Abstract
Exposure to environmental tobacco smoke (ETS) is a major risk to human health, and the home is the greatest single source of ETS for children. The authors investigated fetal exposure to paternal smoking at home during pregnancy. Korean families were included as trios of fathers, mothers, and neonates identified in 2005–2007. Sixty-three trios were finally enrolled in this study after exclusion of those in which the mother was a smoker or was regularly exposed to ETS at places other than the home. Nicotine and cotinine concentrations in hair were measured by using liquid chromatography–tandem mass spectrometry to determine long-term exposure to ETS. The difference between neonatal nicotine concentrations in the smoker and nonsmoker groups was not statistically significant. However, in the indoor-smoker group, neonatal nicotine concentrations were significantly higher than in the outdoor and nonsmoker groups (P < 0.05). Furthermore, neonatal nicotine concentrations in the outdoor-smoker group were not different from those in the nonsmoker group. These findings indicate that paternal smoking inside the home leads to significant fetal and maternal exposure to ETS and may subsequently affect fetal health. Conversely, findings show that paternal smoking outside the home prevents the mother and her fetus from being exposed to ETS.
Keywords: fetus, hair, nicotine, smoking, tobacco smoke pollution
Exposure to environmental tobacco smoke (ETS; also referred to as passive smoking) and active smoking present major risks to human health, and they contribute to the development of various human cancers, respiratory illnesses, and other diseases (1, 2). Studies on ETS have been previously conducted in various environments including the home, the greatest single source of ETS for children (3–5). Children exposed to ETS at home are more prone to respiratory illness and are more likely to be admitted to the hospital (6). In addition, maternal smoking increases the risks of premature birth, spontaneous abortion, and intrauterine growth retardation and of other diseases (7–10). However, there is some debate concerning the clinical impact of fetal exposure to paternal smoking for mothers who do not smoke (4). Moreover, few studies have examined the extent to which the fetus is indirectly exposed to paternal smoking or whether paternal smoking adversely affects fetal well-being (11–14). This lack of study is largely attributable to the difficulties of determining fetal exposure to ETS because of the small quantities involved. Therefore, development of a highly sensitive method to assess ETS exposure is a key step before correlation with fetal health outcomes of paternal smoking can be investigated. The smoking place at home can also be an important factor in identifying smokers to assess exposure of a father's family members to significant ETS (15).
Nicotine and its major metabolite cotinine are commonly used as smoking biomarkers, and their levels can be determined in various biologic specimens. Compared with other biologic specimens, hair has several advantages because it can be easily collected, can be stored for a long time without deterioration, and provides better information on long-term (several months) exposure to tobacco smoke (16–19). However, previously described methods require large quantities of hair (3–50 mg) to determine nicotine and cotinine levels, which has limited its use as a smoking biomarker irrespective of its many advantages (20–22).
We recently developed a method based on highly sensitive liquid chromatography–tandem mass spectrometry that requires as little as 1 mg of hair to measure nicotine and cotinine simultaneously (23). In the present study, we used this method to measure maternal and neonatal nicotine and cotinine concentrations to determine whether the fetuses of mothers who do not smoke are significantly exposed to ETS by paternal smoking and how different this exposure is according to the smoking place at home.
MATERIALS AND METHODS
Subject characteristics
We identified trios of potential subjects consisting of neonates and both parents at the Newborn Care Unit of Inje University Ilsan Paik Hospital in Korea from April 2005 to March 2007. Informed consent was obtained from mothers or fathers, and candidates were asked to complete a questionnaire that detailed the smoking histories of family members. In the present study, we approached 107 families and selected 92 candidate families in which the mother was a nonsmoker and had no regular exposure to ETS at places other than the home. About 2–3 mg of hair was collected from all members of each family. Only 63 of 92 candidate families entered the final analysis after we excluded those who did not complete the questionnaire or provide a sufficient hair sample. Of the 63 families enrolled, 27 were totally nonsmoking and the other 36 had only fathers who smoked. The smoker group was subdivided into an outdoor-smoker group (27 families) and an indoor-smoker group (9 families) according to whether the father smoked inside or outside the home. Smoking on a veranda could be classified as inside or outside, but we decided to classify it as inside smoking for apartments and so classified it in this study.
