Abstract
Higher plasma urate concentration has been linked to lower risk of Parkinson's disease in men, but data are lacking on women and African Americans. The authors examined plasma urate in relation to Parkinson's disease in the biracial, population-based Atherosclerosis Risk in Communities (ARIC) cohort. Between 1987 and 1989, 15,792 participants, aged 45–64 years, were recruited from 4 US communities and have since been followed with 3 triennial visits and annual surveillance. Plasma urate was measured at visits 1 and 2, and the concentrations were highly correlated. From visit 1 through 2004, 95 potential cases of Parkinson's disease were identified from multiple sources. Odds ratios and 95% confidence intervals were calculated from multivariate logistic regression models. Plasma urate concentration was inversely associated with Parkinson's disease occurrence. The odds ratios between extreme quartiles of plasma urate were 0.4 (95% confidence interval: 0.2, 0.8) in the overall analysis, 0.3 (95% confidence interval: 0.1, 0.7) for men, and 0.4 (95% confidence interval: 0.2, 1.0) for Caucasians. Such an association was also suggested among women and African Americans but was not statistically significant because of small sample sizes. These data support the previous finding that urate may be a protective factor against Parkinson's disease.
Keywords: cohort studies, Parkinson disease, uric acid
Accumulating evidence supports that oxidative stress contributes to loss of dopaminergic neurons in the substantia nigra of Parkinson's disease patients (1). Uric acid is a potent endogenous antioxidant that effectively scavenges reactive nitrogen and oxygen radicals and has thus been hypothesized to protect against neurodegeneration in Parkinson's disease (2–4). Recent results from 3 prospective cohorts seem to support this hypothesis (5–7). The Honolulu Asian Aging Study first reported that higher plasma urate concentration measured at midlife was associated with lower Parkinson's disease risk decades later among Japanese-American men (5). Similar observations were then reported by the Health Professionals Follow-up Study (6) and the Rotterdam study (7). While these studies showed promising evidence for men, they provided little data on women. The first 2 studies included men only (5, 6), and the third had a total of 68 cases and presented no sex-specific results (7). Furthermore, none of the previous studies included African Americans, who, on average, have higher levels of plasma urate than do Caucasians (8). In the current study, we examined the relation between plasma urate and risk of Parkinson's disease in the Atherosclerosis Risk in Communities (ARIC) Study, a biracial cohort followed prospectively for approximately 20 years comprising 55% women and 27% African Americans.
MATERIALS AND METHODS
Study population
Details of the ARIC cohort design and objectives have been published previously (9). Briefly, this ongoing, multicenter, longitudinal study includes over 20 years of follow-up. ARIC was designed to examine cardiovascular risk factors in 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; Washington County, Maryland; and the northwest suburbs of Minneapolis, Minnesota (9). Between 1987 and 1989, the study recruited 15,792 participants aged 45–64 years via probability list or area sampling. At baseline, all enrollees who provided consent participated in a comprehensive in-home interview on personal characteristics and history, lifestyle, and diet using a food frequency questionnaire. Participants were also invited to a clinical examination focusing mostly on cardiovascular diseases and provided a blood sample.
After baseline, participants received 3 additional clinical examinations approximately every 3 years, with the most recent in 1996–1998. At each study visit, participants were asked to bring the bottles of all medications they had taken during the past 2 weeks, and the medications were later electronically coded. At visit 4, all participants were asked whether they had ever been diagnosed with Parkinson's disease and, if yes, the year of diagnosis. In addition to triennial visits, the study has contacted participants annually by telephone and has set up a comprehensive community surveillance network involving all area hospitals. By doing so, all hospitalizations during follow-up were identified with discharge diagnoses recorded according to the International Classification of Diseases (ICD; the Ninth Revision was used during early follow-up, and the Tenth Revision was used later). Finally, deaths during follow-up were identified via an annual search of the National Death Index, and causes of death were recorded and ICD coded. To remove potential prevalent cases from the analysis, we excluded 15 cases who used anti-Parkinson medication at visit 1 or self-reported a date of diagnosis before visit 1, or were hospitalized because of or died from Parkinson's disease before 1990. After further excluding participants whose race was other than Caucasian or African American and who had missing values on urate and potential confounders, a total of 15,036 participants were included in the primary analysis.
