Dichotomous | |
| Cigarette consumption of father 3 months before conception | Continuous (cigarettes/day) | |
| Smoking status of others in the house before birth | Dichotomous | |
| Smoking status of others in the house after birth | Dichotomous | |
| Household cigarette consumption during the month before dust collection | Continuous (cigarettes/day) | |
| Parental demographics | Mother's age | Continuous (years) |
| Father's age | Continuous (years) | |
| Mother's education | Categorical | |
| Father's education | Categorical | |
| Household annual income | Categorical | |
| House characteristics | Residence is apartment | Dichotomous |
| Residence is townhouse | Dichotomous | |
| Residence is mobile home | Dichotomous | |
| Age of residence | Categorical | |
| Child-specific variables | Case-control status | Dichotomous |
| Sex | Dichotomous | |
| Age at reference date | Continuous (years) | |
| Down syndrome status | Dichotomous | |
| Birth weight | Continuous (g) | |
| Breastfeeding duration | Continuous (months) | |
| Sampling conditions | Sampling method | Dichotomous |
| Sampling temperature | Continuous (°Fa) | |
| Sampling humidity | Continuous (% relative humidity) | |
| Size of sampling area | Continuous (m2) | |
| Age of carpet sampled | Continuous (years) | |
| Room throughway distinction | Dichotomous | |
| Vacuum use frequency | Categorical | |
| Interview respondent | Dichotomous | |
| Time effects | Time from diagnosis to initial interview | Continuous (days) |
| Time from diagnosis to dust collection | Continuous (days) | |
| Year of dust collection | Continuous | |
| Season is spring | Dichotomous | |
| Season is summer | Dichotomous | |
| Season is fall | Dichotomous | |
| Residence stability | Continuous (years) | |
| Ethnicity | Child is white | Dichotomous |
| Child is other ethnicity | Dichotomous | |
| Mother is white | Dichotomous | |
| Mother is other ethnicity | Dichotomous | |
| Father is white | Dichotomous | |
| Father is other ethnicity | Dichotomous |
°C = (5/9)(°F – 32).
References
- 1.Stjernfeldt M, Berglund K, Lindsten J, et al. Maternal smoking during pregnancy and risk of childhood cancer. Lancet. 1986;1(8494):1350–1352. doi: 10.1016/s0140-6736(86)91664-8. [DOI] [PubMed] [Google Scholar]
- 2.John EM, Savitz DA, Sandler DP. Prenatal exposure to parents’ smoking and childhood cancer. Am J Epidemiol. 1991;133(2):123–132. doi: 10.1093/oxfordjournals.aje.a115851. [DOI] [PubMed] [Google Scholar]
- 3.Infante-Rivard C, Krajinovic M, Labuda D, et al. Parental smoking, CYP1A1 genetic polymorphisms and childhood leukemia (Québec, Canada) Cancer Causes Control. 2000;11(6):547–553. doi: 10.1023/a:1008976116512. [DOI] [PubMed] [Google Scholar]
- 4.Cnattingius S, Zack M, Ekbom A, et al. Prenatal and neonatal risk factors for childhood myeloid leukemia. Cancer Epidemiol Biomarkers Prev. 1995;4(5):441–445. [PubMed] [Google Scholar]
- 5.Mucci LA, Granath F, Cnattingius S. Maternal smoking and childhood leukemia and lymphoma risk among 1,440,542 Swedish children. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1528–1533. [PubMed] [Google Scholar]
- 6.Shu XO, Ross JA, Pendergrass TW, et al. Parental alcohol consumption, cigarette smoking, and risk of infant leukemia: a Childrens Cancer Group Study. J Natl Cancer Inst. 1996;88(1):24–31. doi: 10.1093/jnci/88.1.24. [DOI] [PubMed] [Google Scholar]
- 7.Ji BT, Shu XO, Linet MS, et al. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J Natl Cancer Inst. 1997;89(3):238–244. doi: 10.1093/jnci/89.3.238. [DOI] [PubMed] [Google Scholar]
- 8.Chang JS, Selvin S, Metayer C, et al. Parental smoking and the risk of childhood leukemia. Am J Epidemiol. 2006;163(12):1091–1100. doi: 10.1093/aje/kwj143. [DOI] [PubMed] [Google Scholar]
- 9.Lee KM, Ward MH, Han S, et al. Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk Res. 2009;33(2):250–258. doi: 10.1016/j.leukres.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudant J, Menegaux F, Leverger G, et al. Childhood hematopoietic malignancies and parental use of tobacco and alcohol: the ESCALE study (SFCE) Cancer Causes Control. 2008;19(10):1277–1290. doi: 10.1007/s10552-008-9199-5. [DOI] [PubMed] [Google Scholar]
- 11.Severson RK, Buckley JD, Woods WG, et al. Cigarette smoking and alcohol consumption by parents of children with acute myeloid leukemia: an analysis within morphological subgroups—a report from the Childrens Cancer Group. Cancer Epidemiol Biomarkers Prev. 1993;2(5):433–439. [PubMed] [Google Scholar]