Measurement of hair nicotine and cotinine levels
Hair nicotine and cotinine levels were determined blinded to the smoking status of study subjects, and the liquid chromatography–tandem mass spectrometry method, previously described (23), was used. Hair samples of 1 mg were finely cut, washed for 1 hour with 2 mL of dichloromethane, and then digested for 90 minutes with 0.9 mL of 1 mol/L sodium hydroxide at 60°C. The digested samples were mixed with internal standards of nicotine and cotinine extracted with 2 mL of diethyl ether, and extracts were evaporated for 40 minutes at 50°C, redissolved in 50 μL of mobile phase (methanol-water (80:20, v/v)), and injected (10 μL) into the liquid chromatography–tandem mass spectrometry system. The high-pressure liquid chromatography unit used was an HP 1100 (Agilent Technologies, Inc., Santa, Clara, California), and the tandem mass spectrometer was an API 4000 machine (Applied Biosystems, Foster City, California/MDS Inc., Concord, Ontario, Canada; Sciex) equipped with an atmospheric pressure chemical ionization interface.
Statistical analysis
The Mann-Whitney test was used to compare groups regarding hair nicotine and cotinine levels. Spearman rank correlation coefficients (r) were calculated for correlation between nicotine levels at the family level. P values of <0.05 were regarded as significant, and data in this paper are expressed as mean (standard deviation (SD)). All analyses were performed by using SPSS software (release 12.0; SPSS, Inc., Chicago, Illinois).
RESULTS
Paternal, maternal, and neonatal nicotine concentrations (ng/mg hair) in the smoker group were 30.21 (SD, 32.75), 1.39 (SD, 1.83), and 0.20 (SD, 0.39) and in the nonsmoker group were 2.67 (SD, 3.01), 0.51 (SD, 0.49), and 0.12 (SD, 0.15), respectively (Table 1, Figure 1). Paternal, maternal, and neonatal cotinine concentrations (ng/mg hair) in the smoker group were 2.96 (SD, 1.79), 0.14 (SD, 0.17), and 0.04 (SD, 0.10) and in the nonsmoker group were 0.22 (SD, 0.19), 0.05 (SD, 0.05), and 0.04 (SD, 0.06), respectively. According to the Mann-Whitney test, fathers who smoked had significantly higher hair nicotine and cotinine levels than fathers who did not smoke (P < 0.05) (Table 2). Maternal nicotine and cotinine levels in the smoker group were also significantly higher than those in the nonsmoker group (P < 0.05). However, differences between neonatal nicotine or cotinine levels in the smoker and nonsmoker groups were not statistically significant.
Table 1.
Nicotine and Cotinine Concentrationsa in Neonatal, Maternal, and Paternal Hair in the Study Groups, Korea, 2005–2007
| Smoker Group | No. | Nicotine | Cotinine | ||||
| Neonate | Mother | Father | Neonate | Mother | Father | ||
| No smoker | 27 | 0.12 (0.15) | 0.51 (0.49) | 2.67 (3.01) | 0.04 (0.06) | 0.05 (0.05) | 0.22 (0.19) |
| Smoker father | 36 | 0.20 (0.39) | 1.39 (1.83) | 30.21 (32.75) | 0.04 (0.10) | 0.14 (0.17) | 2.96 (1.79) |
| Outdoor | 27 | 0.09 (0.11) | 0.80 (0.81) | 25.89 (32.23) | 0.02 (0.05) | 0.10 (0.09) | 2.82 (1.69) |
| Indoor | 9 | 0.53 (0.69) | 3.18 (2.77) | 43.18 (29.18) | 0.11 (0.17) | 0.29 (0.28) | 3.38 (2.09) |
Values are expressed as mean (standard deviation) concentration, ng/mg hair.
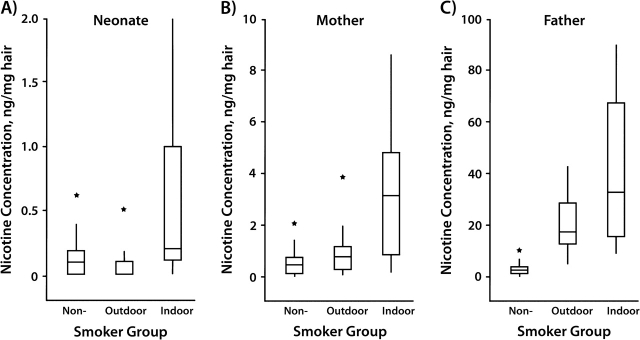
Figure 1.