The ARIC Study was approved by the institutional review boards of all study sites, and informed consent was obtained from all study participants. This particular analysis was approved by the Human Subjects Committee of the National Institute of Environmental Health Sciences.
Identification of Parkinson's disease cases
Possible Parkinson's disease cases through 2004 were initially identified if they had any of the following: use of typical antiparkinsonian medications (carbidopa/levodopa (Sinemet; DuPont Pharma, Wilmington, Delaware) or dopamine agonists (e.g., pramipexole (Mirapex; Pharmacia & Upjohn Company, Kalamazoo, Michigan), ropinirole (Requip; GlaxoSmithKline, Middlesex, United Kingdom), and pergolide (Permax; Athena Neurosciences, Inc., South San Francisco, California)), a monamine oxidase B inhibitor such as selegiline (Eldepryl; Somerset Pharmaceuticals, Inc., Tampa, Florida), or amantadine (Symmetrel; Endo Pharmaceuticals, Inc., Wilmington, Delaware) or anticholinergic drugs (e.g., trihexyphenidyl (Artane; Lederle Laboratories, Pearl River, New York), benztropine (Cogentin; Merck & Co., Inc., West Point, New York)) at any of the 4 visits; self-reported Parkinson's disease at the fourth study visit; or an ICD code of Parkinson's disease (332.0 for ICD-9 or G20 for ICD-10) on the hospitalization discharge chart or death certificate. A total of 173 possible Parkinson's disease cases were identified. Self-reported medication use by these 173 potential Parkinson's disease cases, along with the ICD diagnosis and the status of self-reported Parkinson's disease, were individually reviewed by a movement disorder specialist (X. H.).
Individuals who used drugs that could induce parkinsonism, such as neuroleptics or lithium, were excluded (n = 41). Furthermore, we excluded 20 individuals who used only amantadine and/or anticholinergic drugs without additional supporting evidence of Parkinson's disease. Finally, 112 participants were considered potential Parkinson's disease cases, and information on confounders was missing for 2 of them. As explained earlier, 15 additional cases were likely prevalent cases at baseline, leaving 95 cases in the primary analysis. Inclusion of these 15 cases in the analyses did not materially change the results (data not shown).
Measurement of plasma urate and potential confounders
Fasting blood was collected at visits 1 and 2 from all eligible participants. Details on blood collection, handling, and storage have been published previously (10, 11). Briefly, specimens were drawn into vacuum tubes containing a serum separator gel (for glucose and chemistries), sodium citrate (for hemostatic factors), or ethylenediaminetetraacetic acid (for lipids). The tubes of blood for glucose and chemistries were allowed to clot for 30–45 minutes, centrifuged at 3,000 × g for 10 minutes at 4°C, and then quickly frozen at −70°C until analysis within a few weeks (10, 11). Plasma urate was measured by using the uricase method at the central laboratories of the ARIC Study. The reliability coefficient of the urate assay was 0.91 as evaluated among 40 participants with repeated measurements at least 1 week apart, and the within-person variability was 7.2% (12). Plasma level of creatinine was also measured as part of the standard laboratory assay (12).
Information on potential confounders was obtained from a baseline in-home interview, including basic demographics, smoking status, and consumption of caffeinated coffee and alcohol. Body weight was measured to the nearest pound (1 pound = 0.45 kg), and height was measured to the nearest centimeter. Body mass index was computed as weight (kilograms) divided by height (meters) squared.