- 12.Schuz J, Kaatsch P, Kaletsch U, et al. Association of childhood cancer with factors related to pregnancy and birth. Int J Epidemiol. 1999;28(4):631–639. doi: 10.1093/ije/28.4.631. [DOI] [PubMed] [Google Scholar]
- 13.Brondum J, Shu XO, Steinbuch M, et al. Parental cigarette smoking and the risk of acute leukemia in children. Cancer. 1999;85(6):1380–1388. [PubMed] [Google Scholar]
- 14.Menegaux F, Steffen C, Bellec S, et al. Maternal coffee and alcohol consumption during pregnancy, parental smoking and risk of childhood acute leukaemia. Cancer Detect Prev. 2005;29(6):487–493. doi: 10.1016/j.cdp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Menegaux F, Ripert M, Hemon D, et al. Maternal alcohol and coffee drinking, parental smoking and childhood leukaemia: a French population-based case-control study. Paediatr Perinat Epidemiol. 2007;21(4):293–299. doi: 10.1111/j.1365-3016.2007.00824.x. [DOI] [PubMed] [Google Scholar]
- 16.Pang D, McNally R, Birch JM. Parental smoking and childhood cancer: results from the United Kingdom childhood cancer study. Br J Cancer. 2003;88(3):373–381. doi: 10.1038/sj.bjc.6600774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrick DL, Cheadle A, Thompson DC, et al. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84(7):1086–1093. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein JD, Thomas RK, Sutter EJ. Self-reported smoking in online surveys: prevalence estimate validity and item format effects. Med Care. 2007;45(7):691–695. doi: 10.1097/MLR.0b013e3180326145. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins RA, Counts RW. Personal exposure to environmental tobacco smoke: salivary cotinine, airborne nicotine, and nonsmoker misclassification. J Expo Anal Environ Epidemiol. 1999;9(4):352–363. doi: 10.1038/sj.jea.7500036. [DOI] [PubMed] [Google Scholar]
- 20.Boyd NR, Windsor RA, Perkins LL, et al. Quality of measurement of smoking status by self-report and saliva cotinine among pregnant women. Matern Child Health J. 1998;2(2):77–83. doi: 10.1023/a:1022936705438. [DOI] [PubMed] [Google Scholar]
- 21.Walsh RA, Redman S, Adamson L. The accuracy of self-report of smoking status in pregnant women. Addict Behav. 1996;21(5):675–679. doi: 10.1016/0306-4603(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 22.Caraballo RS, Giovino GA, Pechacek TF, et al. Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2001;153(8):807–814. doi: 10.1093/aje/153.8.807. [DOI] [PubMed] [Google Scholar]
- 23.Hammond SK, Sorensen G, Youngstrom R, et al. Occupational exposure to environmental tobacco smoke. JAMA. 1995;274(12):956–960. [PubMed] [Google Scholar]
- 24.Rylander E, Pershagen G, Eriksson M, et al. Parental smoking, urinary cotinine, and wheezing bronchitis in children. Epidemiology. 1995;6(3):289–293. doi: 10.1097/00001648-199505000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Haley NJ, Hoffmann D. Analysis for nicotine and cotinine in hair to determine cigarette smoker status. Clin Chem. 1985;31(10):1598–1600. [PubMed] [Google Scholar]
- 26.Hein HO, Suadicani P, Skov P, et al. Indoor dust exposure: an unnoticed aspect of involuntary smoking. Arch Environ Health. 1991;46(2):98–101. doi: 10.1080/00039896.1991.9937435. [DOI] [PubMed] [Google Scholar]
- 27.Matt GE, Quintana PJ, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control. 2004;13(1):29–37. doi: 10.1136/tc.2003.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willers S, Hein HO, Jansson L. Assessment of environmental tobacco smoke exposure: urinary cotinine concentrations in children are strongly associated with the house dust concentrations of nicotine at home. Indoor Air. 2004;14(2):83–86. doi: 10.1046/j.1600-0668.2003.00211.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Aung T, Berkeley E, et al. Measurement of nicotine in household dust. Environ Res. 2008;108(3):289–293. doi: 10.1016/j.envres.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Colt JS, Gunier RB, Metayer C, et al. Household vacuum cleaners vs. the high-volume surface sampler for collection of carpet dust samples in epidemiologic studies of children. Environ Health. 2008;7:6. doi: 10.1186/1476-069X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singer BC, Revzan KL, Hotch T, et al. Sorption of organic gases in a furnished room. Atmos Environ. 2004;38:2483–2494. [Google Scholar]
- 32.Hatcher L. A Step-by-Step Approach to Using the SAS System for Factor Analysis and Structural Equation Modeling. Cary, NC: SAS Institute, Inc; 1994. Principal component analysis. [Google Scholar]