Comparative box plots of nicotine concentrations (ng/mg) in A) neonatal, B) maternal, and C) paternal hair by smoker group, Korea, 2005–2007. Y-axes in each of the 3 parts are scaled differently depending on distribution of nicotine concentrations in each group. Asterisks denote outliers. The vertical line indicates the range of concentrations observed within each group, the box indicates the 25th and 75th percentiles, and the horizontal line in each box indicates the median concentration in each group.
Table 2.
Comparison of Nicotine and Cotinine Concentrationsa in the Study Groups, Korea, 2005–2007
| Smoker Group | Nicotine | Cotinine | ||||
| Neonate | Mother | Father | Neonate | Mother | Father | |
| No smoker vs. smoker father | 0.529 | 0.019 | <0.001 | 0.280 | 0.008 | <0.001 |
| Outdoor vs. indoor | 0.008 | 0.009 | 0.056 | 0.205 | 0.016 | 0.517 |
| No smoker vs. outdoor vs. indoor | 0.023 | 0.004 | <0.001 | 0.13 | 0.001 | <0.001 |
| No smoker or outdoor vs. indoor | 0.006 | 0.002 | 0.001 | 0.229 | 0.001 | 0.006 |
| No smoker vs. outdoor | 0.746 | 0.142 | <0.001 | 0.090 | 0.087 | <0.001 |
Values are expressed as P values based on the Mann-Whitney test.
Maternal and fetal exposure to ETS showed large differences according to whether the father smoked inside or outside the home. Respective paternal nicotine and cotinine concentrations (ng/mg hair) were 25.89 (SD, 32.23) and 2.82 (SD, 1.69) in the outdoor-smoker group and 43.18 (SD, 29.18) and 3.38 (SD, 2.09) in the indoor-smoker group, which were not statistically different. Maternal and neonatal nicotine concentrations (ng/mg hair) in the outdoor-smoker group were 0.80 (SD, 0.81) and 0.10 (SD, 0.09), and cotinine concentrations were 0.09 (SD, 0.11) and 0.02 (SD, 0.05), respectively; these findings were no different from those for the nonsmoker group. However, in the indoor-smoker group, maternal and neonatal nicotine concentrations (ng/mg hair) were 3.18 (SD, 2.77) and 0.29 (SD, 0.28), and cotinine concentrations were 0.53 (0.69) and 0.11 (SD, 0.17), respectively; all, except for neonatal cotinine concentrations, were significantly higher than those in the outdoor-smoker and nonsmoker groups (P < 0.05).
In the indoor-smoker group, neonatal nicotine and maternal nicotine levels showed a mild correlation (Spearman rank correlation coefficient, r = 0.638). However, we found a weak correlation between paternal nicotine and maternal nicotine (r = 0.250) or neonatal nicotine (r = 0.162) levels.
DISCUSSION
The results of this study show that fathers who smoke indoors expose their pregnant spouses and unborn children to significant ETS. Overall, the ratio of hair nicotine concentrations indicates that a pregnant woman is exposed to 7.4% (ratio of maternal to paternal nicotine level) of the total smoke consumed by her husband all day long and that this amount will be increased when smoking only at home is considered. Moreover, 16.7% (ratio of neonatal to maternal nicotine level) of smoke inhaled by the mother is then transferred to her fetus. Thus, the ETS generated by a father who smokes may affect fetal and maternal health. Further investigations are required to clarify the subsequent health effects. Paternal nicotine concentrations in hair in the outdoor-smoker group were lower than those in the indoor-smoker group, but this difference was not significant. Therefore, we conclude that differences between maternal and neonatal nicotine/cotinine concentrations in the outdoor-smoker and indoor-smoker groups are not due to differences in paternal smoking but to exposure at home.
Maternal and neonatal nicotine/cotinine levels in the outdoor-smoker group were not significantly higher than those in the nonsmoker group. Therefore, outside smoking substantially reduces maternal and fetal exposure.