Data analysis
Participants were categorized by baseline (visit 1) plasma urate concentration into quartiles for the primary analysis. We used unconditional logistic regression models to derive odds ratios and 95% confidence intervals, adjusting for baseline age (years), sex, race (Caucasian vs. African American), smoking (never, past, current), caffeine intake (continuous), body mass index (continuous), alcohol intake (continuous), and plasma creatinine (continuous). Statistical significance for linear trend was tested by including the median of each urate quartile as a continuous variable in the regression model. To further minimize the potential impact of prevalent or imminent cases on the analysis, the primary analysis was repeated by excluding 19 cases using antiparkinson medication before visit 2, having a self-reported date of diagnosis before 1995, or having been hospitalized because of or died from Parkinson's disease before 1995. Stratified analyses were further conducted according to baseline age (<55 vs. ≥55 years), sex (men vs. women), race (Caucasian vs. African American), and smoking status (never vs. ever). Partial Pearson correlation coefficients between urate levels at visits 1 and 2 were calculated separately for cases and controls, adjusting for age, sex, race, alcohol intake, body mass index, and plasma creatinine. We also used linear regression models to evaluate whether having Parkinson's disease was associated with change in plasma urate from visit 1 to visit 2, adjusting for potential confounders. All statistical analyses were performed by using SAS software (version 9.1; SAS Institute, Inc., Cary, North Carolina). Statistical tests were considered significant with a 2-sided P < 0.05.
RESULTS
A total of 95 potential Parkinson's disease patients were included in the primary analyses. As expected, smoking and caffeine intake were each associated with a lower risk of Parkinson's disease: after adjusting for age, sex, and race, the odds ratio for current smokers compared with never smokers was 0.6 (95% confidence interval: 0.3, 1.0) and the odds ratio comparing the highest versus lowest quartiles of caffeine intake was 0.4 (95% confidence interval: 0.2, 0.7).
Population characteristics according to urate quartiles at visit 1 are presented in Table 1. Individuals with higher plasma urate concentrations were more likely to be older, men, African American, and past smokers but less likely to be current smokers. As expected, they also had a higher body mass index, higher alcohol consumption, and higher plasma creatinine. Caffeine intake was similar across urate quartiles with the exception of the highest quartile.
Table 1.
Population Characteristics, According to Quartiles of Baseline Plasma Urate Concentration, of Participants in the Atherosclerosis Risk in Communities Study, 1987–1989ab
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| Plasma urate at visit 1, mg/dL | 4.14 (0.57) | 5.36 (0.28) | 6.39 (0.31) | 8.08 (1.02) |
| Plasma urate at visit 2, mg/dL | 4.87 (0.96) | 5.96 (0.92) | 6.88 (1.03) | 8.11 (1.45) |
| Age, years | 53.1 (5.7) | 54.1 (5.7) | 54.5 (5.6) | 54.9 (5.8) |
| Men | 14.2 | 35.3 | 57.7 | 69.4 |
| African American | 21.2 | 24.9 | 25.6 | 31.8 |
| Former smoker | 22.6 | 28.2 | 35.4 | 42.2 |
| Current smoker | 27.8 | 27.7 | 25.4 | 23.4 |
| Caffeine intake, mg/day | 291 (301) | 296 (303) | 293 (293) | 270 (280) |
| Body mass index, kg/m2 | 25.2 (4.4) | 27.1 (5.0) | 28.4 (5.4) | 29.7 (5.4) |
| Total alcohol consumption, g/week | 25.3 (65.6) | 32.7 (72.1) | 47.5 (100.1) | 63.1 (125.0) |
| Plasma creatinine, mg/dL | 0.99 (0.22) | 1.06 (0.21) | 1.13 (0.33) | 1.24 (0.65) |
Means (standard deviations) are provided for continuous variables and percentages for categorical variables.
All variables were statistically different across urate quartiles (P < 0.001). One-way analysis of variance was conducted for continuous variables and chi-square statistics for categorical variables.