Paternal nicotine and cotinine concentrations in hair were found to be weakly correlated with neonatal or maternal nicotine and cotinine concentrations. Although average maternal/neonatal concentrations in the indoor-smoker group were significantly higher than those in the outdoor-smoker group or nonsmoker group, the weak quantitative correlation observed among these family members in the indoor-smoker group is likely to have been caused by factors other than exposure to ETS; hair nicotine and cotinine concentrations are known to be affected by various individual factors such as nicotine metabolism, hair growth, and number of cigarettes smoked (18, 24). The impact of these individual factors can be supported by our finding that a correlation between even maternal nicotine and neonatal nicotine was not very strong. In addition, maternal exposure to ETS in this study came from only paternal smoking at home, but paternal nicotine concentration itself is related to total cigarette smoke. Therefore, it is suggested that, overall, maternal or fetal nicotine concentrations were higher in the indoor-smoker group than in other groups but, for these reasons, might show a weak correlation with paternal nicotine concentration itself at the family level.
Neonatal hair cotinine levels have been suggested to be a useful marker of fetal exposure to maternal smoking (25, 26). However, in terms of fetal exposure to paternal smoking, our study suggests that hair nicotine rather than hair cotinine is a useful marker. This finding can be explained by a difference in neonatal hair nicotine level and cotinine level. Average neonatal nicotine level (0.53 ng/mL) in the indoor-smoker group was much higher than its detection limit of 0.16 ng/mL, but cotinine level (0.11 ng/mL) was slightly higher than its detection limit of 0.07 ng/mL. Therefore, cotinine level may not represent fetal exposure below a certain level. The usefulness of hair cotinine seems to be questionable in the study of fetal exposure to ETS by paternal smoking because of its low concentration.
Summarizing, our findings indicate that paternal smoking inside the home leads to significant fetal and maternal exposure to ETS. We also found that paternal smoking outside the home helpfully reduces levels of ETS to which the smoker's wife and her fetus are exposed.
Acknowledgments
Author affiliations: Department of Laboratory Medicine, National Cancer Center, Goyang, Korea (Moon-Woo Seong, Hye-Jung Ryu, Sun-Young Kong, Do-Hoon Lee); Department of Pediatrics, Inje University Ilsan Paik Hospital, Goyang, Korea (Jong Hee Hwang, Jin Soo Moon); Department of Laboratory Medicine, Inje University Ilsan Paik Hospital, Goyang, Korea (Tae Hyun Um); and Cancer Research Institute and Cancer Research Center, Seoul National University, Seoul, Korea (Jae-Gahb Park).
This work was supported by research grants from the National Cancer Center (N0610010), Korea.
Conflict of interest: none declared.
Glossary
Abbreviations
- ETS
environmental tobacco smoke
- SD
standard deviation
References
- 1.Tobacco Smoke and Involuntary Smoking. Lyon, France: International Agency for Research on Cancer; 2004. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; vol 83. [Google Scholar]
- 2.Spitzer WO, Lawrence V, Dales R, et al. Links between passive smoking and disease: a best-evidence synthesis. A report of the Working Group on Passive Smoking. Clin Invest Med. 1990;13(1):17–42. discussion 3–6. [PubMed] [Google Scholar]
- 3.The GTSS Collaborative Group. A cross country comparison of exposure to secondhand smoke among youth. Tob Control. 2006;15(suppl 2) doi: 10.1136/tc.2006.015685. ii4–ii19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. ( http://www.surgeongeneral.gov/library/secondhandsmoke/) [PubMed] [Google Scholar]
- 5.Rushton L. Health impact of environmental tobacco smoke in the home. Rev Environ Health. 2004;19(3–4):291–309. [PubMed] [Google Scholar]
- 6.Samet JM, Lewit EM, Warner KE. Involuntary smoking and children's health. Future Child. 1994;4(3):94–114. [PubMed] [Google Scholar]
- 7.Bearer C, Emerson RK, O'Riordan MA, et al. Maternal tobacco smoke exposure and persistent pulmonary hypertension of the newborn. Environ Health Perspect. 1997;105(2):202–206. doi: 10.1289/ehp.97105202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Key AP, Ferguson M, Molfese DL, et al. Smoking during pregnancy affects speech-processing ability in newborn infants. Environ Health Perspect. 2007;115(4):623–629. doi: 10.1289/ehp.9521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell EA, Thompson JM, Robinson E, et al. Smoking, nicotine and tar and risk of small for gestational age babies. Acta Paediatr. 2002;91(3):323–328. doi: 10.1080/08035250252834003. [DOI] [PubMed] [Google Scholar]
- 10.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663–737. [PMC free article] [PubMed] [Google Scholar]
- 11.Eskenazi B, Prehn AW, Christianson RE. Passive and active maternal smoking as measured by serum cotinine: the effect on birthweight. Am J Public Health. 1995;85(3):395–398. doi: 10.2105/ajph.85.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaakkola JJ, Jaakkola N, Zahlsen K. Fetal growth and length of gestation in relation to prenatal exposure to environmental tobacco smoke assessed by hair nicotine concentration. Environ Health Perspect. 2001;109(6):557–561. doi: 10.1289/ehp.01109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nafstad P, Fugelseth D, Qvigstad E, et al. Nicotine concentration in the hair of nonsmoking mothers and size of offspring. Am J Public Health. 1998;88(1):120–124. doi: 10.2105/ajph.88.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebagliato M, Florey C, du V, Bolumar F. Exposure to environmental tobacco smoke in nonsmoking pregnant women in relation to birth weight. Am J Epidemiol. 1995;142(5):531–537. doi: 10.1093/oxfordjournals.aje.a117671. [DOI] [PubMed] [Google Scholar]
- 15.Al-Delaimy WK, Crane J, Woodward A. Is the hair nicotine level a more accurate biomarker of environmental tobacco smoke exposure than urine cotinine? J Epidemiol Community Health. 2002;56(1):66–71. doi: 10.1136/jech.56.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahlsen K, Nilsen OG. Nicotine in hair of smokers and non-smokers: sampling procedure and gas chromatographic/mass spectrometric analysis. Pharmacol Toxicol. 1994;75(3–4):143–149. doi: 10.1111/j.1600-0773.1994.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 17.Tuomi T, Johnsson T, Reijula K. Analysis of nicotine, 3-hydroxycotinine, cotinine, and caffeine in urine of passive smokers by HPLC-tandem mass spectrometry. Clin Chem. 1999;45(12):2164–2172. [PubMed] [Google Scholar]
- 18.Al-Delaimy WK, Crane J, Woodward A. Questionnaire and hair measurement of exposure to tobacco smoke. J Expo Anal Environ Epidemiol. 2000;10(4):378–384. doi: 10.1038/sj.jea.7500102. [DOI] [PubMed] [Google Scholar]
- 19.Seong MW, Nam MH, Ryu HJ, et al. The comparison of two smoking biomarkers in various biological samples. Clin Chim Acta. 2007;383(1–2):180–181. doi: 10.1016/j.cca.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 20.Kalinić N, Skender L, Karacić V, et al. Passive exposure to tobacco smoke: hair nicotine levels in preschool children. Bull Environ Contam Toxicol. 2003;71(1):1–5. doi: 10.1007/s00128-003-0121-2. [DOI] [PubMed] [Google Scholar]
- 21.Chetiyanukornkul T, Toriba A, Kizu R, et al. Hair analysis of nicotine and cotinine for evaluating tobacco smoke exposure by liquid chromatography-mass spectrometry. Biomed Chromatogr. 2004;18(9):655–661. doi: 10.1002/bmc.369. [DOI] [PubMed] [Google Scholar]
- 22.Klein J, Blanchette P, Koren G. Assessing nicotine metabolism in pregnancy—a novel approach using hair analysis. Forensic Sci Int. 2004;145(2–3):191–194. doi: 10.1016/j.forsciint.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 23.Ryu HJ, Seong MW, Nam MH, et al. Simultaneous and sensitive measurement of nicotine and cotinine in small amounts of human hair using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20(18):2781–2782. doi: 10.1002/rcm.2659. [DOI] [PubMed] [Google Scholar]
- 24.Dempsey D, Jacob P, III, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301(2):594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 25.Eliopoulos C, Klein J, Phan MK, et al. Hair concentrations of nicotine and cotinine in women and their newborn infants. JAMA. 1994;271(8):621–623. [PubMed] [Google Scholar]
- 26.Jacqz-Aigrain E, Zhang D, Maillard G, et al. Maternal smoking during pregnancy and nicotine and cotinine concentrations in maternal and neonatal hair. BJOG. 2002;109(8):909–911. doi: 10.1111/j.1471-0528.2002.01322.x. [DOI] [PubMed] [Google Scholar]