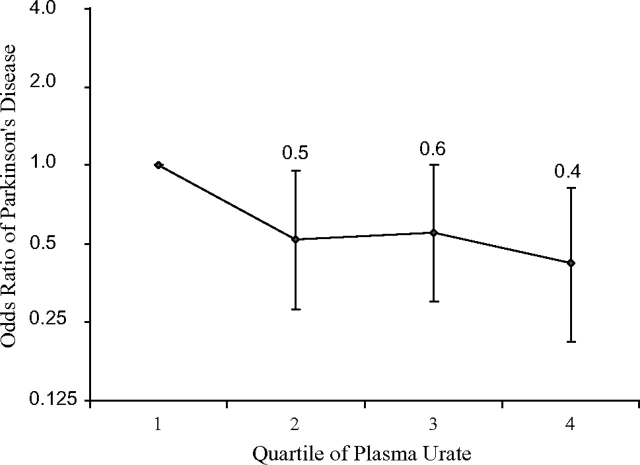
Higher plasma urate concentration at visit 1 was associated with lower occurrence of Parkinson's disease (Figure 1). After we adjusted for age and other potential confounders, compared with individuals whose plasma urate was in the lowest quartile, those in the highest quartile had approximately a 60% lower risk (P for trend = 0.03). The odds ratio estimates were essentially unchanged after excluding cases identified during the early years of follow-up, but the linear trend was attenuated and became nonsignificant because of the reduced number of cases (odds ratio = 0.5, 95% confidence interval: 0.2, 1.1; P for trend = 0.1). Stratified analysis showed similar results by age, sex, race, and smoking status (Table 2). Although the trend test was not statistically significant because of small sample sizes, an inverse association between urate and Parkinson's disease was suggested for women and African Americans, as was shown for men and Caucasians.
Figure 1.
Odds ratios and 95% confidence intervals for Parkinson’s disease, according to quartiles of baseline plasma urate concentration adjusted for age, sex, race, smoking, caffeine intake, body mass index, alcohol intake, and plasma creatinine, in participants in the Atherosclerosis Risk in Communities Study, 1987–2004. P for linear trend across plasma urate quartiles = 0.03.
Table 2.
Odds Ratios and 95% Confidence Intervalsa for Parkinson's Disease, According to Plasma Urate Quartiles in Stratified Analyses, for Participants in the Atherosclerosis Risk in Communities Study, 1987–2004
| Quartile 1 |
Quartile 2 |
Quartile 3 |
Quartile 4 |
P for Trend | ||||||||
| No.b | OR | No.b | OR | 95% CI | No.b | OR | 95% CI | No.b | OR | 95% CI | ||
| Sex | ||||||||||||
| Men | 9/481 | 1.0 | 12/1,332 | 0.5 | 0.2, 1.1 | 18/2,245 | 0.4 | 0.2, 0.9 | 18/2,650 | 0.3 | 0.1, 0.7 | 0.02 |
| Women | 17/2,954 | 1.0 | 8/2,456 | 0.5 | 0.2, 1.3 | 9/1,648 | 0.9 | 0.4, 2.1 | 4/1,175 | 0.5 | 0.2, 1.7 | 0.4 |
| Race | ||||||||||||
| Caucasian | 19/2,709 | 1.0 | 18/2,843 | 0.6 | 0.3, 1.2 | 23/2,892 | 0.6 | 0.3, 1.2 | 18/2,606 | 0.4 | 0.2, 1.0 | 0.05 |
| Black | 7/726 | 1.0 | 2/945 | 0.2 | 0.04, 1.0 | 4/1,001 | 0.4 | 0.1, 1.6 | 4/1,219 | 0.4 | 0.1, 1.6 | 0.3 |
| Baseline age, years | ||||||||||||
| <55 | 10/2,070 | 1.0 | 7/2,026 | 0.5 | 0.2, 1.3 | 11/1,968 | 0.6 | 0.2, 1.5 | 8/1,789 | 0.4 | 0.1, 1.2 | 0.2 |
| ≥55 | 16/1,365 | 1.0 | 13/1,762 | 0.5 | 0.3, 1.1 | 16/1,925 | 0.5 | 0.2, 1.1 | 14/2,036 | 0.4 | 0.2, 1.0 | 0.07 |
| Smoking status | ||||||||||||
| Never | 11/1,706 | 1.0 | 11/1,666 | 0.8 | 0.3, 1.8 | 12/1,525 | 0.7 | 0.3, 1.7 | 8/1,318 | 0.5 | 0.2, 1.3 | 0.1 |
| Ever | 15/1,729 | 1.0 | 9/2,122 | 0.4 | 0.2, 0.9 | 15/2,368 | 0.5 | 0.2, 1.0 | 14/2,507 | 0.4 | 0.2, 0.9 | 0.08 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Adjusted for age, sex, race, smoking, caffeine intake, plasma creatinine, body mass index, and alcohol intake when appropriate.
Values are expressed as number of participants with Parkinson's disease/number of participants without Parkinson's disease in each quartile.
Because of aging of the study participants, urate level increased from visit 1 to visit 2 in those with (6.01–6.52 mg/dL) and without (6.04–6.48 mg/dL) Parkinson's disease. The partial correlation coefficient of plasma urate between visit 1 and visit 2 was 0.56 for Parkinson's disease cases and 0.68 for individuals without Parkinson's disease. Parkinson's disease status was not related to change in urate over a 3-year period between visits 1 and 2 (P = 0.7).
DISCUSSION
In this community-based, biracial cohort, we found that higher plasma urate concentration was associated with lower occurrence of Parkinson's disease. This association was evident among men and Caucasians but was also suggested for women and African Americans. All study participants were 45–64 years of age at study visit 1 and have been followed for nearly 20 years. Because study participants were all younger than 65 years of age at baseline and we excluded possible prevalent cases from the primary analysis, most cases of Parkinson's disease in this analysis were likely incident. Further excluding cases identified during the early years of follow-up did not change the results. Finally, Parkinson's disease status was not related to change in urate levels from visit 1 to visit 2, suggesting that reverse causality is less likely to explain the observed urate–Parkinson's disease relation.
Results of this study are in line with those from previous cohorts of mostly male participants, where an association between higher urate level and lower Parkinson's disease risk was consistently reported (5–7). Only the Rotterdam study included female participants, and the authors reported no difference by sex but did not provide any details (7). Recent analyses of 2 clinical trials among Parkinson's disease patients that were initially designed to investigate other potential neuroprotective agents showed that higher levels of serum or cerebrospinal fluid urate were associated with slower Parkinson's disease progression in men (13, 14); for women, however, the association was not as evident. A sex difference was also observed in the analysis of the General Practice Research Database, in which gout or the use of gout medication was associated with a lower Parkinson's disease risk for men but not for women (15). Although these results are informative, they could not be directly extrapolated to urate and Parkinson's disease development. It is well known that by the time of Parkinson's disease diagnosis, a majority of the dopaminergic neurons have already died. In the case of gout, only a small portion of individuals with high plasma urate concentrations develop gout, and gout risk is much lower for women (16); therefore, a null association regarding gout and Parkinson's disease might not necessarily mean that urate is not related to Parkinson's disease in women. The current analysis, albeit limited by sample size, suggests that the finding on plasma urate and risk of developing Parkinson's disease may be generalizable to women and African Americans.
Prospective data on biomarkers and Parkinson's disease risk are rare (5–7, 17, 18) because such research requires following tens of thousands of biospecimen donors for a long period. This is particularly true for women, who have a lower incidence of Parkinson's disease than men do. Compared with previous studies, the current study included more female Parkinson's disease cases and, also for the first known time, included African Americans. In addition, unlike previous studies, this study measured plasma urate for every eligible cohort participant twice 3 years apart. Although repeated measures over a longer period of time are desirable, the current analysis showed that Parkinson's disease status was not related to change in plasma urate level. Finally, although the possibility of residual confounding cannot be excluded, known risk factors for Parkinson's disease were adjusted throughout the analyses.
The consistency of a urate–Parkinson's disease relation across all available studies suggests that this association is unlikely due to chance. Even though none of the studies could exclude the possibility that the association was caused by unknown common genetic or environmental factors that underlie both urate and Parkinson's disease, the hypothesis that urate protects against Parkinson's disease development and progression should be carefully evaluated. Unlike other mammals, humans and apes have high levels of plasma urate as a result of mutations of uricase occurring millions of years ago (4). The fact that plasma urate increases with age may be advantageous to human aging because urate is a potent scavenger of iron and free radicals, particularly against peroxynitrite, which kills dopaminergic neurons via oxidative stress (4, 19). While epidemiologic data have been consistently supportive of a potential benefit of urate on Parkinson's disease, to our knowledge this issue has received little attention in experimental research. An earlier study found that urate protected dopaminergic neurons from the toxicity of iron and rotenone in mice (20); however, this study was not designed to evaluate the role of urate in animal parkinsonism. An alternative explanation for the current epidemiologic findings is that one or more urate precursors such as adenosine or inosine, rather than urate itself, modulate dopaminergic neuron survival. Each of these possibilities should be evaluated in future investigations, and, if urate or its precursors are etiologically linked to Parkinson's disease, the potential mechanisms should be elucidated.
The major limitations of this study include the lack of a systematic strategy to identify and validate Parkinson's disease diagnosis and the lack of information on date of diagnosis and onset. In the current study, Parkinson's disease cases were identified from multiple sources at various time points. Inevitably, some cases were not identified and some noncases were misclassified as cases. On the other hand, studies on Parkinson's disease based on self-report or medication data have made valuable contributions to the literature (21–24). To evaluate the potential effects of self-reported Parkinson's disease in this particular study, we assessed the relation between Parkinson's disease and smoking or caffeine intake, 2 factors known to be related to lower Parkinson's disease risk. As expected, results obtained were similar to those reported in previous investigations in which systematic approaches to identifying Parkinson's disease were applied (25, 26). Although these findings indirectly support the validity of our study, a systematic strategy to identify and validate Parkinson's disease diagnoses is preferred. Because of the lack of information on date of diagnosis, we were unable to clearly differentiate incident from prevalent cases, nor to calculate Parkinson's disease incidence in the cohort. For the same reason, we used logistic regression but not proportional hazards modeling and therefore were not able to control for selective survival in the analysis. Finally, the small number of female and African-American cases limited the power of statistical analyses; further large studies are needed to confirm these preliminary findings.
Acknowledgments
Author affiliations: Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Honglei Chen); Division of Geriatric Medicine, University of Mississippi Medical Center, Jackson, Mississippi (Thomas H. Mosley); Department of Epidemiology, University of Minnesota, Minneapolis, Minnesota (Alvaro Alonso); and Departments of Neurology, Radiology, Neurosurgery, Pharmacology, Kinesiology & Bioengineering and Hershey Brain Analysis Research Laboratory for Neurodegenerative Disorders, Pennsylvania State University-Milton S. Hershey Medical Center, Hershey, Pennsylvania (Xuemei Huang).
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (grant Z01ES101986). The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022.
The authors thank the staff of the ARIC Study for their important contributions, Dr. Xuguang Guo from Westat Inc. (Durham, North Carolina) for statistical support, and Dr. Richard B. Mailman for his thoughtful comments.
Conflict of interest: none declared.
Glossary
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- ICD
International Classification of Diseases
References
- 1.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53:S26–S38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 2.Ames BN, Cathcart R, Schwiers E, et al. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glantzounis GK, Tsimoyiannis EC, Kappas AM, et al. Uric acid and oxidative stress. Curr Pharm Des. 2005;11(32):4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 4.Kutzing MK, Firestein BL. Altered uric acid levels and disease states. J Pharmacol Exp Ther. 2007;324(1):1–7. doi: 10.1124/jpet.107.129031. [DOI] [PubMed] [Google Scholar]
- 5.Davis JW, Grandinetti A, Waslien CI, et al. Observations on serum uric acid levels and the risk of idiopathic Parkinson's disease. Am J Epidemiol. 1996;144(5):480–484. doi: 10.1093/oxfordjournals.aje.a008954. [DOI] [PubMed] [Google Scholar]
- 6.Weisskopf MG, O'Reilly E, Chen H, et al. Plasma urate and risk of Parkinson's disease. Am J Epidemiol. 2007;166(5):561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lau LM, Koudstaal PJ, Hofman A, et al. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58(5):797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 8.Foley RN, Wang C, Ishani A, et al. NHANES III: influence of race on GFR thresholds and detection of metabolic abnormalities. J Am Soc Nephrol. 2007;18(9):2575–2582. doi: 10.1681/ASN.2006121411. [DOI] [PubMed] [Google Scholar]
- 9.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 10.Iribarren C, Folsom AR, Eckfeldt JH, et al. Correlates of uric acid and its association with asymptomatic carotid atherosclerosis: the ARIC study. Ann Epidemiol. 1996;6(4):331–340. doi: 10.1016/s1047-2797(96)00052-x. [DOI] [PubMed] [Google Scholar]
- 11.Navaneethan SD, Beddhu S. Associations of serum uric acid with cardiovascular events and mortality in moderate chronic kidney disease. Nephrol Dial Transplant. doi: 10.1093/ndt/gfn621. Advance Access: November 25, 2008. (DOI: 10.1093/ndt/gfn621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118(5):496–500. [PubMed] [Google Scholar]
- 13.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol. 2008;65(6):716–723. doi: 10.1001/archneur.2008.65.6.nct70003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ascherio A, LeWitt PA, Watts A, et al. CSF as well as serum urate are predictors of Parkinson's disease progression [abstract LB-2] Presented at the 10th International Conference of Parkinson's Disease and Movement Disorders, Kyoto, Japan, November 26–29, 2006. [Google Scholar]
- 15.Alonso A, Rodríguez LA, Logroscino G, et al. Gout and risk of Parkinson disease: a prospective study. Neurology. 2007;69(17):1696–1700. doi: 10.1212/01.wnl.0000279518.10072.df. [DOI] [PubMed] [Google Scholar]
- 16.Terkeltaub RA. Clinical practice. Gout. N Engl J Med. 2003;349(17):1647–1655. doi: 10.1056/NEJMcp030733. [DOI] [PubMed] [Google Scholar]
- 17.de Lau LM, Koudstaal PJ, Hofman A, et al. Serum cholesterol levels and the risk of Parkinson's disease. Am J Epidemiol. 2006;164(10):998–1002. doi: 10.1093/aje/kwj283. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, O'Reilly EJ, Schwarzschild MA, et al. Peripheral inflammatory biomarkers and risk of Parkinson's disease. Am J Epidemiol. 2007;167(1):90–95. doi: 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- 19.Tieu K, Ischiropoulos H, Przedborski S. Nitric oxide and reactive oxygen species in Parkinson's disease. IUBMB Life. 2003;55(6):329–335. doi: 10.1080/1521654032000114320. [DOI] [PubMed] [Google Scholar]
- 20.Duan W, Ladenheim B, Cutler RG, et al. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson's disease. J Neurochem. 2002;80(1):101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 21.Kamel F, Tanner CM, Umbach DM, et al. Pesticide exposure and self-reported Parkinson's disease in the Agricultural Health Study. Am J Epidemiol. 2007;165(4):364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- 22.Hu G, Jousilahti P, Bidel S, et al. Type 2 diabetes and the risk of Parkinson's disease. Diabetes Care. 2007;30(4):842–847. doi: 10.2337/dc06-2011. [DOI] [PubMed] [Google Scholar]
- 23.Logroscino G, Sesso HD, Paffenbarger RS, Jr, et al. Body mass index and risk of Parkinson's disease: a prospective cohort study. Am J Epidemiol. 2007;166(10):1186–1190. doi: 10.1093/aje/kwm211. [DOI] [PubMed] [Google Scholar]
- 24.Driver JA, Kurth T, Buring JE, et al. Parkinson disease and risk of mortality: a prospective comorbidity-matched cohort study. Neurology. 2008;70(16 pt 2):1423–1430. doi: 10.1212/01.wnl.0000310414.85144.ee. [DOI] [PubMed] [Google Scholar]
- 25.Ross GW, Abbott RD, Petrovitch H, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA. 2000;283(20):2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 26.Hernán MA, Zhang SM, Rueda-deCastro AM, et al. Cigarette smoking and the incidence of Parkinson's disease in two prospective studies. Ann Neurol. 2001;50(6):780–786. doi: 10.1002/ana.10028. [DOI] [PubMed] [Google Scholar]